Abstract
Controlled mechanical ventilation (CMV) plays a key role in triggering the impaired diaphragm muscle function and the concomitant delayed weaning from the respirator in critically ill intensive care unit (ICU) patients. To date, experimental and clinical studies have primarily focused on early effects on the diaphragm by CMV, or at specific time points. To improve our understanding of the mechanisms underlying the impaired diaphragm muscle function in response to mechanical ventilation, we have performed time-resolved analyses between 6 h and 14 days using an experimental rat ICU model allowing detailed studies of the diaphragm in response to long-term CMV. A rapid and early decline in maximum muscle fibre force and preceding muscle fibre atrophy was observed in the diaphragm in response to CMV, resulting in an 85% reduction in residual diaphragm fibre function after 9–14 days of CMV. A modest loss of contractile proteins was observed and linked to an early activation of the ubiquitin proteasome pathway, myosin:actin ratios were not affected and the transcriptional regulation of myosin isoforms did not show any dramatic changes during the observation period. Furthermore, small angle X-ray diffraction analyses demonstrate that myosin can bind to actin in an ATP-dependent manner even after 9–14 days of exposure to CMV. Thus, quantitative changes in muscle fibre size and contractile proteins are not the dominating factors underlying the dramatic decline in diaphragm muscle function in response to CMV, in contrast to earlier observations in limb muscles. The observed early loss of subsarcolemmal neuronal nitric oxide synthase activity, onset of oxidative stress, intracellular lipid accumulation and post-translational protein modifications strongly argue for significant qualitative changes in contractile proteins causing the severely impaired residual function in diaphragm fibres after long-term mechanical ventilation. For the first time, the present study demonstrates novel changes in the diaphragm structure/function and underlying mechanisms at the gene, protein and cellular levels in response to CMV at a high temporal resolution ranging from 6 h to 14 days.
Key points
Weaning from mechanical ventilation (MV) of long-term intensive care unit (ICU) patients is delayed by impaired respiratory muscle function; however, the mechanisms that cause the impairment are not fully understood.
A novel experimental rat ICU model was used for time-resolved analyses (6 h to 2 weeks) of the effects of MV on diaphragm muscle fibre structure and function, and on gene and protein expression.
A prompt and progressive decline of diaphragm muscle fibre function, preceding atrophy, occurred with MV, and at the end of 2 weeks residual diaphragm muscle fibre function was <15% of control levels.
Cellular and subcellular analyses indicated that oxidative stress-triggered protein modifications had significantly diminished diaphragm muscle fibre function.
The novel finding that activation of proteolytic pathways and regulation of contractile protein synthesis were different in diaphragm and limb muscles has direct implications for the design of muscle-specific intervention strategies.
Introduction
Delayed weaning from the mechanical ventilator is frequently observed in critically ill intensive care unit (ICU) patients, increasing the ICU stay by 50% with significant negative social and economic consequences for patients and health care providers (Esteban et al. 2002). The understanding of the mechanisms underlying this delay is incomplete, but recent studies indicate that prolonged controlled mechanical ventilation (CMV) is a key factor triggering the impairment in diaphragm muscle function (Le Bourdelles et al. 1994; Anzueto et al. 1997; Powers et al. 2002; Radell et al. 2002; Yang et al. 2002; Gayan-Ramirez et al. 2004; De Jonghe et al. 2007). A rapid severe atrophy and impaired diaphragm muscle function has been reported in ICU patients and in experimental ICU models in response to CMV (Le Bourdelles et al. 1994; Anzueto et al. 1997; Powers et al. 2002; Radell et al. 2002; Yang et al. 2002; Gayan-Ramirez et al. 2003; De Jonghe, 2007). To date, however, clinical and experimental studies have primarily focused on the early effects of CMV (Le Bourdelles et al. 1994; Powers et al. 2002; Gayan-Ramirez et al. 2004), or at specific time points (Anzueto et al. 1997; Radell et al. 2002; Yang et al. 2002). This is, at least in part, due to logistic limitations in clinical studies and in experimental studies using large mammals. In experimental rodent studies, the technical limitations of commercially available ventilators typically restrict measurements to short durations of 1–2 days or less due to the inability to maintain life support for longer durations. However, the effects of mechanical ventilation on diaphragm muscle function involve multiple mechanisms, including specific alterations in synthesis, degradation and post-translational modifications of contractile proteins, i.e. proteins with slow turnover rates and with half-lives typically longer than one week. Thus, in order to improve our understanding of underlying mechanisms, it is of vital importance to study the time course with high temporal resolution ranging from short (hours) to long (weeks) durations of mechanical ventilation-induced changes at the gene and protein levels as well as consequences for diaphragm muscle structure and function.
The present study aims at improving our understanding of the mechanisms underlying the impaired diaphragm muscle function in response to mechanical ventilation by performing temporal analyses of diaphragm muscle contractile gene/protein expression and post-translational modifications, as well as the effects on regulation of muscle contraction in membrane-permeabilized single muscle fibres and high resolution analyses of contractile protein organization using X-ray diffraction analyses. An experimental rat model not limited by early mortality was used, allowing time-resolved studies for long durations (multiple weeks), i.e. a unique experimental model where animals undergo postsynaptic neuromuscular blockade (NMB), are sedated, mechanically ventilated and extensively monitored for durations varying between 6 h and 14 days. It was hypothesized that diaphragm muscle fibre size and function would be more severely affected by the ICU intervention than the changes we have previously observed in limb muscles (Ochala et al. 2011b), but our results only partially confirmed our initial hypotheses. In accordance with our hypotheses, a more severe and earlier decline in specific force was observed in the diaphragm than in limb muscle fibres, and preceded diaphragm muscle fibre atrophy observed first after 5 days and longer exposure to the ICU intervention. The mechanisms underlying the loss in muscle function in the diaphragm differed from our previous observations in limb muscles with significant consequences for the design of future specific intervention strategies.
Methods
Animals
The Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine and the Ethical committee at Uppsala University approved all aspects of this study.
A total of 15 sham-operated controls and 46 anaesthetized and mechanically ventilated female Sprague–Dawley rats treated with α-cobrotoxin (the neuromuscular blocker used in our experimental rats) for durations varying from 6 h to 14 days were included in the original study (Ochala et al. 2011b) and a subsample of four animals from the control, 0.25–4 day (0.25, 0.80, 1.13 and 4 days), 5–8 day (5, 5, 8 and 8 days), and the 9–14 day (9, 10, 11 and 14 days) groups were included in this study. Animals were maintained in protein and fluid balance, i.e. (1) an intra-arterial solution (0.6 ml h−1) consisting of 50 ml H2O, 50 ml 0.5 n lactated Ringer, 1.25 g oxacillin sodium, 2.8 mg α-cobratoxin, 0.3 mg vitamin K (Synkavite) and 20 mequiv K+ (as KCl); (2) an intravenous solution (0.6 ml h−1) consisting of 50 ml H2O, 50 ml 0.5 n lactated Ringer, 20% glucose (Baxter, Deerfield, IL, USA) and 1.25 g oxacillin sodium for the initial 48 h, then 8.5% Travasol amino acids (Baxter) and 20% Intralipid (Kabi, Uppsala, Sweden) were added subsequently to provide adequate nutrients (Dworkin & Dworkin, 1990, 2004). This regimen provides a total caloric intake of approximately 8 kcal day–1. The sham-operated control animals underwent the same interventions as the controls, but neuromuscular transmission was not blocked with α-cobrotoxin. That is, sham-operated controls were anaesthetized (isoflurane), allowed to breath spontaneously, given intra-arterial and intravenous solutions, and killed within 2 h after the initial anaesthesia and surgery.
During surgery or any possible irritating manipulation, the anaesthetic isoflurane level was at >1.5%, which maintained the following states: (1) the electroencephalogram (EEG) was synchronized and dominated by high-voltage slow-wave activity; (2) mean arterial pressure was 100 mmHg and heart rate was 420 beats min−1; and (3) there was no evident EEG, blood pressure or heart rate responses to surgical manipulation. Isoflurane was delivered accurately into the inspiratory gas stream by a precision mass-flow controller. After the initial surgery, isoflurane concentration was gradually reduced (over 1–2 days) and then, during periods when the rat was undisturbed, maintained at <0.5% for the duration of the experiment. Rats were ventilated through a per os coaxial tracheal cannula at 72 breaths min−1 with an inspiratory and expiratory ratio of 1:2 and a minute volume of 180–200 ml, and gas concentrations of 49.5% O2, 47% N2 and 3% CO2, delivered by a precision (volume drift <1% week–1) volumetric respirator. Intermittent hyperinflations (6 per hour at 15 cmH2O), positive end-expiratory pressure (1.5 cmH2O), and expiratory CO2 monitoring were continuous. Neuromuscular block was induced on the first day (100 μg i.v., α-cobrotoxin) and maintained by continuous infusion (250 μg day−1, i.v.). Mechanical ventilation started immediately after the NMB induction. Continuous monitoring in the laboratory, and via a remote computer connection, of vital signs, including: heart rate, blood pressure, EEG, respiratory resistance and urine output ensured that the rats were stable and not in distress. Dworkin & Dworkin (2004; see Figs 1 and 2) in an extensive series of experiments demonstrated that rats maintained with the procedures used here remain stable, with normal baseline cardiovascular levels, and normal cyclic variability of cardiovascular function during experiments of 30 days or more. The neuromuscularly blocked rats also maintain normal sleep and other ultradian cycles, with concomitant variability in autonomic reflexes. The rats were studied one at a time, monitored, and attended around the clock. Experiments were terminated at durations varying between 6 h and 14 days. All animals were killed as follows: while under deep isoflurane anaesthesia (>2.0%), via a midline thoracotomy, a blood sample was obtained from the heart, the ascending aorta bisected, and the heart removed. In no case did animals show any signs of infections or septicaemia.
Figure 1. Muscle fibre size, specific force, myosin protein contenst and mRNA expression of actin and MyHC isoforms.

