Abstract
The effect of daily work stress on the next morning’s awakening cortisol level was determined in a sample of 124 mothers (M age = 49.89, SD= 6.33) of adolescents and adults with developmental disabilities and compared to 115 mothers (M age = 46.19, SD = 7.08) of individuals without disabilities. Mothers participated in 8 days of diary telephone interviews and provided saliva samples. Multilevel models revealed that mothers of individuals with developmental disabilities had lower awakening cortisol levels than comparison mothers. Work stress interacted with parental status to predict the awakening cortisol level on the following morning. When mothers of individuals with developmental disabilities experienced a work stressor, their awakening cortisol level was significantly higher on the subsequent morning, but for comparison mothers, work stressors were not significantly associated with cortisol level. Findings extend understanding of the differential impacts of specific types of stressors on physiological functioning of mothers of individuals with and without developmental disabilities.
Keywords: working mothers, work stress, health, cortisol
Work Stress on Physiological Functioning
There are unique challenges and opportunities in parenting an adolescent or adult with a developmental disability (DD). Although there is evidence of resiliency as well as vulnerability (e.g., Bekhet, Johnson, & Zauszniewski, 2012), parenting an individual with a DD often translates into higher stress levels, poorer psychological functioning, and more physical symptoms as compared to parents of typically developing children (Smith, Seltzer, & Greenberg, 2012). Recent studies have shown that child-related stressors can take a toll on neuroendocrine activity in parents of adolescents and adults with DD but have less impact on the physiological functioning of parents of individuals without disabilities (Seltzer et al., 2009). Less attention has been directed toward the effects of nonfamily stress on the physiological functioning of parents of individuals with disabilities. This study investigated the impact of work stress on the awakening cortisol level in mothers of adolescents and adults with and without DD. Specifically, we studied mothers of adolescents and adults with autism spectrum disorders (ASD) and mothers of those with Fragile X syndrome (FXS) and compared them with mothers of nondisabled similar-age children.
Caregiving and well-being
The life expectancy of individuals with DD has increased over the past several decades (e.g., Janicki, Dalton, Henderson, & Davidson, 1999), and many rely on family members throughout adulthood (Seltzer, Krauss, Orsmond, & Vestal, 2000). Thus, caring for a son or daughter with a DD often lasts into the parent’s old age. Smith et al. (2010) demonstrated that mothers of individuals with ASD are exposed to more daily stressors than mothers of individuals without disabilities. Greater exposure to stressors often leads to poorer psychological status, impaired physical health, and worse life course outcomes for these parents. Smith and colleagues (2012) also found that the mothers of a son or daughter with a DD (either ASD or FXS) experienced more days with headaches, backaches, and fatigue than mothers of a child without a disability. Seltzer, Greenberg, Floyd, Pettee, and Hong (2001) demonstrated that, as compared to mothers of individuals without DD, mothers of children with DD had lower rates of employment and social participation and were more likely than parents of typically developing children to report that family matters reduced the time available for their job. Together, these findings indicate that caring for a son or daughter with a DD takes a toll on multiple aspects of a parent’s life, including work as well as family.
Work and caregiving
The interplay of work and family in shaping psychological and physical well-being has been studied extensively (e.g., Grzywacz, Almeida, & McDonald, 2002; Moen, Kelly, & Huang, 2008). Although employment offers caregivers psychological and physical benefits (e.g., Hong & Seltzer, 1995), working caregivers are likely to experience strain when balancing work and family demands. Job strain in working care-givers has been linked to negative psychological and physical outcomes, including burden and worry (e.g., Molloy et al., 2008). These findings suggest that the impacts of work stressors on health may be accentuated by caregiving responsibilities, and that the strain of balancing work and family responsibilities is likely to increase working caregivers’ risk for dysregulated stress physiology. This study investigates how stress at work affected the physiological functioning, as measured by awakening cortisol levels, of mothers of adolescents and adults with and without DD.
Work stress and cortisol
Some attention already has been focused on determining whether work stress has a potent psychosocial influence on physiological outcomes, especially on autonomic and endocrine activity. Cortisol is an important marker of stress reactivity (Adam & Gunnar, 2001) and is frequently used as a general indicator of neuroendocrine regulation. Some studies of work stress and various parameters of adrenal function and cortisol secretion utilized samples that are homogenous with respect to occupation (e.g., teachers, nurses; Bellingrath, Weigl, & Kudielka, 2008; Wingenfeld, Schulz, Damkroeger, Rose, & Driessen, 2009), whereas others studied populations with diverse occupations (e.g., Alderling, Theorell, de la Torre, & Lundberg, 2006). Findings have been mixed, with some studies documenting a positive association whereas others showed a negative or no association between work stress and neuroendocrine functioning (e.g., Chandola, Heraclides, & Kumari, 2009; Steptoe, Cropley, Griffith, & Kirschbaum, 2000). For example, in their sample of teachers, Bellingrath and colleagues (2008) did not find any associations between burnout or vital exhaustion and basal cortisol activity. Conversely, Alderling et al. (2006), who utilized a more diverse sample of workers, found that women in high-strain jobs exhibited a significantly higher morning cortisol level at 30 minutes post-awakening than women in low-strain jobs. Furthermore, many of the available studies focused solely on work conditions and often overlooked how other aspects of life, such as family-related factors, may also shape physiological functioning.
