Abstract
Cellular homeostasis requires the balance of a multitude of signaling cascades that are contingent upon the essential proteins being properly synthesized, folded and delivered to appropriate subcellular locations. In eukaryotic cells the endoplasmic reticulum (ER) is a specialized organelle that is the central site of synthesis and folding of secretory, membrane and a number of organelletargeted proteins. The integrity of protein folding is enabled by the presence of ATP, Ca++, molecular chaperones, as well as an oxidizing redox environment. The imbalance between the load and capacity of protein folding results in a cellular condition known as ER stress. Failure of these pathways to restore ER homeostasis results in the activation of apoptotic pathways. Protein disulfide isomerases (PDI) compose a superfamily of oxidoreductases that have diverse sequences and are localized in the ER, nucleus, cytosol, mitochondria and cell membrane. The PDI superfamily has multiple functions including, acting as molecular chaperones, protein-binding partners, and hormone reservoirs. Recently, PDI family members have been implicated in the regulation of apoptotic signaling events. The complexities underlying the molecular mechanisms that define the switch from pro-survival to pro-death response are evidenced by recent studies that reveal the roles of specific chaperone proteins as integration points in signaling pathways that determine cell fate. The following review discusses the dual role of PDI in cell death and survival during ER stress.
Keywords: PDI, ER, ER stress, Apoptosis, Misfolded proteins, UPR, Oxidative stress, Redox, Conformational disease
Introduction
Cellular homeostasis is supported by a symphonic balancing act involving protein synthesis, folding, modification, trafficking and degradation. In eukaryotic cells the endoplasmic reticulum (ER) is a specialized organelle that is the central site of synthesis and folding of secretory, membrane and a number of organelletargeted proteins. Correct folding and post-translational modifications of these proteins are enabled by the presence of ATP, Ca++, molecular chaperone proteins, and an oxidizing environment that promotes disulphide bond formation [1]. Properly folded proteins are efficiently exported to the Golgi apparatus and other intracellular organelles, while misfolded proteins are recognized by ER quality control machinery (ERQC), translocated to the cytoplasm and targeted for degradation by the ER-associated degradation (ERAD) ubiquitin-proteosome system [2,3]. A hypothesized 30% of newly synthesized, potentially misfolded proteins are targeted for degradation under normal cellular physiology [4]. Given this surprising preexisting burden, even acute perturbations in the ER lumen environment and/or cellular events that disrupt chaperone activity may result in the severe accumulation and aggregation of unfolded or misfolded proteins.
The imbalance between the load and capacity of protein folding results in a cellular condition known as ER stress. Accumulating evidence demonstrates the importance of ER stress signaling pathways in maintaining function and differentiation of secretory cells such as B lymphocytes, pancreatic beta-cells, osteoblasts, and liver cells (reviewed elsewhere, [5]). However, these same pathways are linked to the etiology and progression of a number of human diseases, including Alzheimers, Parkinsons, diabetes mellitus, atherosclerosis, ischemia, liver and heart disease, as well as in cancer progression and the activation of inflammatory processes in and around the tumor microenvironment [6] (Table 1). ER stress is accompanied be the activation of a number of highly conserved integrated transcriptional and translational signaling cascades aimed at restoring ER homeostasis. Collectively, these signal-transduction pathways are termed the unfolded protein response (UPR).
Table 1.