Single muscle fibre cross-sectional area measured at fixed sarcomere length (A) and specific force (B) in control animals (white bars) and animals exposed to CMV for durations of 0.25–4, 5–8 and 9–14 days (black bars). Haematoxylin and Eosin staining of cross-sections (C, top) (×20) and single muscle fibres (C, bottom) of rat diaphragm in control (Ctl), and 1, 5 and 10 days of CVM. All fibres were fixed at 2.65–2.75 μm sarcomere length. Cross-striation pattern was weaker at longer durations due to a general loss of myofibrillar proteins, but it was possible to measure sarcomere length at high magnification. At the electron microscope level, sarcomeres were intact at all durations (not shown). D, myosin protein content normalized to total protein content. E, actin (black), and MyHC type IIa (white) and type IIx (grey) mRNA expression in the diaphragm muscle from control animals and animals exposed to CMV for 0.25–4, 5–8 and 9–14 days. Values are means + SEM. *P < 0.05, ***P < 0.001: significant difference compared with controls. Scale bars represent 50 μm.
Figure 2. Intracellular lipid content in individual diaphragm muscle fibres.

A, Oil-red-O staining of diaphragm muscle cross-sections in a control animal and in animals exposed to 6, 24, 32 and 180 h of CMV and neuromuscular blockade. The horizontal bar corresponds to 50 μm. B, the average total single muscle fibre intracellular lipid droplet volumes normalized to cell volume in type I (•) and type II (○) fibres classified according to myofibrillar ATPase enzyme histochemistry. Values are means ± 95% confidence intervals. C, log-transformed data from controls and animals exposed to mechanical ventilation for 6, 18, 24 and 32 h. The fitted line is a transition function (extreme value cumulative function): y = a + b × e∧(–e∧(–(x – d × ln(ln2) – c)/d)); a = −5.983, b = 1.375, c = 18.349, d = 0.214. r2 = 0.464, F3,236 = 68.21, P < 0.001. Each circle represents a single muscle fibre.
Muscle biopsies and permeabilization of fibres
The diaphragm muscle was dissected immediately after the animals were killed. One half of the diaphragm muscle was quickly frozen in liquid propane cooled by liquid nitrogen and stored at −160°C for further analyses. The other half was placed in relaxing solution at 4°C and bundles of ∼50 fibres were dissected free and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. The bundles were then treated with skinning solution (relaxing solution containing glycerol; 50:50 v/v) for 24 h at 4°C, after which they were transferred to –20°C. The muscle bundles were treated with sucrose, a cryo-protectant, within 1–2 weeks for long-term storage (Frontera & Larsson, 1997). After the sucrose treatment, muscle bundles were detached from the capillary tubes and snap frozen in liquid nitrogen-chilled propane and stored at −160°C. One day before the experiments, a bundle was transferred to a 2.0 m sucrose solution for 30 min, subsequently incubated in solutions of decreasing sucrose concentration (1.5–0.5 m) and finally kept in a skinning solution at −20°C.
Single muscle fibre experimental procedure
On the day of an experiment, a fibre segment 1–2 mm long was left exposed to the experimental solution between connectors leading to a force transducer (model 400A, Aurora scientific, Watertown MA, US) and a lever arm system (model 308B, Aurora Scientific) (Moss, 1979). The total compliance of the attachment system was carefully checked and remained similar for all the single muscle fibres tested (5 ± 0.5% of the fibre length). The apparatus was mounted on the stage of an inverted microscope (model IX70, Olympus, Edgmont PA, US). While the fibre segments were in relaxing solution, the sarcomere length was set to 2.65–2.75 μm by adjusting the overall segment length (Larsson & Moss, 1993). The diameter of the fibre segment between the connectors was measured through the microscope at a magnification of ×320 with an image analysis system prior to the mechanical experiments. Fibre depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fibre. The focusing control of the microscope was used as a micrometer. Fibre cross-sectional area (CSA) was calculated from the diameter and depth, assuming an elliptical circumference, and was corrected for the 20% swelling that is known to occur during skinning (Moss, 1979).
Relaxing and activating solutions contained (in mm) 4 Mg-ATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate, and KCl to adjust the ionic strength to 180 mm. The pH was adjusted to 7.0. The concentrations of free Ca2+ were 10−9 m (relaxing solution) and 10−4.5 m (activating solution), expressed as pCa values (i.e. –log [Ca2+]). Apparent stability constants for Ca2+-EGTA were corrected for temperature (15°C) and ionic strength (180 mm). The computer program of Fabiato (1988) was used to calculate the concentrations of each metal, ligand and metal–ligand complex.
At 15°C, immediately preceding each activation, the fibre was immersed for 10–20 s in a solution with a reduced Ca2+-EGTA buffering capacity. This solution was identical to the relaxing solution except that the EGTA concentration was reduced to 0.5 mm, which results in more rapid attainment of steady force during subsequent activation. Force was measured by the slack-test procedure (Edman, 1979). This was calculated as the difference between the maximal steady-state isometric force in activating solution and the resting force measured in the same segment while in the relaxing solution. Maximal force production was normalized to CSA (specific force, P0/CSA).
For contractile measurements, strict acceptance criteria were applied. First, the sarcomere length was checked during the experiments using a high-speed video analysis system (model 901A HVSL, Aurora Scientific). A muscle fibre was accepted and included in the analyses: (i) if the sarcomere length of a single muscle fibre changed <0.10 μm between relaxation and maximum activation, (ii) if maximal force changed <10% from first to final activation (Moss, 1979).
After the mechanical measurements, each fibre was placed in urea buffer (120 g urea, 38 g thiourea, 70 ml H2O, 25 g mixed bed resin, 2.89 g dithiothreitol, 1.51 g Trizma base, 7.5 g SDS, 0.004% bromophenol blue) in a plastic microcentrifuge tube and stored at −80°C.
Myosin heavy chain (MyHC) isoform expression
The MyHC isoform composition of fibres was determined by 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Sample loads were kept small (equivalent to ∼0.05 mm of fibre segment) to improve the resolution of the MyHC bands (slow and fast MyHC: type I, IIa, IIx and IIb). Electrophoresis was performed at 120 V for 24 h with a Tris–glycine electrode buffer (pH 8.3) at 15°C (SE 600 vertical slab gel unit, Hoefer Scientific Instruments, San Fransisco CA, US). The gels were silver-stained and subsequently scanned in a soft laser densitometer (Molecular Dynamics, Sunnyvale CA, US) with a high spatial resolution (50 μm pixel spacing) and 4096 optical density levels.
MyHC quantification
Diaphragm muscle tissue was homogenized in SDS-gel sample buffer (50 mm Tris-HCl, 2% SDS, 0.1% bromophenol blue, 10% glycerol and 2% β-mercaptoethanol, pH 8.8) and resolved on 14% SDS-PAGE with an acrylamide:bisacrylamide ratio of 180:1. After staining with Coomassie blue R-250, the gel was scanned at 600 dpi and the image densitometry was analysed using ImageJ Software (Amersham Biosciences, Freiburg, Germany). The relative level of total MyHC was quantified by normalization to the whole lane densitometry that represents the amount of total protein input.
Protein carbonylation detection
To assess the formation of protein carbonyl groups, the OxyBlot protein oxidation detection kit (Chemicon, Chandlers Ford, UK) was used as previously described (Dalla Libera et al. 2009). In brief, about 10 muscle cryosections (12 μm) were solubilized at 4°C in 100 μl of 0.01% tetrafluoroacetic acid containing protease inhibitors, 5 mm EDTA and 2% β-mercaptoethanol. About 12 μg of total protein was then used for derivatization with 2,4-dinitrophenylhydrazine (DNPH) according to the manufacturer's detailed protocol and processed for Western blot analysis. A positive control included derivatization of 3 μg of oxidized bovine serum albumin (BSA), whereas the negative control was performed with an equal amount of total protein reacted in the absence of DNPH. Levels of oxidated protein were quantified using the NIH ImageJ analysis software and normalized as described in Dalla Libera et al. (2009).
Mass spectrometry
In-gel digestion
The type I, IIa, IIx and, in some instances, IIb, MyHC isoforms were separated on a 6% SDS-PAGE gel. The bands were cut out and stored at −80°C before the sample preparation took place. A thawed gel piece was first washed with 100 mm NH4HCO3 and then reduced with 10 mm dithiothreitol at 56°C for 30 min. Alkylation was initiated by adding iodoacetamide in 2.2-fold excess and the sample was then incubated in darkness at room temperature for 40 min. The solution was removed and the gel piece was destained by rinsing with 50 mm NH4HCO3 in 50% acetonitrile (ACN). The solution was removed and the gel piece was rinsed with ACN. The gel piece was dried at 50°C before the addition of 0.025 μg ml−1 trypsin. The gel piece was allowed to soak at 8°C for 1 h before the addition of 100 mm NH4HCO3. The sample was then kept at 37°C overnight. On the following morning, the sample was sonicated for 5 min and the liquid collected and dried under vacuum. The peptides were stored at −20°C until further analysis, when they were redissolved in 0.1% trifluoroacetic acid.
Liquid chromatography (LC)/mass spectrometry (MS) analysis
The peptides were analysed using an LTQ-Orbitrap Velos Pro (Thermo Scientific). Separation of the peptides was performed using an EASY-nLC II system (Thermo Scientific, Bremen Germany). An EASY-Column, 2 cm, i.d. 100 μm, 5 μm, C18-A1 (Thermo Scientific) was used as a pre-column and an EASY column, 10 cm, i.d. 75 μm, 3 μm C18-A2 (Thermo Scientific) was used as an analytical column. The mass analyser and the separation system were controlled using Tune 2.6.0 and Xcalibur 2.1. The sample was loaded on the pre-column using Solvent A (0.1% formic acid) with 2% Solvent B (0.1% formic acid in acetonitrile). After the loading, 2 min of 2% B using a flow rate of 200 nl min−1 followed. The first step of the gradient was an increase from 2% Solvent B to 35% Solvent B during 15 min. The second step was an increase from 35% Solvent B to 50% Solvent B during 5 min. The third step was an increase from 50% Solvent B to 80% Solvent B during 5 min. The flow rate was then increased from 200 nl min−1 to 400 nl min−1 during 3 min, and 80% Solvent B with a flow rate of 400 nl min−1 was then kept for 8 min.