Caregiving stress and cortisol
There have been a growing number of studies examining family-related factors and cortisol reactivity. Seltzer et al. (2009) reported that parents of adolescents and adults with disabilities exhibited a flatter daily decline of cortisol on days when they spent more time with their child with a disability, a pattern not evident in parents of similar-age children without disabilities. A profile of blunted or flattened cortisol changes across the day also was observed in another study of mothers of individuals with ASD (Seltzer et al., 2010); if the adolescent or adult with ASD had a history of clinically significant behavior problems, the mother had a less pronounced cortisol awakening response on the morning after a day when the son or daughter with ASD had more behavior problems. Similarly, Hartley et al. (2012) reported a lower cortisol level in genetically vulnerable mothers on mornings following a day when their child with FXS had more behavior problems. Together, these studies indicate that the chronic stress of parenting a child with DD affects the hypothalamic-pituitary-adrenal axis (HPA), specifically resulting in an atypical pattern of cortisol, especially in the context of challenging care-related events.
Diminished adrenal activity concurs with reports of blunted or flattened cortisol responses in other subgroups of individuals, including those with posttraumatic stress disorder (PTSD), certain forms of depression, occupational stress, and chronic disease conditions (Pruessner, Hellhammer, & Kirschbaum, 1999; Yehuda, 2000). However, there is evidence that different types of stressors may exert different effects. For instance, in some studies, individuals with PTSD display low cortisol levels in response to “reminder triggers” of the original traumatizing event, but conversely they can have an elevated cortisol response to stressful stimuli that are not related to the precipitating traumatic event (Bremner et al., 2003; Heim, Newport, Bonsall, Miller, & Nemeroff, 2001). The complexity of the linkage between psychological and physiological functioning highlights the need to better understand how parents of adolescents or adults with a DD react physiologically to different types of stress.
Work stress, caregiving stress, and cortisol
Some research already has attempted to bridge the interplay of work and caregiving stress, but the findings regarding cortisol levels have not been consistent. Luecken et al. (1997), in a between-group analysis, found that working women with at least one child exhibited higher cortisol levels over a 24-hour period than working women without children. However, in a within-group analysis, Adam and Gunnar (2001) reported lower average daily cortisol levels in working mothers who had more children in the household and in mothers who engaged in longer hours of employment. Eller, Kristiansen, and Hansen (2011) did not find significant effects of work and home factors on cortisol responses.
This Study
As we designed this study, we were mindful of methodological considerations raised by previous studies. For example, in some studies (e.g., Eller et al., 2011), cortisol was measured only on one day. Due to the variation in cortisol across days (Dahlgren, Kecklund, Theorell, & Akerstedt, 2009), it is now recommended that samples on multiple days be evaluated to reliably generate the typical picture for an individual, and this study includes cortisol measured on four consecutive days. Another methodological issue pertains to how best to classify employment status. In Adam and Gunnar (2001), for example, homemakers were combined with individuals who worked for pay outside the home, and this study was limited to mothers who worked outside of the home. Furthermore, the causal and proximal influences of stressful life events on cortisol levels are often difficult to determine, thereby making it challenging to identify antecedents and temporal relationships. By examining work stress that occurred on the day prior to the cortisol measurement, the time-order associations were clarified.
This study builds on and extends the extant research by investigating how stressful work events influence cortisol reactivity in mothers of adolescents and adults with and without DD. In line with past research that showed stronger cortisol reactivity on a work day (e.g., Kunz-Ebrecht, Kirschbaum, Marmot, & Steptoe, 2004), we focused specifically on how stressful work days affected cortisol levels upon awakening the subsequent morning.
We posed two hypotheses. In line with past research (Hartley et al., 2012; Seltzer et al., 2009; Seltzer et al., 2010) where it was shown that parents of adolescents and adults with disabilities had a low or flattened cortisol profile, we hypothesized a main effect whereby mothers of adolescents and adults with DD would evince lower cortisol at awakening than comparison mothers (Hypothesis 1). We also predicted an interaction effect between parent status and work stress on the level of cortisol at awakening (Hypothesis 2). Specifically, we hypothesized that mothers of adolescents and adults with DD would have a higher level of cortisol at awakening the day following a stressful event at work than on the morning after a day without a work stressor. This prediction was based on the expectation that mothers of adolescents and adults with DD would be particularly reactive to work stress due to greater work–family strain, and on evidence from PTSD research indicating that there might be greater cortisol reactivity to stressful events not specifically related to the trigger event. Although mothers of adolescents and adults with DD are hypothesized to be more reactive to work stressors than the comparison mothers, we also predicted that the comparison mothers will exhibit an elevation in cortisol level the morning following a work stressor, but to a substantially lesser extent. In spite of the mixed findings of work stress and cortisol (e.g., Chandola et al., 2010), we predicted an elevation to work stressor in the comparison mothers due in part to the increased cortisol level that is typically observed in response to acute stressors.
Method
Sample
The mothers of adolescents and adults with DD were obtained from two linked longitudinal studies: Adolescents and Adults with Autism (the AAA study; Barker et al., 2011) and Families of Adolescents and Adults with Fragile X Syndrome (the FXS study; Hartley et al., 2012). The comparison group of parents was derived from the Midlife in the United States Survey (MIDUS; Brim, Ryff, & Kessler, 2004). All three studies consisted of interviews and questionnaires followed by a Daily Diary Study and cortisol collection. The identical Daily Diary and cortisol paradigms, and sample collection protocols, were employed across the AAA, FXS, and MIDUS studies.
We used a common set of criteria to select sample members across the three studies. To be included, all mothers had to be employed at the time of the Daily Diary Study (see the operational definition of employment under the Measures section) and had to be at work, as defined by spending time on business or paid work, on the day prior to each cortisol collection day. In addition, mothers had to be co-residing with their child with a DD (in AAA and FXS) or with at least one child (in MIDUS).
Mothers of Adolescents and Adults with Developmental Disabilities
Mothers in the AAA and FXS studies were recruited through service agencies, clinics, and research registries using similar procedures. To qualify for the AAA study, families met the following three criteria: (a) included a child age 10 or older; (b) child received an independent diagnosis of ASD from a professional, as reported by parents; and (c) scores on the Autism Diagnostic Interview–Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003), administered by research staff, were consistent with the parental diagnostic report.