The role of ER stress pathways in pathology
| Role of ER stress pathways | References | |
|---|---|---|
| Cancer | UPR proteins are upregulated; protect tumor cells from hypoxia-induced apoptosis, cell surface PDI expression mediates tumor invasion |
[8,90,97,113–115] |
| Alzheimers disease | Plaque formation and mutant presenilin-1 induces ER stress and neurodegeneration | [59,116,117] |
| Parkinsons disease | Mutation of parkin results in ER stress/UPR and neurodegeneration | [60,118–120] |
| Hutington’s Disease | Mutant huntingtin accumulation changes ER calcium homeostasis; results in UPR mediated neurodegeneration |
[121,122] |
| Amyotrophic lateral sclerosis | Mutant SOD disrupts ERAD and activates ASK1 | [123] |
| Prion Disease | Prion infection induces protective UPR pathways | [58,67,124] |
| Cerebral Ischemia | UPR mediated CHOP activation and neuronal death | [125] |
| Viral Infection | Infection induces UPR | [126] |
| Bipolar disease | Current treatments induce UPR pathways, proposed role for XBP1 polymorphisms in predisposition |
[127,128] |
| Diabetes | Impaired UPR pathways (PERK): Type 1; obesity induced ER stress and UPR mediated β-cell apoptosis: Type 2 |
[74,75,77,129–131] |
| Inflammation | Mediated through UPR sensor, CREBH | [132,133] |
| Atherosclerosis | Oxidized lipids and homocysteins induce ER stress/UPR in vascular cells | [78,79,82,134–136] |
| Autoimmune disease | ER stress pathways and overloaded ER linked to autoantigen production | [137,138] |
| Cardiovascular disease | Myocardial infarction and/or cardiac schemia induces ER stress/ UPR protective pathways |
[84,85,139–141] |
| Z- α1-antitrypsin deficiency | Accumulation of mutant protein induces stress and early onset liver disease | [142] |
The UPR initially counters ER stress by transiently arresting protein synthesis, Figure 1. This is followed by the activation of ERAD pathways and increased transcription of chaperone genes, such as the glucose-regulated protein GRP78 (BiP), calreticulin, calnexin, and protein disulfide isomerases (PDI), each with a role in promoting protein folding and alleviating protein aggregation. Failure of these pathways to restore ER homeostasis results in the activation of apoptotic pathways. The complexities underlying the molecular mechanisms that define this switch from pro-survival to pro-death response are evidenced by recent studies that reveal the roles of specific chaperone proteins as integration points in signaling pathways that determine cell fate [7–12]. The following review discusses the dual role of PDI in cell death and survival during ER stress.
Figure 1.
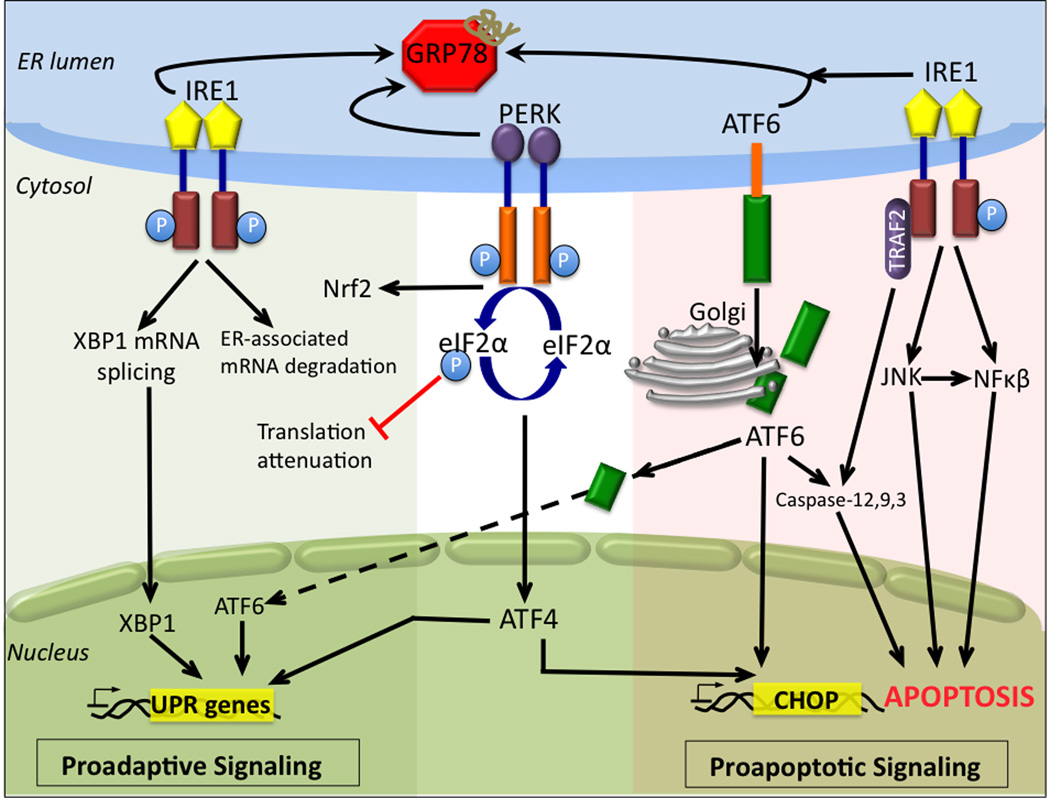
Signal transduction pathways in ER stress-induced apoptosis. The aggregation of misfolded/unfolded proteins in the ER results in GRP78 dissociation and the sequential activation of the three ER stress receptors: pancreatic ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). Activated PERK phosphorylates eukaryotic initiation factor 2α (eIF2α), which suppresses global mRNA translation and protein synthesis and activates ATF4 translation, which induces the transcription of genes aimed at restoring ER homeostasis. PERK phosphorylation of nuclear factor-E2-related factor 2 (Nrf2) regulates the expression of a number of genes involved in antioxidant defense pathways. Under chronic ER stress, activation of PERK induces the expression pro-apoptotic pathways involving ATF4-dependent CHOP expression. ATF6 is activated by proteolysis mediated by proteases after its translocation from the ER to the Golgi apparatus. Following proteolysis, active ATF6 translocates to the nucleus and regulates the expression of ER chaperones to facilitate protein folding, secretion, and degradation in the ER. Under severe ER stress ATF6 induces CHOP expression and mediates caspase activity. Activated IRE1 induces the expression of genes involved in ER-associated mRNA protein degradation and mediates the splicing of XBP1 mRNA. Spliced XBP1 translocates to