For the MS analysis, a survey scan was performed for m/z from 400 to 2000 at a resolution of 60,000 using the orbitrap. Following this event, 10 data-dependent MS/MS spectra were recorded using the 10 most intense ions from the survey scan. This MS/MS analysis was performed using the linear ion trap. The precursor ions were isolated within a 1Da window and fragmented by collision-induced dissociation with 35% normalized collision energy. The activation time was set to 10 ms and q = 0.25. Dynamic exclusion was set to 30 s and the exclusion mass width was set to 5 p.p.m. relative to the reference mass.
Data analysis
The acquired data (.RAW-files) were processed using Proteome Discoverer (Thermo Scientific, version 1.4.0.288), where SEQUEST was used to search the results against the Uniprot-Swissprot database for Rattus norvegicus taxonomy. For all searches, the enzyme specificity was trypsin; two missed cleavages were allowed; error tolerances of 0.02 and 0.7 were set for the survey scan and the MS/MS analysis, respectively; carbamidomethylation (C) was set as a static modification; oxidation (M) and deamidation (N, Q) were set as variable modifications. In order to search for many post-translational modifications (PTMs), the processing was performed in four blocks with following variable modifications: (1) methylation (C-terminal, D, E), carbamylation (K); (2) oxidation (F, H, K, P), phosphorylation (S, T, Y); (3) oxidation (D, N, R, W, Y); (4) methylation (C-terminal), acetylation (K, S), nitration (W, Y). The search results from these blocks were merged and validated using Percolator (embedded in Proteome Discoverer), in which the target false discovery rate was set to 0.1 (strict) and 0.5 (relaxed). Out of the resulting peptides, only those with medium or high confidence were taken into account for further processing. All peptides with found PTMs were further processed as follows. First, all peptides containing only carbamidomethylations and/or methionine oxidations and/or carbamylations, as well as all peptides belonging to proteins other than myosin, were excluded. Second, only PTM sites reported in at least three CMV samples and absent in controls were taken into account. Third, all remaining peptides were verified, i.e. their mass spectra were manually checked for PTM confirmation. The latter was done based on knowledge of basic theory of peptide fragmentation, as well as considering possible misinterpretations (description of the details can be found in Bergström Lind et al. 2013).
Immunoblotting
SDS-PAGE was performed by using Mini-PROTEAN 3 Cell (Bio-Rad Laboratories, Hercules, CA, USA). For all samples, 2.5 μg of total protein was loaded per lane on the stacking gel with 4% (w/v) acrylamide concentration and 12% in the running gel; the gel matrix included 10% glycerol. Electrophoresis was performed at 120 V for 90 min and the gels were immediately transferred to polyvinylidene fluoride (PVDF) membranes (GE Healthcare, Buckinghamshire, UK). Membranes were incubated with calpain (Cell Signaling technology, Beverly, MA, USA), anti-microtubule-associated protein 1 light chain 3β (anti-LC3B) (L7543, Sigma-Aldrich, MO, USA), muscle-specific RING finger protein 1 (MuRF1; AF5366, R&D Systems, Minneapolis, MN, USA), rabbit polyclonal anti-caspase 3 (06-735, Millipore, Bedford, MA, USA), α-actinin (A7732, Sigma-Aldrich, St Louis, MO, USA), and actin (sc-1616, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) primary antibodies. The membranes were incubated with secondary antibodies NXA931 or NA934 (GE Healthcare) or sc-2020 (Santa Cruz Biotechnology, Inc.) and using the ECL Advance Western blotting detection kit (RPN 2135, GE Healthcare, Buckinghamshire, UK) according to manufacturer's instructions. The immunoblots were subsequently scanned in a soft laser densitometer (Molecular Dynamics, Sunnyvale, CA, USA). The signal intensities were quantified using the volume integration function (arbitrary units) and normalized to actin.
X-ray diffraction recordings and analyses
On the day of X-ray recordings, fibre bundles were placed in a plastic dish containing a relaxing solution and washed thoroughly to remove the glycerol. They were then transferred to a specimen chamber filled with the same relaxing solution. Each fibre bundle was then fixed (clamped ends) and slightly stretched. Subsequently, X-ray diffraction patterns were recorded at 15°C.
For each fibre bundle, approximately 20–40 diffraction patterns were recorded at the BL45XU beamline of SPring-8 and were analysed as described previously (Iwamoto et al. 2001, 2002, 2003; Iwamoto, 2009). The wavelength was 0.09 nm and the specimen-to-detector distance was 2 m. As a detector, a cooled CCD camera (C4880, Hamamutsu photonics, Hamamatsu, Japan) was used in combination with an image intensifier (VP5445, Hamamatsu Photonics). To minimize radiation damages, the exposure time was kept low (2 s) and the specimen chamber was moved by 200 μm after each exposure. Moreover, we placed an aluminium plate upstream of the specimen chamber. Following X-ray recordings, background scattering was subtracted, and reflection intensities were determined as described elsewhere (Iwamoto et al. 2001, 2002, 2003; Yagi, 2003; Iwamoto, 2009).
Total RNA isolation and quantification
Total RNA was extracted from frozen diaphragm muscle tissue (10–30 mg) using QiagenRNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). Muscle tissue was homogenized using a motor homogenizer (Eurostar Digital, IKA-Werke, Staufen, Germany). QIAshredder columns (Qiagen Inc., Valencia, CA, USA) were used to disrupt DNA. Total RNA was eluted from RNeasy Mini columns with 30 μl of RNase-free water. RNA concentrations were then quantified using the fluorescent nucleic acid stain Ribogreen (Molecular Probes, Eugene, OR, USA) on a Chameleon PLC IIs (Hidex, Turku, Finland) fluorescence spectrophotometer.
Quantitative real-time PCR
qRT-PCR was used to quantify the mRNA levels for rat type IIa and IIx MyHCs, and skeletal α-actin (GenBank accession numbers X15939, L13606 and L24897, respectively).
Quantities of 100 ng of total RNA from diaphragm samples were reverse transcribed to cDNA using Qscript cDNA supermix (Quanta Biosciences, Gaithersburg, MD, USA). cDNA was amplified in triplicate using the MyiQ single colour real-time PCR detection system (Bio-Rad Laboratories). The thermal cycling conditions include 95°C for 10 min, followed by 50 cycles of a two-step PCR with denaturation at 95°C for 15 s and a combined annealing and extension step at 60°C for 1 min. Each reaction was performed in a 25 μl volume with 0.4 μm of each primer and 0.2 μm of SYBR Green. When optimizing each PCR, the PCR products were run on 2% agarose gels to ensure that primer–dimer formation was not occurring. Taqman primers were designed using the software Primer Express (Applied Biosystems, Foster City, CA, USA). Primer sequences for MyHC IIa were: forward GGCCAGAGTGCGTGAACTG, reversed AAGCCCTTTGACAGCCTCAA, amplicon size 73; for MyHC IIx: forward TCGCCGAGTCCCAGGTC, reversed CGCTTATGATTTTGGTGTGAACC, amplicon size 66; and for actin: forward AGGTCATCACCATCGGCAAT, reversed AAGGAAGGCTGGAAGAGCGT, amplicon size 61. Probes labelled with FAM (N-(3-fluoranthyl) maleimide) and primers were purchased from Thermo Electron (Thermo Electron, Ulm, Germany). All probes and primers were purified by high-performance liquid chromatography. Threshold cycle (Ct) data obtained from running qRT-PCR was related to a standard curve to obtain the starting quantity (SQ) of the template cDNA. The values were normalized against 18S (GenBank accession number AF102857).
Immunohistochemistry
Serial consecutive transverse cryosections were assayed for immunohistochemistry using antimyosin heavy chain (MyHC) antibodies as previously described (Tarricone et al. 2008). The following antibodies were used: clone BA-D5 for type I-β MyHC, clone SC-71 for type IIa MyHC, and clone BF-35, which labels all MyHCs except for type IIx, and therefore allows the identification of the unreacting myofibres as type IIx.
DNPH immunohistochemistry
Serial cryosections to those labelled with antimyosin antibodies were reacted with DNPH for 1 h at room temperature (Vitadello et al. 2003). After a 10 min rinse in 50 mm Tris pH 7.6 added with 0.9% NaCl, sections were saturated with 3% BSA for 20 min, and incubated with the anti-DNPH antibody. Bound antibody was revealed by peroxidase immunohistochemistry
NADPH-d histochemistry
Nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) activity was demonstrated by histochemistry following previously described procedures (Hope et al. 1991; Rothe et al. 2005) with minor modifications (Vitadello et al. 2014). In brief, 12 μm-thick cryosections were fixed for 10 min with 4% paraformaldehyde. After rinsing with PBS, sections were incubated in a medium containing 50 mm Tris–HCl pH 8.00, 0.2% Triton X-100, 0.5 mm nitroblue tetrazolium chloride (Serva, Crescent Chemical Co., Islandia, NY, USA) and 1 mm β-NADPH (Sigma) for 45 min at 37°C. Reaction was stopped by rinsing briefly with distilled water. Coverslips were mounted with glycerine jelly.
Consistency of the labelling was validated by independent analysis of adjacent cryosections. Histochemical and immunohistochemical analysis was performed using the Zeiss Axioplan microscope and ×10 and ×40 Plan-Neofluar objectives (Zeiss, Milan, Italy). Images were acquired using a Leica digital DFC 300FX camera and the IM50 software (Leica Microsystems SRL, Milan, Italy). Myofibre cross-sectional circumference (CSC) was evaluated using ImageJ software. The value of NADPH-d-positive CSC was expressed as percentage of the corresponding entire myofibre CSC. Values of CSC percentage for each myofibre population corresponded to the mean of the values of about 15 fibres in the same muscle (the experimental unit).
Confocal microscopy
After a 10 min fixation at room temperature with 4% buffered paraformaldehyde, cryosections were incubated overnight at 4°C with rabbit anti-neuronal nitric oxide synthase (anti-nNOS) antibodies pAb (H-299; Santa Cruz Biotechnology, Inc.), extensively rinsed with phosphate-buffered saline (PBS) and incubated for 2 h at room temperature with goat anti-rabbit Ig conjugated with Alexa 568. Slides were observed with a Leica SP5 confocal microscope equipped with a helium 543 nm laser (Leica Microsystems). Images were recorded at 1024 × 1024 pixel density after Kalman 4 normalization.