Families of 406 individuals with ASD participated at the start of the study in 1998. Data were collected primarily from mothers nine times between 1998 and 2012 (see Barker et al., 2011). At Time 5 (2006–2007), the AAA Daily Diary Study was conducted with mothers whose son or daughter with ASD still lived at home. By Time 5, 135 individuals with ASD remained co-resident with their mothers. Ninety-two mothers completed the Daily Diary protocol and cortisol collection. Mothers who did not meet our employment definition (n = 29; see below for the definition) were excluded. Respondents with missing data on key variables or noncompliant with complete saliva collection (n = 11) were dropped. Our final sample consisted of 52 mothers of adolescents and adults with ASD.
The FXS study required that the mothers be the biological parent of a son or daughter with FXS age 12 or older. The FXS study is a longitudinal study of 147 mothers of adolescents and adults with the full mutation of FXS. For this analyses, we utilized the first wave of data (2008–2009) in the FXS study. All but seven mothers were carriers of the “premutation” of the gene that causes FXS, and to reduce heterogeneity, these seven mothers were excluded. Of the 111 mothers who participated in the Daily Diary protocol and cortisol collection (2009–2010), 29 who did not meet our employment definition were excluded and an additional five mothers who did not work on the days prior to all of the cortisol collection days were excluded. Respondents with missing data or saliva sampling noncompliance also were dropped (n = 5). Thus, 72 mothers of adolescents and adults with FXS met the selection criteria. Although our analytic sample of mothers of individuals with FXS were significantly younger and had higher total household income than mothers of individuals with ASD, these two groups of mothers did not differ in other demographic characteristics, exposure to work stressors, or awakening cortisol level. The 72 mothers of individuals with FXS were combined with the 52 mothers of individuals with ASD to create our sample of 124 mothers of adolescents and adults with DD. The decision to combine the two groups of mothers is in keeping with past research (e.g., Lovell, Moss, & Wetherell, 2012; Seltzer et al., 2009), enhances statistical power, and concurs with prior results confirming similar cortisol patterns in mothers of individuals with ASD (e.g., Seltzer et al., 2010) and FXS (e.g., Hartley et al., 2012).
Mothers of Adolescents and Adults without Disabilities
A comparison sample of mothers of co-residing adolescents and adults without disabilities was drawn from MIDUS-II (2004–2006). A sample of 1,265 individuals participated in the MIDUS-II Daily Diary Study (2004–2009). Because the AAA and FXS studies consisted only of mothers, we excluded 548 MIDUS male respondents. Respondents who did not have any children living in their home (n = 421) were dropped. Another 66 respondents who had a child with a DD or a mental health condition were excluded. Respondents who did not participate in the cortisol collection were dropped (n = 59), resulting in 171 mothers of co-residing, similar-age children without disabilities. Mothers who did not meet our employment definition (n = 41) and who did not work on the day prior to all of the cortisol collection days (n = 12) were excluded. Three respondents with missing data or had saliva sampling noncompliance (n = 3) were dropped. Our comparison sample consisted of 115 mothers of adolescents and adults without disabilities.
Procedure
The Daily Diary Study protocol consisted of telephone interviews conducted with mothers across eight consecutive evenings. The telephone interview included questions on daily stressors, time use, and mood experienced in the previous 24 hours (Almeida, Wethington, & Kessler, 2002). The Daily Diary interviews were conducted by Pennsylvania State University’s Survey Research Center to ensure identical procedures. All mothers were asked the same core set of questions. Mothers of an individual with a DD additionally were asked each day about the behavior problems of that child.
On Days 2 through 5 of the Daily Diary Study, mothers provided four saliva samples on each day. Participants received a Home Saliva Collection Kit, which included 16 salivettes (Sarstedt, Nümbrecht, Germany), an instruction sheet, and a collection time log, one week prior to the telephone interview. Respondents were instructed to record the time they provided each sample; to collect their first sample before eating, drinking, or brushing their teeth; not to consume any caffeinated products before taking their subsequent samples; and to store all samples in the refrigerator.
Measures
Cortisol
Saliva was collected upon awakening, 30 minutes postawakening, before lunch, and before bedtime on Days 2 to 5 of the Daily Diary Study. The 16 samples were assayed for cortisol via a commercially available luminescence immunoassay (IBL, Hamburg, Germany), with intra-assay coefficients of variation below 5%. Cortisol data were log transformed to correct for positively skewed distributions. Before log transformation, salivary cortisol values higher than 60 nmol/l were recoded as 61 to minimize the influence of extreme outliers, following the Winsorization statistical approach (Dixon & Yuen, 1974).
Although there are different approaches to examining cortisol, including the complete daily rhythms, this study focuses on awakening cortisol levels, consistent with our prior analysis of the effects of stressful life events and daily stress in mothers of children with ASD (Wong et al., 2012). The morning cortisol level reflects the body’s ability to mobilize energy to handle the tasks of the day (Clow, Thorn, Evans, & Hucklebridge, 2004). Other research (e.g., Seltzer et al., 2010) has showed that low or blunted cortisol level in the morning is linked to stress on the day before. Further, focusing on awakening cortisol levels allowed us to more confidently interpret the time-order effects of prior work stressors on the next day’s cortisol level, whereas later values might have been contemporaneous with exposure to new stress.
Employment
Workers in our analytic sample were selected on the basis of three sources of employment information: (a) self-reported employment status assessed at the interview/questionnaire portion of the study; (b) information from the Daily Diary Study on “how much time was spent on activities related to business, paid work, or school” during the Diary Study period; and (c) notes from the open-ended questions of the Daily Diary Study.