Statistical analysis
One-way analyses of variance (ANOVA) and Tukey's post hoc test were used when comparing multiple groups and P < 0.05 was considered statistically significant. Sigma Stat software was used. The non-linear functions were fitted using TableCurve 2002 software (TableCurve 2D version 5.01. SYSTAT Software Inc., Erkrath, Germany), which has 3200 build-in functions. The functions were sorted by r-square and the functions having the highest r-square were selected. Data are presented as means ± standard error of the mean (SEMs) or standard deviations (SDs).
Results
Single diaphragm muscle fibre size, force-generation capacity (specific force) and lipid contents
A total of 160 diaphragm muscle fibres met the acceptance criteria and were included in the analyses, i.e. 10 fibres were analysed in each animal corresponding to 40 fibres in each group. A maintained cross-sectional fibre area was observed during the initial 4 days of CMV and followed by a progressive decline in fibre size (Fig. 1A). That is, the cross-sectional areas (CSAs) were 29% smaller (P < 0.05, 740 ± 100 vs. 1080 ± 80 μm2) after 5–8 days and 50% smaller (P < 0.001, 510 ± 60 vs. 1080 ± 80 μm2) after 9–14 days CMV (Fig. 1A and C) than in controls.
Maximum diaphragm muscle fibre force normalized to cross-sectional area, i.e. specific force, showed a different temporal pattern from that of fibre size and a 25% lower (P < 0.001) specific force was observed by 6 h to 4 days CMV compared with controls (16.3 ± 0.6 vs. 21.6 ± 0.9 N cm−2). Specific force declined progressively at the longer durations and was 50% lower (P < 0.001, 10.8 ± 0.6 vs. 21.6 ± 0.9 N cm−2) after 5–8 days and 70% lower (P < 0.001, 6.6 ± 0.7 N cm−2 vs. 21.6 ± 0.9 N cm−2) after 9–14 days of CMV (Fig. 1B). Thus, the combined effects of mechanical ventilation on fibre size and specific force indicate a 25, 64 and 85% reduction of diaphragm muscle function in response to 0.25–4, 5–8 and 9–14 days of CMV compared with control fibres, respectively. In addition to the 40 fibres included in each group, 0, 3, 9 and 15 fibres in controls, 0.25–4, 5–8 and 9–14 day experimental groups, respectively, did not generate any force upon maximum calcium activation (pCa 4.5) and were not included in the analyses, suggesting an even larger decline in aggregate diaphragm muscle function in response to CMV than indicated above.
Increased intracellular lipid content was observed in response to CMV in individual diaphragm muscle fibres (Fig. 2A). By taking advantage of the autofluorescence properties of the Oil-Red-O staining, the total volume of the oil droplets in each muscle fibre was measured and normalized to the fibre volume. According to these semiquantitative analyses, there was a relatively rapid increase in intracellular lipid content independent of muscle fibre type (Fig. 2B). To study this in more detail, a transition function was fitted to lipid contents in all muscle fibres from control animals and animals exposed to CMV for 6, 18, 24 and 32 h. According to these analyses, the transition width is 20 min suggesting a fast process starting shortly after 18 h of exposure to CMV (Fig. 2C). A large inter-fibre variability in Oil-O-Red staining was observed at all durations and, except for CMV for 32 and 60 h, there was no significant difference in intracellular lipid content between fibre types. At 32 and 60 h CMV, a higher lipid content was observed in slow- (type I) compared to fast-twitch (type II) muscle fibres, classified according to myosin ATPase enzyme histochemistry. However, the large variability in intracellular lipid content between muscle fibres and lack of a consistent fibre-type effect on lipid content reduces the interpretative value of this observation and it cannot be ruled out that it represents a bias with no or limited biological significance.
X-ray diffraction experiments
Small-angle X-ray diffraction experiments were conducted on diaphragm skinned fibre bundles to determine myofilament structure in response to CMV and NMB. A maintained structural integrity of myofibrils was observed in rat diaphragm muscle fibres at CMV durations varying between 6 h and 14 days (Fig. 3A). The intensity ratio of the (1,1) and (1,0) reflections in the relaxed state did not change significantly over a CMV duration between 6 h and 14 days, and this ratio increased when the muscle was put into rigor at all durations, including the 14 day muscle (Fig. 3B). This shows that myosin can bind to actin in an ATP-dependent manner even after 14 days of exposure to CMV. The total intensity of scatter relative to the 6th actin layer line intensity increased (P < 0.001) with CMV duration (Fig. 3C). This is consistent with the accumulation of intracellular lipid droplets as well as myofibrillar protein loss, i.e. muscle fibre atrophy and the parallel myosin and actin protein loss. The intensity of the first myosin meridional reflection (1MM) decreased (P < 0.01) with CMV duration, but the intensity of 2MM and the 4th myosin layer line (4ML) reflections did not decrease and showed a trend towards an increased intensity (P < 0.1). (Fig. 3D). This suggests a change in the contrast of density along the thick filament without a loss of the helical order and supports a post-translational modification of the thick filament backbone. In addition, a slight but statistically significant increase (P < 0.001) in the (1,0) filament lattice spacing was observed with increasing CMV duration. This may suggest degradation of some proteins that hold the filaments together such as titin, but we did not observe a consistent degradation of titin (not shown). Another possible cause may be a change in the ionic interaction between filaments caused by protein modifications.
Figure 3. Results of X-ray diffraction analyses.

A, Small-angle X-ray diffraction patterns from rat diaphragm muscle bundles slightly stretched in relaxing solution and during rigor in controls or rats exposed to CMV for 4 days and 14 days. Background scatter has been subtracted from the diffraction patterns except for the area of equatorial reflections. First and second myosin meridional reflections at spacings of 14.3 and 7.2 nm, respectively (1MM and 2MM), 4th myosin layer line (4ML), the (1,0) and (1,1) equatorial reflections from the myosin filament lattice, and the 6th actin layer line are indicated. B, ratio of the intensities (I) of the (1,1) and (1,0) equatorial reflections. C, total intensity of scatter (reflectionsand background scattering) relative to the 6th actin layer line (6ALL), measured in the small-angle region up to the 4.8 nm spacing, increased (P < 0.001) with CMV duration. D, intensity of the 1MM (black circles and black regression line), 2MM (red squares and red line) and 4ML (blue triangles and blue regression line) normalized to the 6th actin layer line. E, the (1,0) spacing of the hexagonal lattice of the thick filament.
Myosin and actin mRNA expression
Actin mRNA expression did not differ between controls and the experimental groups.
The mRNA expression of type IIa and IIx MyHC isoforms showed a slight trend towards decreased levels with increasing duration of CMV, but a statistically significant difference was only observed at the longest duration (9–14 days) and was restricted to the IIx MyHC isoform (one-way ANOVA, P < 0.05; Fig. 1E). This is in sharp contrast to the strong transcriptional down-regulation of actin and all MyHC isoforms in limb muscles from animals exposed to CMV for 5 days and longer (Norman et al. 2006; Ochala et al. 2011b).
Myosin and actin protein expression
In accordance with MyHC mRNA expression, there was no significant change in MyHC isoform proportions between control (17 ± 2% type I, 44 ± 3% type IIa and 39 ± 3% type IIx), 0.25–4 day (23 ± 2% type I, 40 ± 4% type IIa and 37 ± 2% type IIx), 5–8 day (22 ± 1% type I, 47 ± 1% type IIa and 30 ± 1% type IIx) and 9–14 day (21 ± 2% type I, 37 ± 1% type IIa and 39 ± 2% type IIx) groups. Myosin quantity normalized to total protein quantity declined slightly over time according to linear regression analyses (r2 = 0.32, P < 0.05), but according to one-way ANOVA there was no statistically significant difference between the different groups (Fig. 1D). Thus, the 70% lower specific force in the 9–14 day group cannot solely be explained by the 18% lower myosin quantity in this group. Furthermore, in accordance with our previous observations, the diaphragm muscle myosin and actin ratio was preserved throughout the experimental period (Norman et al. 2006). This is in sharp contrast to the preferential myosin loss observed in limb muscle in response to long-term immobilization and mechanical ventilation in ICU patients (Larsson et al. 2000; Llano-Diez et al. 2012) as well as in experimental animal models (Norman et al. 2006; Ochala et al. 2011b).
Protein degradation pathways
Markers of the calcium-activated (calpain-1, 75 kDa fragment), pro-apoptotic (pro-caspase 3 and cleaved caspase 3), autophagy (LC3B) and ubiquitin proteasome (MuRF1) proteolytic pathways were investigated at the protein level by immunoblotting. Similar levels of the active calpain-1 (75 kDa) fragment was observed in controls and after 0.25–4 days CMV, followed by higher (P < 0.05) levels after 5–8 and 9–14 days CMV (Fig. 4C). Significantly higher (P < 0.05) pro-calpain-1 (80 kDa) fragment levels were only observed at the longest duration (9–14 days) of CMV (Fig. 4D). Pro-caspase 3 levels did not change significantly during the observation period, but a transient increase was observed in the active cleaved caspase 3 after 5–8 days CMV (Fig. 4E and F). The autophagy marker 16 kDa to 18 kDa LC3B protein ratio was higher (P < 0.05) after 5–8 days of CMV and remained elevated at the longest 9–14 day duration (P < 0.05; Fig. 2B). Thus, activation of both the calcium-activated and the autophagy pathways were preceded by the decline in specific force but paralleled the atrophy. The MuRF1 content, on the other hand, showed an early increase (0.25–4 days) and remained elevated (P < 0.05) throughout the whole observation period (Fig. 4A).
Figure 4. Protein degradation pathways.

Western blot analyses of MuRF1 (A), LC3B I/II ratio (B), calpain-1 75 kDa (C), calpain-1 80 kDa (D), pro-caspase 3 35 kDa (E), and active caspase 3 17 kDa (F). Controls (open bars) and animals exposed to CMV durations corresponding to 0.25–4, 5–8 and 9–14 days (filled bars) normalized to actin. Values are means + SEM. *P < 0.05, significant difference compared with controls.