We began by identifying respondents who self-reported as being employed in the interview or questionnaire and reported spending time on business, paid work, or school during the Daily Diary Study. Mothers who self-identified as retired were excluded even if they also reported spending time on business or paid work during the Daily Diary Study to avoid postretirement employees for whom the work role might not have been as salient. However, because of the time lapse between the interview/questionnaire portion and the Daily Diary Study, it was possible that employment status changed. Mothers who were not working at the time of the interview/questionnaire (but who did not self-identify as retired at that time) and reported spending time on business or paid work during the Daily Dairy Study were included only if there was clear evidence of employment from the notes from the open-ended questions. Finally, we eliminated cases in which individuals were in school, given our focus on work stress.
Work stressors
Exposure to daily work stressors was assessed using the Daily Inventory of Stressful Events (DISE; Almeida et al., 2002). The DISE comprises a series of stem questions that identify whether certain types of stressful events (e.g., arguments, avoided arguments, work-related) occurred in the past 24 hours and with whom the stressful event occurred. Any work-related stressors included having an argument or avoided an argument with a coworker, boss, employee/supervisee, or clients/customers/patients, as well as other stressful events that happened at work (e.g., equipment malfunction at work, schedule changes). Each work day was coded in a binary manner to categorize whether a work-related stressful event occurred (1) or not (0).
Control variables
We controlled for several characteristics of the mother. Maternal age was coded in years, and marital status was a contrast between unmarried and married. Highest education level was coded from 1 = less than high school to 4 = BA/Associate Degree or higher. Total household income and number of children in the household were controlled. These demographic characteristics were included as controls because of significant differences between mothers of individuals with and without DD (see Results, below). To account for potential medication effects on cortisol (Granger, Hibel, Fortunato, & Kapelewski, 2009), mothers reported whether they took any allergy, steroid, birth control/hormonal, or antidepressant/antianxiety medications (0 = none, 1 = at least one medication) across the Daily Diary Study period. Saliva collection time (Keenan, Licinio, & Veldhuis, 2001) and the number of hours spent on work the previous day (Klumb, Hoppmann, & Staats, 2006) was coded in hours. In past research, negative affect often explained the observed association between daily stress and cortisol (e.g., Smyth et al., 1998); therefore, average negative affect from the previous day was controlled. To measure negative affect, respondents were asked how frequently (0 = none to 4 = all of the time) they felt each 14 negative emotions (e.g., hopeless, angry) over the past 24 hours (Ready, Akerstedt, & Mroczek, 2011).
Results
Descriptive Findings
Descriptive statistics for the study participants are presented in Table 1. Mothers of adolescents and adults with DD were significantly older, were more likely to be married, had higher levels of education, and had more total household income than the comparison mothers. However, the two groups did not differ in race or number of children in the household. Additionally, in our sample of mothers of individuals with DD, 64.71% reported taking at least one medication during the Daily Diary Study period, which was significantly higher than the comparison mothers (52.60%, p < .01). Importantly, the collection time for awakening saliva sample was similar for mothers of individuals with DD (M = 6:30 am, SD = 73.20 minutes) and without DD (M = 6:32 am, SD = 80.68 minutes).
Table 1.
Descriptive Statistics of Demographic Characteristics by Parent Status
| Mothers of Adolescents and Adults without Disabilities (n = 115) | Mothers of Adolescents and Adults with Developmental Disabilities (n = 124) | p | ||
|---|---|---|---|---|
| Age of mother | M | 46.19 | 49.89 | <0.001 |
| SD | 7.08 | 6.33 | ||
| Range | 34–71 | 36–70 | ||
| Race | ||||
| White | % | 96.12 | 90.55 | n.s. |
| Other | % | 3.88 | 9.45 | |
| Marital status | ||||
| Married | % | 75.00 | 81.51 | <0.05 |
| Unmarried | % | 25.00 | 18.49 | |
| Educationa | M | 3.10 | 3.58 | <0.001 |
| SD | 0.90 | 0.70 | ||
| Range | 2–4 | 2–4 | ||
| Total household incomeb | M | 6.39 | 6.79 | <0.01 |
| SD | 2.19 | 1.70 | ||
| Range | 1–8 | 2–8 | ||
| Number of children in the household | M | 2.55 | 2.55 | n.s. |
| SD | 1.38 | 1.24 | ||
| Range | 1–10 | 1–6 | ||
Education: 1 = less than high school, 2 = high school degree or Graduate Equivalency Diploma, 3 = some college, and 4 = Bachelor or Associate degree or higher.
Household income: 1 = $0 to $9,999, 2 = $10,000 to $19,999, 3 = $20,000 to $29,000, 4 = $30,000 to $39,999, 5 = $40,000 to $49,999, 6 = $50,000 to $59,999, 7 = $60,000 to $69,999, and 8= $70,000 and greater.
*p < .05; **p < .01; ***p < .001.
Mothers of adolescents and adults with DD reported working significantly fewer hours on the previous day (M = 6.34, SD = 3.44) than the comparison group (M = 7.22, SD = 3.19, p < .001). The level of negative affect on the previous day also was significantly higher for mothers of individuals with DD (M = .46, SD = .51) than the comparison mothers (M = .24, SD = .30, p < .001). These variables were included in the multilevel models presented below.
Mothers of adolescents and adults with DD reported at least one work stressor from the previous day on 28.85% of the study days as compared to the 23.38% reported by the comparison mothers, but this small difference was not significant. The mean log awakening cortisol level was significantly lower for mothers of individuals with DD (M = 2.49 nmol/l, SD = .56) than the comparison mothers (M = 2.75 nmol/l, SD = .56, p < .001).
Multivariate Findings
For our 239 mothers, we started with a total of 1,004 days of cortisol data. Days when the previous day was a nonwork day (ndays = 279) were excluded. When cortisol values were flagged as problematic due to a failure to follow collection procedures or when mothers woke unusually late or remained awake for more than 20 hours (ndays = 54) were dropped. Missing data for the temporal predictors/covariates resulted in the exclusion of an additional 6 days, resulting in a total of 665 days of cortisol data for the present analyses.
To assess the effects of parental status and daily work stressors on awakening cortisol level the following morning, we utilized a set of two-level multilevel models (SAS Proc Mixed), with days nested within persons. The analyses were carried out in two separate models: main effects only (Model 1) and interaction effect (Model 2). Continuous variables at Level 1 (within-person) were group-mean centered and grand-mean centered at Level 2 (between-person). Daily work stressors were grand-mean centered. The within-person estimate represents the difference in cortisol from a nonstressor day to a stressor day and the between-person estimate reflects the incremental effect of the person over and above the day. Analyses showed that a random intercept only model had acceptable fit, and that the random effect of daily work stressor did not improve the model fit. Analyses indicated that education and household income had no significant effects on awakening cortisol level and did not change any of the estimates; therefore, these variables were dropped in the final models. Preliminary analyses also were carried out to examine whether findings differed when mothers of individuals with DD were analyzed separately (ASD vs. FXS) or together as one group (DD). Multilevel models showed that the findings were similar between these two groups of mothers of individuals with DD, and thus, the models with the combined groups of mothers of individuals with DD are presented.
In our multilevel models, we first examined the main effects of parental status and prior day within-person work stressor exposure on awakening cortisol level (see Table 2, Model 1). In support of Hypothesis 1, there was a significant main effect of parental status. Mothers of adolescents and adults with DD had lower awakening cortisol levels than the comparison mothers (b = −0.234, SE = .067, p < .001).
Table 2.
Multilevel Model of Parent Status and Daily Work Stressor Predicting Log Awakening Cortisol Level
| Model 1 | Model 2 | |
|---|---|---|
| Fixed effects | ||
| Intercept | 2.675 (0.079)*** | 2.699 (0.079)*** |
| Age | 0.004 (0.005) | 0.004 (0.005) |
| Parent statusa | −0.234 (0.067)*** | −0.282 (0.071)*** |
| Taking at least one medicationb | 0.008 (0.064) | 0.006 (0.064) |
| Marital statusc | 0.030 (0.083) | 0.030 (0.082) |
| Number of hours worked-previous day (WP) | −0.009 (0.008) | −0.009 (0.008) |
| Number of hours worked-previous day (BP) | −0.011 (0.012) | −0.010 (0.012) |
| Awakening collection time (WP) | −0.050 (0.022)* | −0.050 (0.022)* |
| Awakening collection time (BP) | −0.010 (0.029) | −0.009 (0.029) |
| Negative affect-previous day (WP) | −0.064 (0.068) | −0.063 (0.067) |
| Negative affect-previous day (BP) | −0.172 (0.095)# | −0.164 (0.094)# |
| Work stressor-previous day (WP) | 0.048 (0.047) | −0.056 (0.066) |
| Work stressor-previous day (BP) | −0.028 (0.140) | −0.045 (0.140) |
| Parent Status x Work Stressor-previous day (WP) | 0.190 (0.085)* | |
| Random effects (variance components) | ||
| BP intercept (level 2) | 0.153 (0.021)*** | 0.153 (0.021)*** |
| df = 231 | df = 231 | |
| WP (level 1) | 0.156 (0.011)*** | 0.155 (0.011)*** |
Note: BP = between-person; WP = within-person.
Parent status: 0 = mothers of adolescents and adults without disabilities, 1 = mothers of adolescents and adults with developmental disabilities.
Taking at least one medication: 0 = no, 1 = yes.
Marital status: 0 = not married, 1 = married.
p < .10;
p < .05;
p < .01;
p < .001.
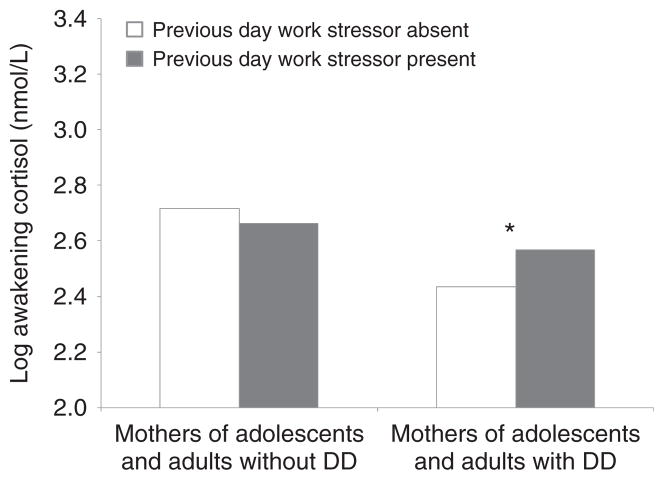
To examine Hypothesis 2, we tested the interaction effect of parental status and within-person work stressor from the prior work day on awakening cortisol level. The results portrayed in Table 2, Model 2, indicate a significant interaction between parental status and within-person work stressor from the prior work day (b = .190, SE = .085, p < .05) on awakening cortisol level. In keeping with the Hypothesis 2 prediction, the awakening cortisol level was higher when mothers of adolescents and adults with DD experienced a work stressor the previous day as compared to days without stressful work events (see Figure 1). In contrast, no difference in awakening cortisol level was observed for the comparison mothers on mornings following stressful and nonstressful work days. For the comparison mothers, a test of simple slopes showed that awakening cortisol level was not significantly different between the two types of days. These patterns support the core hypothesis that mothers of adolescents and adults with DD would be more reactive to work-related stressful events.
Figure 1.