Subsarcolemmal nNOS expression and activity
Since nNOS deregulation has been shown to influence both atrogene (via FoxO; Suzuki et al. 2007) and calpain activation (Tidball et al. 1998), representing a major factor of atrophy development, the distribution of the enzyme and the presence of subsarcolemmal activity was investigated by means of immunofluorescence and enzyme histochemistry. Confocal analysis showed weak subsarcolemmal nNOS immunoreactivity in control animals and an increased subsarcolemmal immunoreactivity with increasing CMV duration (Fig. 5A), which paralleled Western blot evidence of increased protein expression (Fig. 5B and C). This is similar to our previous observations in limb muscles from ICU patients (Llano-Diez et al. 2012), but the nNOS shuttling to the cytoplasm observed in ICU patients after 10 days of immobilization was not observed in the diaphragm (Fig. 5A). To investigate whether the increased signal was related to the presence of active nNOS enzyme at this site, NADPH-diaphorase (NADPH-d) histochemistry was performed. Nitroblue tetrazolium staining, corresponding to the presence of the active nNOS, appeared concentrated in discrete sarcolemmal regions of control diaphragm myofibres in accordance with previous observations in the soleus muscle (Rothe et al. 2005; Vitadello et al. 2014). Measurements of the labelling distribution were expressed as a percentage of fibre cross-sectional circumference (CSC). A large variation in the percentage of positive CSC for NADPH-d histochemistry was observed among diaphragm fibre-type populations, identified in serial consecutive sections using anti-myosin antibodies in indirect immunoperoxidase (Fig. 5D). Positive NADPH-d-labelled CSC reached approximately 50% of the total value in control type I and IIa fibres, whereas only 10% of myofibre CSC showed histochemical activity among IIx and IIb fibres, the large majority lacking subsarcolemmal staining (Fig. 5D, Table 1). Sarcolemmal distribution of NADPH-d histochemistry declined significantly in type I and IIa fibres in response to CMV, disappearing almost completely from type IIa fibres after 4 days of CMV and from type I fibres after 8 days (Fig. 5D, Table 1). Since significantly reduced levels of the endoplasmic reticulum chaperone Grp94, i.e. the product of the Hsp90B1 gene, have been described to occur in the presence of muscle atrophy and have been related to untethering of nNOS from myofibre sarcolemma (Vitadello et al. 2014), protein levels of Grp94 were investigated. A decline (P < 0.05) in Grp94 protein content was observed in the 0.25–4 day group compared with controls, followed by a return to control levels at longer durations (Fig. 5E).
Figure 5. Subsarcolemmal nNOS expression and activity during CMV and neuromuscular blockade for 0.25–4, 5–8 and 9–14 days.
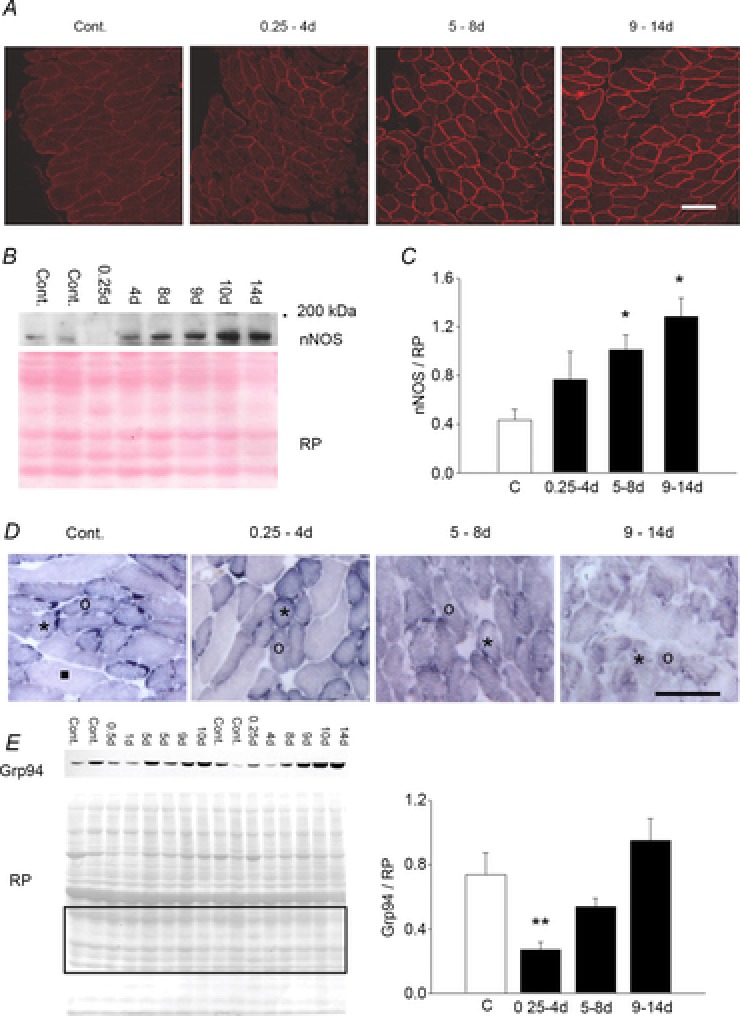
A, confocal immunofluorescence shows increasing nNOS subsarcolemmal reactivity with chronic CMV compared to control diaphragm (Cont.). B, Western blot of total diaphragm homogenates shows increased nNOS polypeptide immunolabelling after 5–14 days of CMV and neuromuscular blockade. ‘200 kDa’ indicates reference mobility of molecular mass standards. Red Ponceau (RP) staining of the 42–25 kDa region of the same blot is shown for loading reference. C, histogram depicts normalized nNOS relative amounts. *P < 0.05, significant difference vs. control. D, NADPH-diaphorase histochemistry. Enzyme activity of subsarcolemmal nNOS corresponds to a dark, discontinuous staining which becomes hardly detectable during CMV and neuromusclar blockade. Symbols indicate the representative staining pattern of each fibre-type population, identified by comparison of serial sections labelled with antimyosin antibodies (see Table 2). Asterisks indicate type I fibres, circles indicate type IIa fibres and the black square corresponds to a representative type IIx fibre. Scale bars represent 100 μm. E, left side: representative Western blot of total diaphragm homogenates with anti-Grp94 antibodies. RP staining of the 42–25 kDa region of the same blot was used for quantification. Right side: histograms depicting normalized Grp94 relative amounts. **P < 0.05, significant difference vs. all the groups.
Table 1.
Effects of CMV on sarcolemmal nNOS activitya in diaphragm fibre-type populations (%CSC)
| Control | 0.25–4 days | 5–8 days | 9–14 days | |
|---|---|---|---|---|
| Type I | 52.37 ± 1.10 | 43.76 ± 6.44* | 16.22 ± 9.59** | 7.93 ± 7.93** |
| Type IIa | 40.85 ± 4.55 | 17.69 ± 9.19# | 2.19 ± 2.19** | 6.35 ± 6.35** |
| Type IIx | 2.61 ± 2.61 | 0 | 0 | 0 |
Enzyme activity was assessed using NADPH-diaphorase histochemistry and expressed as the percentage of reacting cross-sectional circumference (CSC). Data are expressed as means ± SEM (at least 10–15 fibres were evaluated per fibre type population in each diaphragm.
P ≤ 0.05 vs. 5–8 days and 9–14 days values;
P < 0.01 vs. control values;
P = 0.06 vs. control value.
Protein modifications
Quantification of carbonyl groups in amino acid side chains in the diaphragm was used as a marker of oxidative stress. Protein carbonylation levels increased significantly at the shortest duration (0.25–4 days) and continued to increase until 5–8 days of CMV, but declined to control values in the atrophic diaphragm after 9–14 days (Fig. 6A). Derivatization with DNPH of cryosections was used to identify diaphragm myofibres exposed to oxidative stress. Carbonylated myofibres were detected in the control diaphragm (Fig. 6B) and corresponded to about 35% of the total fibre number, although differing for prevalence among fibre types (approximately 60, 30 and 20% of type IIa, I and IIx being carbonylated). The proportion of carbonylated fibres increased in response to CMV and all fibres were carbonylated irrespective of MyHC isoform expression at CMV durations longer than 8 days (Fig. 6B, Table 2).
Figure 6. Changes in muscle protein carbonylation during CMV and neuromuscular blockade for 0.25–4, 5–8 and 9–14 days.

A, left side: representative OxyBlot showing time-related changes in protein carbonylation. Red Ponceau (RP) staining of the same blot was used for loading reference (left side). Right side: histograms depicting relative amounts of carbonylated proteins (Oxy) after normalization with RP. *P < 0.05, significant difference vs. all groups; #P = 0.05 vs. control. B, in situ cryosection derivatization with DNPH to demonstrate carbonylate adduct distribution among different fibre-type populations (see Table 2). Symbols indicate the representative DNPH reactivity of fibre types, identified by comparison of serial sections labelled with antimyosin antibodies. Asterisk, circle and black square indicate type I, type IIa and type IIx fibres, respectively. Scale bar represents 100 μm. Values are means + SEM.
Table 2.
Effects of CMV on the percentage of carbonylated diaphragm myofibresa
| Control | 0.25–4 days | 5–8 days | 9–14 days | |
|---|---|---|---|---|
| Type I | 29.40 ± 7.30 | 31.31 ± 5.39 | 94.78 ± 0.38* | 98.48 ± 1.51** |
| Type IIa | 56.66 ± 23.33 | 56.31 ± 16.68 | 96.80 ± 2.23* | 97.22 ± 2.78** |
| Type IIx | 18.08 ± 5.82 | 7.10 ± 0.20 | 50.56 ± 5.29* | 95.11 ± 2.83** |
Evaluated by means of DNPH immunoreactivity and expressed relative to each fibre-type population. Data are expressed as means ± SEM (at least 300 fibres were evaluated for each muscle).
P ≤ 0.05 vs. control and 0.25–4 days values;0
P < 0.01 vs. control and 0.25–4 days values.