Log Awakening Cortisol Level by Parent Status and Previous Day Work Stressor
DD = development disabilities. *p < .05
Discussion
Much of the prior caregiving literature focused on stress physiology has emphasized the impacts of child- or family-related stressors (e.g., Hartley et al., 2012; Seltzer et al., 2009; Wong et al., 2012). Less attention has been directed toward the influence of nonfamilial demands, specifically work stress, on cortisol reactivity. This study bridged the research on parental demands and occupational stress to better understand the overall toll on parents caring for adolescents or adults with DD.
In accordance with our first hypothesis, we found a significant effect of parental status on cortisol levels at awakening. Mothers of adolescents and adults with DD had lower cortisol at awakening than mothers in the comparison group. The difference between the two groups of mothers is consistent with past literature demonstrating a pattern of blunted or flattened cortisol in parents of adolescents and adults with disabilities but not in parents of similar-age children without disabilities (e.g., Barker, Greenberg, Seltzer, & Almeida, 2012; Seltzer et al., 2009; Seltzer et al., 2010). Similar blunted or flattened cortisol rhythms have been described in individuals with PTSD, for some types of job burnout, and also in some patients with chronic illnesses, such as chronic pain disorders (e.g., Pruessner et al., 1999; Yehuda, 2000). Thus, the current findings highlight the prolonged toll that caring for a son or daughter with a DD can have on a parent’s physiological functioning.
Although mothers of adolescents and adults with DD had lower overall awakening cortisol levels than the comparison group, these same mothers also had elevated levels of awakening cortisol on mornings following a stressful work day, in keeping with our second hypothesis. The finding is consistent with the literature on individuals with a history of PTSD indicating that despite lower overall levels, they may exhibit an elevated cortisol response to stressful events that are distinct from the original trauma (e.g., Bremner et al., 2003; Heim et al., 2001); by analogy, work stressors are distinct from child-related stress for mothers of individuals with DD. This study focused on nonfamily work stress and thus extends prior research on blunted or flattened cortisol patterns in the context of child- or family-related stress for parents of sons or daughters with disabilities (e.g., Seltzer et al., 2009; Seltzer et al., 2010; Wong et al., 2012). The results position us to better understand how different sources of stressors affect the health and physiology of adults experiencing a sustained life of emotional and personal demands (i.e., parenting an adolescent or adult offspring with a disability). The results also suggest that the low cortisol profile characteristic of mothers of individuals with DD does not signify a malfunctioning HPA axis, as adrenal secretion of cortisol was able to rise following work stress. Rather, the main effect of low cortisol likely reflects chronic exposure to child-related stress, and it is thus possible that low cortisol is a compensatory adaptive response or possibly a cognitive adjustment (e.g., maturation of emotion regulation strategies) to chronic child-related stress. These findings also point to the need for future studies to examine the long-term benefits and consequences of cortisol levels and day-to-day cortisol variability on health.
In this study, mothers in the comparison group did not exhibit an elevation in awakening cortisol level the morning after a day with a stressful event at work as compared with comparison group mothers who did not experience a work stressor. The research of Kunz-Ebrecht, Kirschbaum, and Steptoe (2004) suggests that the relationship between work stress and cortisol response may depend on the specific nature of the job stress. They found that job demands but not job control were associated with cortisol awakening response, suggesting that not all work stress is the same. This study design did not allow us to classify the nature of the work stress into its various dimensions. Nor were we able to delineate the unique effects of different types of employment (e.g., full-time, part-time) or the heterogeneity of low- and high-status employment with its differential reward and affirmation values; this would be another fruitful line of inquiry.
Together, findings from this study suggest that mothers of individuals with DD are more vulnerable and were physiologically affected when they were exposed to the demands of work, which were superimposed on their considerable parenting burdens. Work-related stress had more pronounced effects in the context of caring for an adolescent or adult with DD.
This study is not without limitations. Due to the study design, we could not examine how family structures shape caregiving and employment processes (e.g., dual careers, division of labor in caregiving, and paid employment). Future research using a mixed-methods design may help to capture the family processes that underlie caregiving and employment decisions and behaviors, and the subsequent impacts on physiological functioning. Other protective factors, such as the effectiveness of familial or workplace support systems or adaptive cognitive strategies, also could help to buffer against the challenges of caring for a son or daughter with a DD and should be considered. Especially for working mothers of co-residing adolescents or adults with DD, prospective longitudinal research would be important to determine the long-term consequences for health of sustaining low levels of cortisol at awakening. There is already some evidence that it may be associated with more fatigue and reduced alertness, as well as predisposing for inflammatory disorders (e.g., Buske-Kirschbaum, Ebrecht, & Hellhammer, 2010). It is also known that circulating levels of cortisol can influence alertness and the formation of memories as well as the capacity to recall emotionally laden information, which would be of significance for women experiencing many different types of life stress (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson, 2003).
There are also several unique strengths of this study. A naturalistic sampling of cortisol and daily stress was used in this study, thus, furthering the study of physiological phenomena in everyday life. We did not identify a priori the types of events that constitute work stressors. Instead, the DISE instrument offers respondents the opportunity to report what they perceive to be stressful work events. The Daily Diary methodology also allowed us to more accurately capture the temporal relationship between the daily events and subsequent cortisol levels. In addition to the between-group differences that were found, each person also served as her own control using the within-person analytic strategy of contrasting hormonal responses after stressful and nonstressful work days. Past studies (e.g., Dykens & Lambert, 2013) often focused solely on parents of individuals with DD, however, the inclusion of a comparison group of mothers in this study further extends our ability to generalize the findings and more clearly delineates the vulnerability of caregivers for individuals with DD.
Implications
Findings from this study highlight the need for programs and services to help parents caring for adolescents and adults with DD to better manage the dual challenges of parenting demands and work stress. Even though the majority of parental caregivers are employed, programs and services for families of individuals with disabilities have tended to focus on helping these families to learn parenting skills related to reducing the stress emanating from their child’s behavior problems and care needs (e.g., Farmer & Reupert, 2013; see Singer, Ethridge, & Aldana, 2007, for a review). Parents of children with DD also would benefit equally from programs that address stress management skills in the workplace. Existing support and educational programs for parents of adults with disabilities could be expanded to address coping and resilience with workplace stress. Previous work has demonstrated the important role of positive affect in moderating stress and health risks (e.g., Song et al., 2013), and thus the promotion of positive affect should be emphasized in these programs.
Our findings also highlight the opportunity for employers to create and promote a more caregiver-friendly workplace to help reduce work stress. Studies have shown greater work-place flexibility is associated with lower levels of conflict in working caregivers (e.g., Fredriksen-Goldsen & Scharlach, 2006), thereby, suggesting that the option to work flexibly may offer working caregivers an effective way to help reduce and prevent stress transmission between home and work. However, there are studies indicating that even when employees have access to workplace flexibility or leave options, many do not utilize these resources out of worry and fear (Still & Strang, 2003). Barriers to utilization of workplace resources also must be examined. Although not all employers are able to offer workplace resources, employers can help to create a more caregiver-friendly workplace by fostering better communication and enhancing support among supervisors, coworkers, and employees such that a better understanding of the needs and demands of being a working care-giver of an individual with a DD are known and made visible. Our recommendation of programs and services aimed at the reduction of work-place stressor exposure is not intended to place an additional burden on parents of individuals with DD. Rather, the goal is to increase visibility of the challenges faced by working caregivers of adolescents and adults with DD. Together, these programs and services may help to decrease work–family stress and ultimately reduce the negative employment and economic impacts of caring for an individual with a DD (e.g., Parish, Seltzer, Greenberg, & Floyd, 2004).
Summary
The physiological data reported in this article reaffirm our longstanding awareness of the personal and emotional toll of caring for sons or daughters with DD. Our findings provide another example of the unique cortisol dysregulation profile, with low levels of cortisol coexisting with a greater responsiveness to specific stimuli, which is reminiscent of PTSD and some types of depression and job burnout (e.g., Bremner et al., 2003; Heim et al., 2001). In conclusion, this study reinforces the view that more social services and familial support should be available to those who provide sustained care for adolescents and adults with DD.
Acknowledgments
This work was supported by the National Institute on Aging (R01 AG08768 to Marsha Mailick; P01 AG020166 to Carol Ryff). We also are grateful for the support from the National Institute of Child Health and Human Development (T32 HD07489 to Marsha Mailick; P30 HD03352 to Marsha Mailick). Support also was provided by a grant from the National Institute of Child Health and Human Development to the IDDRC at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison, and University of Kansas). The present analysis was based on data collected by the UW-Madison Waisman Center site (Marsha Mailick, PI).
Contributor Information
Jen D. Wong, The Ohio State University
Marsha R. Mailick, University of Wisconsin-Madison*
Jan S. Greenberg, University of Wisconsin-Madison**
Jinkuk Hong, University of Wisconsin-Madison***.
Christopher L. Coe, University of Wisconsin-Madison****
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/S0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Alderling M, Theorell T, de la Torre B, Lundberg I. The demand control model and circadian saliva cortisol variations in a Swedish population based sample (PART study) BMC Public Health. 2006;27:1–8. doi: 10.1186/1471-2458-6-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Experiences (DISE): An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Barker ET, Greenberg JS, Seltzer MM, Almeida DM. Daily stress and cortisol patterns in parents of adult children with a serious mental illness. Health Psychology. 2012;31:130–134. doi: 10.1037/a0025325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker ET, Hartley SL, Seltzer MM, Floyd FJ, Greenberg JS, Orsmond GI. Trajectories of emotional well-being in mothers of adolescents and adults with autism. Developmental Psychology. 2011;47:551–561. doi: 10.1037/a0021268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhet AK, Johnson NL, Zauszniewski JA. Resilience in family members of persons with autism spectrum disorder: A review of the literature. Issues in Mental Health Nursing. 2012;33:650–656. doi: 10.3109/01612840.2012.671441. [DOI] [PubMed] [Google Scholar]
- Bellingrath S, Weigl T, Kudielka BM. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biological Psychology. 2008;78:104–113. doi: 10.1016/j.biopsycho.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. The MIDUS national survey: An overview. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we? A national study of wellbeing at midlife. Chicago, IL: University of Chicago Press; 2004. pp. 1–36. [Google Scholar]
- Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain, Behavior, and Immunity. 2010;24:1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Chandola T, Heraclides A, Kumari M. Psychophysiological biomarkers of workplace stressors. Neuroscience & Biobehavioral Reviews. 2010;35:51–57. doi: 10.1016/j.neubiorev.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Kecklund G, Theorell T, Akerstedt T. Day-to-day variation in saliva cortisol-relation with sleep, stress and self-rated health. Biological Psychology. 2009;82:149–155. doi: 10.1016/j.biopsycho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Yuen KK. Trimming and Winsorization. Statistical Papers. 1974;15:157–170. [Google Scholar]