LC/MS analysis of tryptic peptides originating from in-gel-digested SDS-PAGE bands corresponding to myosin isoforms revealed the presence of 12 myosin PTMs in the CMV group which were absent in controls. Since the number of controls in this study was three times less than CMV objects (7 vs. 22), only those modifications found in at least three samples from the CMV group were considered (Table 3). Deamidations were the most frequent PTMs (8 out of 12) and they tended to be more frequently observed at the longer durations of CMV. One exception was the deamidated N1833 which was clearly dominating in the 0.25–4 day CMV group. Two oxidation sites related to protein carbonylation were also found and verified; both had a clear tendency to be more pronounced at longer CMV durations. This finding is, to some extent, in agreement with our findings related to oxidative stress (absence of oxidations in the control group and increasing observation frequency in the mechanically ventilated animals). Finally, methylated aspartate and nitrated tryptophan residues were detected in the CMV group, but were not related to CMV duration. All verified PTMs were located in the rod region of the myosin and their positions are demonstrated in Fig. 7.
Table 3.
Post-translational modifications of the myosin heavy chain that are specific to mechanically ventilated rat at durations of 0.25–4 days, 5–8 days and 9–14 days, i.e. modifications not observed in controls
| Frequencya | ||||||
|---|---|---|---|---|---|---|
| Peptide sequence | Associated protein (Rattus norvegicus) | Amino acid modified | 0.25–4 days | 5–8 days | 9–14 days | |
| Deamidation | KKDFELNALNAR | Myosin 7 | N1088 | 3/11 | 1/5 | 3/6 |
| Deamidation | LQDAEEAVEAVNAK | Myosin 6 and myosin 7 | Q1398 | 3/11 | 1/5 | 2/6 |
| Deamidation | LTQESIMDLENDKQQLDER | Myosin 7 | N1071 | 1/11 | 0/5 | 2/6 |
| Deamidation | LTQESIMDLENDKQQLDER | Myosin 7 | Q1074 or Q1075 | 1/11 | 0/5 | 2/6 |
| Deamidation | NAESVKGMR | Myosin 6 and myosin 7 | N1833 | 6/11 | 2/5 | 1/6 |
| Deamidation | NDLQLQVQAEADGLADAEER | Myosin 4 | N889 or Q892 | 1/11 | 1/5 | 2/6 |
| Deamidation | QKYEESQSELESSQK | Myosin 6 and myosin 7 | Q1458 or Q1464 | 0/11 | 1/5 | 2/6 |
| Deamidation | RNNLLQAELEELR | Myosin 7 | N1677 or N1678 | 1/11 | 0/5 | 2/6 |
| Methylation | KKHADSAAELAEQIDNLQR | Myosin 3 | D1199 | 4/11 | 1/5 | 1/6 |
| Nitration | ILAEWK | Myosin 6 | W1457 | 2/11 | 1/5 | 1/6 |
| Oxidation | LAQESILDLENDKQQLDER | Myosin 3 | D1070 | 1/11 | 1/5 | 2/6 |
| Oxidation | NLTEEMAGLDENIVK | Myosin 4 | N992 or K995 | 1/11 | 2/5 | 3/6 |
Example: 3/11 means that this PTM was found in 3 animals out of 11 in total for that specific CMV group.
Figure 7. Post-translational modifications in the myosin heavy chain specific to rats with 0.25–14 days of CMV.

A, schematic overview of the positions of the amino acids with a specific PTM. Green arrows represent deamidation, red arrows represent oxidation, orange arrows represent methylation and blue arrows represent nitration. B, a closer look at the coiled-coil structure of the myosin tail and the position of N889. The image was created with PyMOL (http://www.pymol.org) using the atomic coordinates of Protein Data Bank entry 2FXM (Blankenfeldt et al. 2006).
Discussion
In this study we have, to the best of our knowledge, for the first time conducted time-resolved analyses with high temporal resolution of the effects of controlled mechanical ventilation (CMV) on diaphragm muscle structure and function. A clear temporal progression of gene/protein expression, fibre atrophy, protein modifications, protein–protein interactions and altered regulation of muscle contraction were observed in diaphragm muscle fibres in response to short- to long-term CMV with significant consequences for overall respiratory function. The atrophy and loss of maximum force normalized to cross-sectional fibre area (specific force) were more severe than previously observed in limb muscles of animals exposed to the same type of intervention (Ochala et al. 2011b). Transcriptional regulation and myofibrillar protein expression in response to CMV were, on the other hand, less affected in the diaphragm compared with our earlier observations in limb muscles. That is, the strong transcriptional down-regulation of actin and myosin synthesis, and the preferential myosin loss observed in limb muscles, was not observed in the diaphragm. However, a more severe oxidative stress, as indicated by protein carbonylations, post-translational protein modifications and intracellular lipid accumulation were observed in the diaphragm than in limb muscles (Renaud et al. 2013). The up-regulation of the three major proteolytic pathways in skeletal muscle, i.e. the ubiquitin-proteasome, the calcium-activated and the lysosomal pathways were activated in a similar temporal pattern to that previously reported by our group in limb muscles (Ochala et al. 2011b), i.e. the early up-regulation of the MuRF1 E3 ligase followed the same temporal pattern as the myosin loss, and the up-regulation of calpains and LC3B paralleled the muscle fibre atrophy. It is suggested that the impaired diaphragm muscle function (less than 15% of control values after 9–14 days of mechanical ventilation) represents an important factor underlying the long weaning process in ICU patients, amounting to about 40% of the time spent on the ventilator and being further delayed in 20–30% of the mechanically ventilated patients due to additional problems (Esteban et al. 1995; McConville & Kress, 2012).
Methodological considerations
During the initial 4 days of mechanical ventilation, single fibre size was maintained in accordance with our previous observations in diaphragm muscle fibres using a porcine experimental ICU model, i.e. pigs exposed to sedation and mechanical ventilation for 5 days alone or in combination with neuromuscular blockade, corticosteroids and/or sepsis (Ochala et al. 2011c). In contrast, other laboratories have reported severe atrophy in response to a very short duration of mechanical ventilation in both experimental and clinical studies (Powers et al. 2002; Levine et al. 2008). It is important to emphasize that, as evidence of its physiological competence, the experimental rat model used in this study can sustain mechanical ventilation with paralysis for up to 3 months (Dworkin & Dworkin, 1990). To our knowledge, the duration of the mechanical ventilation in rodents using commercially available ventilators are typically shorter than a day or two, at least in part, due to poor survival rate. This implies that, in such studies when commercially available ventilators are used, even at durations shorter than 24 h, other confounding pathogenetic factors besides competent mechanical ventilation may affect diaphragm muscle structure and function. In clinical studies, surgical biopsies have been obtained from mechanically ventilated patients using ventilators that can maintain respiratory function for long durations, but surgical biopsies from the diaphragm are typically obtained from organ donors with a severe underlying disease/trauma and variable pharmacological treatments, adding further confounding factors to the effects induced by mechanical ventilation per se. The combined effect of critical illness and CMV on the diaphragm is clinically highly relevant. However, it is important to study the effects of mechanical ventilation per se on the diaphragm in order to distinguish the actual CMV-induced changes in muscle structure and function from other factors and thus better design future intervention studies.
Diaphragm muscle fibre size and function
In contrast to muscle fibre size, the force-generation capacity of individual diaphragm muscle fibres was decreased after 6 h to 4 days, in line with our previous observations in diaphragm muscle fibres using a porcine ICU model (Ochala et al. 2011a), and further progressively decreased at longer CMV durations in the rat model. In limb muscles, on the other hand, both fibre size and specific force were maintained in the 0.25–4 day group (Ochala et al. 2011b). After 9–14 days of CMV, the combined effect of decreased fibre size and specific force resulted in an 85% reduction in diaphragm muscle function. Impairments in muscle membrane excitability and excitation–contraction coupling may affect diaphragm muscle function in response to long-term CMV, but it is obvious from the skinned fibre experiments that CMV-induced changes in the regulation of muscle contraction at the contractile protein level play a very important role in impairing diaphragm muscle function in response to long-term mechanical ventilation. Oxidative stress and post-translational modifications appear to play a significantly more important role for diaphragm muscle in response to CMV and immobilization than the loss of myosin and myosin-associated proteins reported in limb muscles (Larsson et al. 2000; Norman et al. 2006; Ochala et al. 2011b).
Degradation pathways
The three major protein degradation pathways in skeletal muscle – the calcium-dependent pathway, the lysosomal pathway and the ATP-dependent ubiquitin-proteasome pathway – were investigated at the protein level, i.e. calpain-1, LC3B and MuRF1. An increased MuRF1 protein content was observed early (0.25–4 days) and was maintained at an elevated level throughout the 14 day experimental period, while calpain-1 and LC3B contents did not change significantly during the first 4 days of CMV. At the gene level, myosin expression remained unchanged until 8 days of CMV, contrary to our previous observation in limb muscles where myosin loss was paralleled by a similar decrease in mRNA expression (Ochala et al. 2011b), suggesting that the transcriptional down-regulation of myofibrillar proteins has a stronger impact on the preferential myosin loss observed in limb muscles than on protein degradation pathways. In addition, the small loss in myosin normalized to total protein content and the unchanged myosin:actin ratio throughout the experiment indicate that the mechanisms underlying the decreased specific force observed in the diaphragm in response to CMV is not primarily due to loss of contractile proteins, in contrast to our previous observations in limb muscles under the same conditions (Ochala et al. 2011b). Similar to our previous studies in limb muscles (Ochala et al. 2011b), autophagy and calcium-dependent pathways were up-regulated later and paralleled the muscle fibre atrophy. The early activation of autophagy (Hussain et al. 2010; Tang et al. 2011), calpains (McClung et al. 2009) or the pro-apoptotic caspase 3 (McClung et al. 2007) in response to less than 24 h of mechanical ventilation were not confirmed in this study. However, as discussed above, critical illness and underlying disease are additional important interfering factors and activators of proteolytic pathways besides mechanical ventilation that may explain these differences.