- Dykens EM, Lambert W. Trajectories of diurnal cortisol in mothers of children with autism and other developmental disabilities: Relations to health and mental health. Journal of Autism and Developmental Disorders. 2013;43:2426–2434. doi: 10.1007/s10803-013-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller NH, Kristiansen J, Hansen AM. Long-term effects of psychosocial factors of home and work on biomarkers of stress. International Journal of Psychophysiology. 2011;79:195–202. doi: 10.1016/j.ijpsycho.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Farmer J, Reupert A. Understanding autism and understanding my child with autism: An evaluation of a group parent education program in rural Australia. Australian Journal of Rural Health. 2013;21:20–27. doi: 10.1111/ajr.12004. [DOI] [PubMed] [Google Scholar]
- Fredriksen-Goldsen KI, Scharlach AE. An interactive model of informal adult care and employment. Community, Work, and Family. 2006;9:441–455. [Google Scholar]
- Granger D, Hibel L, Fortunato C, Kapelewski C. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1348. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Grzywacz JG, Almeida DM, McDonald D. Work-family spillover and daily reports of work and family stress in the adult labor-force. Family Relations. 2002;51:28–36. [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg JS, Almeida D, Coe C, Abbeduto L. Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavioral Development. 2012;36:53–61. doi: 10.1177/0165025411406857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hong J, Seltzer MM. The psychological consequences of multiple roles: The nonnormative case. Journal of Health and Social Behavior. 1995;36:386–398. [PubMed] [Google Scholar]
- Janicki MP, Dalton AJ, Henderson CM, Davidson PW. Mortality and morbidity among older adults with intellectual disability: Health services considerations. Disability Rehabilitation. 1999;21:284–294. doi: 10.1080/096382899297710. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proceedings of the National Academy of Sciences. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumb P, Hoppmann C, Staats M. Work hours affect spouse’s cortisol secretion—For better and for worse. Psychosomatic Medicine. 2006;68:742–746. doi: 10.1097/01.psy.0000233231.55482.ff. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on workdays and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status, and neuroendocrine activation over the working day. Social Science and Medicine. 2004;58:1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- Lovell B, Moss M, Wetherell M. The psychosocial, endocrine and immune consequences of caring for a child with autism or ADHD. Psychoneuroendocrinology. 2012;37:534–542. doi: 10.1016/j.psyneuen.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Suarez EC, Kuhn CM, Barefoot JC, Blumenthal JA, Siegler IC, Williams RB. Stress in employed women: Impact of marital status and children at home on neurohormone output and home strain. Psychosomatic Medicine. 1997;59:352–359. doi: 10.1097/00006842-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Moen P, Kelly E, Huang Q. Work, family, and life-course fit: Does control over work time matter? Journal of Vocational Behavior. 2008;73:414–425. doi: 10.1016/j.jvb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy GJ, Johnston DW, Johnston M, Gao C, Witham MD, Struthers AD, McMurdo ME. Using the demand-control model of job strain to predict caregiver burden and caregiver satisfaction in the informal caregivers of heart failure patients. British Journal of Health Psychology. 2008;13:401–417. doi: 10.1348/135910707X203363. [DOI] [PubMed] [Google Scholar]
- Parish SL, Seltzer MM, Greenberg JS, Floyd F. Economic implications of caregiving at midlife: Comparing parents with and without children who have developmental disabilities. Mental Retardation. 2004;42:413–426. doi: 10.1352/0047-6765(2004)42<413:EIOCAM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol response to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Ready RE, Akerstedt AM, Mroczek DK. Emotional complexity and emotional well-being in older adults: Risks of high neuroticism. Aging and Mental Health. 2011;16:17–26. doi: 10.1080/13607863.2011.602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Couteur AL, Lord C. In: ADI-R: Autism Diagnostic Interview-Revised. WPS, editor. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Hong J, Taylor JL. Psychosocial and biological makers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Floyd FJ, Pettee Y, Hong J. Life course impacts of parenting a child with disability. American Journal on Mental Retardation. 2001;106:265–286. doi: 10.1352/0895-8017(2001)106<0265:LCIOPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith L, Almeida D, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Orsmond GI, Vestal C. Families of adolescents and adults with autism: Uncharted territory. In: Glidden LM, editor. International review of research on mental retardation. Vol. 23. San Diego, CA: Academic Press; 2000. pp. 267–294. [Google Scholar]
- Singer GH, Ethridge BL, Aldana SI. Primary and secondary effects of parenting and stress management interventions for parents of children with developmental disabilities: A meta-analysis. Mental Retardation and Developmental Disabilities. 2007;13:357–369. doi: 10.1002/mrdd.20175. [DOI] [PubMed] [Google Scholar]
- Smith L, Hong J, Seltzer MM, Greenberg JS, Almeida DM, Bishop S. Daily experiences among mothers of adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:167–178. doi: 10.1007/s10803-009-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile X syndrome and mothers of adolescents and adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42:1836–1846. doi: 10.1007/s10803-011-1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Song J, Seltzer MM, Ryff CD, Coe CL, Greenberg JS, Hong J. Allostatic load in parents of children with developmental disorders: Moderating influence of positive affect. Journal of Health Psychology. 2013 doi: 10.1177/1359105312468193. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Cropley M, Griffith J, Kirschbaum C. Job strain and anger expression predict early morning elevation in salivary cortisol. Psychosomatic Medicine. 2000;62:286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Still M, Strang D. Institutionalizing family-friendly policies. In: Moen P, editor. It’s about time: Couples and careers. Ithaca, NY: Cornell University Press; 2003. pp. 288–309. [Google Scholar]
- Wingenfeld K, Schulz M, Damkroeger A, Rose M, Driessen M. Elevated diurnal salivary cortisol in nurses is associated with burnout but not with vital exhaustion. Psychoneuroendocrinology. 2009;34:1144–1151. doi: 10.1016/j.psyneuen.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Wong JD, Seltzer MM, Greenberg JS, Hong J, Almeida DM, Coe CL. Stressful life events and daily stressors affect awakening cortisol level in midlife mothers of individuals with autism spectrum disorders. Aging and Mental Health. 2012;16:939–949. doi: 10.1080/13607863.2012.688191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Biology of post-traumatic stress disorder. Journal of Clinical Psychiatry. 2000;61:14–21. [PubMed] [Google Scholar]