Post-translational protein modifications
Mechanical ventilation has repeatedly been shown to induce oxidative stress and reactive oxygen species (ROS) production (Leijten et al. 1995; Powers et al. 2002; Zergeroglu et al. 2003; Cheung et al. 2006), targeting proteins for degradation. In accordance with those studies, we observed an increased amount of protein carbonylations after only a few hours of CMV. Abnormal ROS levels result in mitochondrial dysfunction (Blakemore et al. 1996; Suliman et al. 1999) that may lead to intrinsic apoptosis and activation of the proteasome pathway targeting ROS-induced post-translational protein modifications (Boudriau et al. 1996; Zergeroglu et al. 2003). It is accordingly suggested that the early increase in protein modifications represents an early oxidative stress while the activation of the calpains and lysosomal pathways may be related to a maintained oxidative stress and ROS accumulation. The calcium-binding chaperone Grp94 is involved in the maintenance of calcium homeostasis and the protection against oxidative stress in muscle cells (Vitadello et al. 2003; Bando et al. 2004; Pizzo et al. 2010) and Barton and co-workers (Barton et al. 2012) recently showed that conditional ablation of Grp94 in fetal muscle results in decreased insulin-like growth factor-1 (IGF-1) production and impaired muscle size that could be rescued by recombinant IGF-1 administration. In addition, Vitadello et al. have recently shown that recombinant Grp94 reduces the muscle fibre atrophy and oxidative stress in response to hind limb suspension by stabilizing subsarcolemmal nNOS (Vitadello et al. 2014). In the diaphragm, Grp94 was transiently depressed early during CMV (0.25–4 days) and then returned to control values. We recently observed a similar transient early decline in Grp94 expression in limb muscles in response to the ICU condition, followed by a compensatory increase in Grp94 expression in response to mechanical loading (Renaud et al. 2013). In spite of the passive mechanical loading of the diaphragm by the ventilator, there was no increase in Grp94 protein levels in the diaphragm and the corresponding protection against oxidative stress/damage. This may, at least partially, explain why the diaphragm is more susceptible to oxidative damage and protein modifications than limb muscles.
Mass spectrometry analyses demonstrate mechanical ventilation induced myosin PTMs, but they were all located in the rod region. This region is essential for the packing of myosin molecules into myosin filaments and for the binding of myosin-associated proteins. Unfortunately, less is known concerning modifications of the α-helical coiled-coil rod domain of the myosin molecule than in the globular S1 head region containing the catalytic domain and the actin-binding site. However, mutations in the rod region of myosin result in specific myopathies (Ochala & Larsson, 2012) and perturbations in this region disturb sarcomeric uniformity with consequences for regulation of muscle contraction (Cammarato et al. 2011).
The muscle atrophy caused by the removal of weight bearing on distal hind limb muscles (typically the slow-twitch soleus), using the hind limb suspension model, has a strong impact on nNOS expression (Tidball et al. 1998; Suzuki et al. 2007). The loss of weight bearing induces the translocation of nNOS from the subsarcolemmal region to the cytoplasm, increased NO production and activation of atrogenes via FoxO transcription factors and of calpains, resulting in muscle atrophy and weakness (Tidball et al. 1998; Suzuki et al. 2007). In addition to unloading effects, nNOS was recently shown to play an important role in loading-induced muscle hypertrophy, suggesting that nNOS plays a crucial role in converting mechanical load into intracellular signalling pathways controlling protein synthesis and degradation (Ito et al. 2013). As mentioned above, recent results from Vitadello and co-workers have shown that Grp94 overexpression counteracts the atrophy associated with hind limb suspension by stabilizing nNOS to the sarcolemma, and Grp94 has also been shown to support muscle growth via local IGF production (Barton et al. 2012; Vitadello et al. 2014). The transient decline in Grp94 expression observed in the diaphragm and previously observed in limb muscles in response to the ICU condition was followed by a significant muscle fibre atrophy and loss of specific force, but it did not induced a shuttling of nNOS to the cytoplasm as previously observed in hind limb suspension models as well as in immobilized ICU patients (Tidball et al. 1998; Suzuki et al. 2007; Llano-Diez et al. 2012). Conversely, in the diaphragm, nNOS accumulation increased in the subsarcolemmal region in response to CMV, concomitantly to the return at physiological levels of Grp94, supporting further the role of the endoplasmic reticulum chaperone in the maintenance of the enzyme at this localization. Strikingly, this accumulation was accompanied by a dramatic decline in nNOS activity according to NADPH-d histochemistry. This finding, together with the confocal results, suggests that nNOS shuttling to the cytoplasm is neither an obligatory nor an exclusive event associated with muscle fibre atrophy. Consistently, the inhibition of nNOS activity by the use of 7-nitroindazole attenuated, but did not abolish, hind limb muscle atrophy due to unloading (Suzuki et al. 2007; Vitadello et al. 2014). Further, the diaphragm was loaded passively by the ventilator and there was complete removal of activation of contractile proteins due to postsynaptic blockade of neuromuscular transmission. In the hind limb suspension model, the external load induced by weight bearing was eliminated, but animals were freely moving their hind limbs and the internal strain induced by activation of contractile proteins is not eliminated in the hind limb suspension model.
Muscle-specific differences
As discussed above, significant differences were observed at the gene/protein levels, protein modifications and regulation of muscle contraction between the diaphragm and distal hind limb muscles in response to the ICU condition. These differences are muscle specific and not muscle fibre-type specific, i.e. similar changes were observed in both fast- and slow-twitch hind limb muscles and different from the diaphragm irrespective of muscle fibre type. Different mechanisms may underlie this difference, such as: (1) intrinsic differences between the diaphragm and hind limb muscles related to embryological background, innervation and activation patterns (Hirasawa & Kuratani, 2013), or (2) the continuous passive mechanical loading of the diaphragm by the ventilator.
Each skeletal muscle may represent a specific ‘allotype’ with inherent differences in gene/protein expression, myogenic factor activation and embryological origin (Hoh, 1992). The evolutionary origin of the diaphragm is not completely resolved, but recent results indicate that the diaphragm is first acquired from forelimb muscles into a primitive diaphragm, followed by entrapment in the region controlled by pulmonary development patterns (Hirasawa & Kuratani, 2013). Intrinsic differences between the diaphragm and, for example, distal hind limb muscles may accordingly have contributed to the muscle-specific differences in response to the ICU condition.
Basal levels of cell tension, i.e. tensegrity, have a strong impact on structure and function in all cells, and muscle cells have a very well developed mechanosensing system affecting transcriptional regulation of both protein synthesis and degradation (Ingber, 1991, 1993, 1997, 2002, 2003). Furthermore, muscle cells have an extremely well developed system for mechanosensing spanning from the extracellular matrix and integrins connected to cytoskeletal actin and the sarcodystroglycan complex, mechanosensing ion channels and caveolin 3 in the sarcolemma to titin kinase at the centre of the sarcomere (Gautel, 2011; Sinha et al. 2011). In experimental and clinical studies, the mechanical silencing associated with the ICU condition, i.e. loss of external load related to weight bearing and internal load related to activation of contractile proteins, has been shown to be a major factor triggering the preferential myosin loss associated with this severe acquired myopathy in ICU patients, i.e. critical illness myopathy or acute quadriplegic myopathy (Ochala et al. 2011b; Llano-Diez et al. 2012). Furthermore, passive full flexion–extension in the ankle joint in ICU patients (6 times per minute for 10 h per day) and in the experimental ICU model (12 times per minute for 12 h per day) attenuated the loss in specific force associated with the ICU condition (Llano-Diez et al. 2012; Renaud et al. 2013). In the experimental model, passive loading also reduced muscle fibre atrophy, the transcriptional down-regulation of contractile proteins and the preferential myosin loss in the distal limb muscles with the highest protein turnover rate (Renaud et al. 2013), but these effects were not observed in the patients (Llano-Diez et al. 2012). However, it remains unknown if this is secondary to species differences or if it is a dose–response effect. The maintained (minimally affected) transcriptional regulation of myofibrillar protein synthesis and myosin:actin ratio in the diaphragm argue in favour of a dose–response effect, i.e. 72 passive mechanical loadings per minute 24 h per day compared with the 12 per min and 12 h per day passive loading of distal hind limb muscles. However, the passive loading of the diaphragm did not eliminate the muscle wasting or the severe decline in single muscle fibre force-generation capacity resulting in a residual 85% reduction in the capacity of diaphragm muscle fibres to generate force after 2 weeks of CMV. In fact the 85% reduction is probably an underestimation since the non-force-generating muscle fibres increased in number with CMV duration and were not included in the final analyses. Finally, other potential factors triggering the diaphragm muscle dysfunction in response to CMV are the oxidative stress with concomitant protein modifications and paracrine influences, e.g. cytokines from the pulmonary region.
Conclusion
A series of novel findings observed in this study are of importance for understanding the mechanisms behind the diaphragm muscle atrophy and weakness induced by CMV. First, CMV induces early structural changes of the sarcomere resulting in the impairment of the force-generating capacity of the diaphragm. Second, the early and maintained oxidative stress in response to CMV is probably a key factor triggering post-translational protein modifications, impaired myofibrillar protein organization and atrophy. Third, the temporal pattern, phenotype and mechanism underlying the loss of contractile proteins differ between the diaphragm and limb muscles in response to CMV and immobilization. This knowledge will have to be taken into account when designing intervention strategies aimed at reducing the loss in muscle function and mass in mechanically ventilated and immobilized ICU patients.
Acknowledgments
We are grateful to Guillaume Renaud for excellent single muscle fibre contractile recordings. Some of the experiments were performed under approval of the SPring-8 Proposal Review Committee (2012B1047 and 2011B1058; Project leader: Dr Julien Ochala).
Glossary
- BSA
bovine serum albumin
- CMV
controlled mechanical ventilation
- CSA
cross-sectional area
- CSC
cross-sectional circumference
- DNPH
2,4-dinitrophenylhydrazine
- EEG
electroencephalogram
- ICU
intensive care unit
- LC
liquid chromatography
- LC3B
microtubule-associated protein 1 light chain 3β
- MM
myosin meridional
- MS
mass spectrometry
- MuRF1
muscle-specific RING finger protein 1
- MyHC
myosin heavy chain
- NADPH-d
nicotinamide adenine dinucleotide phosphate diaphorase
- NMB
neuromuscular blockade
- nNOS
neuronal nitric oxide synthase
- PTM
post-translational modification
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
Additional information
Competing interests
None declared.
Author contributions
The experimental work was performed in the research laboratory of L.L. in the Department of Neuroscience, Clinical Neurophysiology, Uppsala University with the assistance of A.-M.G. and N.C. The study was designed and supervised by L.L. Gene expression analyses were performed by R.C. Protein expression analyses were performed by R.C., H.S., H.-Z.F., J.-P.J. and L.G. Protein carbonylation analyses were performed by B.R. and L.G. Analyses of nNOS immunofluorescence and enzyme histochemistry were performed by Y.H. and M.V. Intracellular lipid quantifications were performed by J.P. X-ray diffraction experiments were conducted by H.I. and N.Y. Mass spectrometry analyses, protein modelling and interpretations were done by H.-M.B., K.A. and J.B. L.L. wrote the manuscript with assistance from R.C., B.D., N.Y., J.-P.J., H.-M.B., K.A., J.B. and L.G. All authors approved the final version of the manuscript.
Funding
This study was supported by grants from the Swedish Research Council (8651), the European Commission (MyoAge, EC Fp7 CT-223756 and COST CM1001), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), King Gustaf V and Queen Victoria's Foundation, and Uppsala University Hospital to L.L., the NIH (AR-048816) to J.-P.J., and the Swedish Research Council (4423) to J.B.
References
- Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med. 1997;25:1187–1190. doi: 10.1097/00003246-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Bando Y, Katayama T, Aleshin AN, Manabe T, Tohyama M. GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis. 2004;9:501–508. doi: 10.1023/B:APPT.0000031446.95532.ad. [DOI] [PubMed] [Google Scholar]
- Barton ER, Park S, James JK, Makarewich CA, Philippou A, Eletto D, Lei H, Brisson B, Ostrovsky O, Li Z, Argon Y. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012;26:3691–3702. doi: 10.1096/fj.11-203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström Lind S, Artemenko KA, Elfineh L, Zhao Y, Bergquist J, Pettersson U. Post translational modifications in adenovirus type 2. Virology. 2013;447:104–111. doi: 10.1016/j.virol.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Rickhuss PK, Watt PW, Rennie MJ, Hundal HS. Effects of limb immobilization on cytochrome c oxidase activity and GLUT4 and GLUT5 protein expression in human skeletal muscle. Clin Sci (Lond) 1996;91:591–599. doi: 10.1042/cs0910591. [DOI] [PubMed] [Google Scholar]
- Blankenfeldt W, Thoma NH, Wray JS, Gautel M, Schlichting I. Crystal structures of human cardiac β-myosin II S2-Δ provide insight into the functional role of the S2 subfragment. Proc Natl Acad Sci U S A. 2006;103:17713–17717. doi: 10.1073/pnas.0606741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudriau S, Cote CH, Vincent M, Houle P, Tremblay RR, Rogers PA. Remodeling of the cytoskeletal lattice in denervated skeletal muscle. Muscle Nerve. 1996;19:1383–1390. doi: 10.1002/(SICI)1097-4598(199611)19:11<1383::AID-MUS2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Cammarato A, Li XE, Reedy MC, Lee CF, Lehman W, Bernstein SI. Structural basis for myopathic defects engendered by alterations in the myosin rod. J Mol Biol. 2011;414:477–484. doi: 10.1016/j.jmb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, Al-Saidi F, Cooper AB, Cook D, Slutsky AS, Herridge MS. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, Vescovo G, Gorza L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:549–557. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Dworkin S. Learning of physiological responses: I. Habituation, sensitization, and classical conditioning. Behav Neurosci. 1990;104:298–319. doi: 10.1037//0735-7044.104.2.298. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Dworkin S. Baroreflexes of the rat. III. Open-loop gain and electroencephalographic arousal. Am J Physiol Regul Integr Comp Physiol. 2004;286:R597–R605. doi: 10.1152/ajpregu.00469.2003. [DOI] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- Esteban A, Frutos F, Tobin MJ, Alía I, Solsona JF, Valverdu I, Fernández R, de la Cal MA, Benito S, Tomás R, Carriedo D, Macías S, Blanco J for the Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Larsson L. Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve. 1997;20:948–952. doi: 10.1002/(sici)1097-4598(199708)20:8<948::aid-mus3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch. 2011;462:119–134. doi: 10.1007/s00424-011-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med. 2003;29:825–833. doi: 10.1007/s00134-003-1688-0. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Kuratani S. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat. 2013;222:504–517. doi: 10.1111/joa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh JF. Muscle fibre types and function. Curr Opin Rheumatol. 1992;4:801–808. [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest. 1991;99:34S–40S. [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signalling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanosensation through integrins: cells act locally but think globally. Proc Natl Acad Sci U S A. 2003;100:1472–1474. doi: 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signalling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19:101–106. doi: 10.1038/nm.3019. [DOI] [PubMed] [Google Scholar]
- Iwamoto H. Evidence for unique structural change of thin filaments upon calcium activation of insect flight muscle. J Mol Biol. 2009;390:99–111. doi: 10.1016/j.jmb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Oiwa K, Suzuki T, Fujisawa T. X-ray diffraction evidence for the lack of stereospecific protein interactions in highly activated actomyosin complex. J Mol Biol. 2001;305:863–874. doi: 10.1006/jmbi.2000.4334. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Oiwa K, Suzuki T, Fujisawa T. States of thin filament regulatory proteins as revealed by combined cross-linkingX-ray diffraction techniques. J Mol Biol. 2002;317:707–720. doi: 10.1006/jmbi.2002.5449. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Wakayama J, Fujisawa T, Yagi N. Static and dynamic x-ray diffraction recordings from living mammalian and amphibian skeletal muscles. Biophys J. 2003;85:2492–2506. doi: 10.1016/s0006-3495(03)74672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45. doi: 10.1097/00003246-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994;149:1539–1544. doi: 10.1164/ajrccm.149.6.8004310. [DOI] [PubMed] [Google Scholar]
- Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–1225. [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- Llano-Diez M, Renaud G, Andersson M, Marrero HG, Cacciani N, Engquist H, Corpeño R, Artemenko K, Bergquist J, Larsson L. Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading. Crit Care. 2012;16:R209. doi: 10.1186/cc11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175:150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37:1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med. 2012;367:2233–2239. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length–tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman H, Nordquist J, Andersson P, Ansved T, Tang X, Dworkin B, Larsson L. Impact of post-synaptic block of neuromuscular transmission, muscle unloading and mechanical ventilation on skeletal muscle protein and mRNA expression. Pflugers Arch. 2006;453:53–66. doi: 10.1007/s00424-006-0110-5. [DOI] [PubMed] [Google Scholar]
- Ochala J, Ahlbeck K, Radell PJ, Eriksson LI, Larsson L. Factors underlying the early limb muscle weakness in acute quadriplegic myopathy using an experimental ICU porcine model. PLoS One. 2011a;6:e20876. doi: 10.1371/journal.pone.0020876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Gustafson AM, Diez ML, Renaud G, Li M, Aare S, Qaisar R, Banduseela VC, Hedström Y, Tang X, Dworkin B, Ford GC, Nair KS, Perera S, Gautel M, Larsson L. Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms. J Physiol. 2011b;589:2007–2026. doi: 10.1113/jphysiol.2010.202044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J. Acquired and hereditary sarcomeric protein diseases. In: Hill JosephA, Stull JamesT, Lee Sweeney H, Olson EricN, Larsson L., editors. Muscle: Fundamental Biology and Mechanisms of Disease. London, UK: Academic Press; 2012. pp. 1023–1030. [Google Scholar]
- Ochala J, Renaud G, Llano Diez M, Banduseela VC, Aare S, Ahlbeck K, Radell PJ, Eriksson LI, Larsson L. Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS One. 2011c;6:e20558. doi: 10.1371/journal.pone.0020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Scapin C, Vitadello M, Florean C, Gorza L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J Cell Mol Med. 2010;14:970–981. doi: 10.1111/j.1582-4934.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- Radell PJ, Remahl S, Nichols DG, Eriksson LI. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med. 2002;28:358–364. doi: 10.1007/s00134-002-1207-8. [DOI] [PubMed] [Google Scholar]
- Renaud G, Llano-Diez M, Ravara B, Gorza L, Feng HZ, Jin JP, Cacciani N, Gustafson AM, Ochala J, Corpeno R, Li M, Hedström Y, Ford GC, Nair KS, Larsson L. Sparing of muscle mass and function by passive loading in an experimental intensive care unit model. J Physiol. 2013;591:1385–1402. doi: 10.1113/jphysiol.2012.248724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe F, Langnaese K, Wolf G. New aspects of the location of neuronal nitric oxide synthase in the skeletal muscle: a light and electron microscopic study. Nitric Oxide. 2005;13:21–35. doi: 10.1016/j.niox.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman IA, Lindgren JU, Gillberg PG, Elhassan AM, Monneron C, Adem A. Alteration of spinal cord IGF-I receptors and skeletal muscle IGF-I after hind-limb immobilization in the rat. Neuroreport. 1999;10:1195–1199. doi: 10.1097/00001756-199904260-00007. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest. 2007;117:2468–2476. doi: 10.1172/JCI30654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel FosFoxO1Stat3-Bim axis. FASEB J. 2011;25:2921–2936. doi: 10.1096/fj.11-183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarricone E, Scapin C, Vitadello M, Esposito F, Margonato V, Milano G, Samaja M, Gorza L. Cellular distribution of Hsp70 expression in rat skeletal muscles. Effects of moderate exercise training and chronic hypoxia. Cell Stress Chaperones. 2008;13:483–495. doi: 10.1007/s12192-008-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol Cell Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- Vitadello M, Gherardini J, Gorza L. The stress proteinchaperone Grp94 counteracts muscle disuse atrophy by stabilizing subsarcolemmal nNOS. Antiox Redox Signaling. 2014;20:2479–2496. doi: 10.1089/ars.2012.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–925. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- Yagi N. An x-ray diffraction study on early structural changes in skeletal muscle contraction. Biophys J. 2003;84:1093–1102. doi: 10.1016/S0006-3495(03)74925-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Luo J, Bourdon J, Lin MC, Gottfried SB, Petrof BJ. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am J Respir Crit Care Med. 2002;166:1135–1140. doi: 10.1164/rccm.2202020. [DOI] [PubMed] [Google Scholar]
- Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol. 2003;95:1116–1124. doi: 10.1152/japplphysiol.00824.2002. [DOI] [PubMed] [Google Scholar]