the nucleus and controls the transcription induction of ER chaperones. If ER load is overwhelming IRE1 activates proapoptotic pathways through c-Jun NH2-terminal kinase (JNK) and nuclear factor-kappa-B (NF-κB). Apoptosis is additionally activated through IRE1 recruitment of TNF-receptor-associated factor 2 (TRAF2), and caspase activation. ATF4/6, activating transcription factor 4/6; CHOP, CCAAT/enhancer-binding protein (C/EBP) homologous protein; ER, endoplasmic reticulum; eIFα2, eukaryotic initiation factorα 2; GRP78, glucose regulated protein 78; IRE1, inositol-requiring enzyme 1; JNK, c-Jun NH2-terminal kinase; NF-κB, nuclear factor-kappa-B; Nrf2, nuclear factor-E2-related factor 2; PERK, pancreatic ER kinase; TRAF2, TNF-receptor-associated factor 2 UPR, unfolded protein response; Xbp1, X-box binding protein 1
The UPR: where do we go from here?
In mammalian cells three main ER trans-membrane receptors modulate ER stress and signal the downstream pathways that define the UPR; PERK (pancreatic ER kinase), IRE1 (inositol requiring enzyme 1) and ATF6 (activating transcription factor 6). Under homeostasis all three ER stress receptors are negatively regulated through direct protein-protein interactions with the ER chaperone GRP78. Accumulation of unfolded or misfolded proteins in the ER lumen induces the dissociation of GRP78 from the three sensors, allowing for activation of their signaling functions. Under ER stress, PERK is activated by auto-phosphorylation and dimerization. UPR mediated PERK phosphorylation alters two integrated signaling pathways, serving to restore ER homeostasis and combat oxidative stress [13,14]. PERK-mediated phosphorylation of eukaryotic initiation factor2 (eIF2α) results in attenuation of transient global mRNA translation in addition to the selective translation of the transcription factors and ER stress genes that carry certain regulatory sequences in their 5’ untranslated regions. These include ATF4 and ER stress genes such as amino acid transporters and cellular redox control genes [13]. Simultaneously, PERK phosphorylation of nuclear factor-E2-related factor 2 (Nrf2) regulates the expression of a number of genes involved in antioxidant defense pathways. Similar to PERK, trans-phosphorylation and homodimerization activate IRE1. The unique endoribonuclease activity of IRE1 catalyzes mRNA splicing of the bZIP transcription factor, X-box binding protein 1 (XBP-1). This forms a stable frameshift splice variant XBP-1s (spliced) that induces a number of genes involved in ER quality control and ERAD components, ER/Golgi biogenesis and activates pathways involved in redox homeostasis and oxidative stress responses [15]. XBP-1s also induces the expression of the HSP40 family member p58IPK, which binds to and inhibits PERK, resulting in a negative feedback loop and translational recovery [16]. p58IPK induction may represent UPR success and the restoration to normal cellular functioning or alternatively allow for the synthesis of pro-apoptotic proteins. Additional roles for IRE1 in translational attenuation and in mediating ER stress responses have been reported [17,18]. Following GRP78 dissociation, ATF6 activation involves redox-mediated regulated intra-membrane proteolysis (RIP) in the Golgi [19]. Proteolytic processing of ATF6 enables transcription factor binding and the regulation of a number of ER stress response genes, including the expression of ER chaperones, ERA components, and gene products with roles in lipid biogenesis and ER expansion [20,21].
ER-stress mediated apoptosis
The UPR pathways outlined above define the early pro-survival response signals that are aimed at restoring ER homeostasis. When the folding capacity is not restored all three UPR pathways contribute to cell apoptosis (Figure 1). Recent studies suggest that the degree of ER stress and the differential stability of pro-survival and pro-death mRNAs/proteins may partially regulate cell fate [22]. PERK, IRE1 and ATF6 mediated apoptosis have been linked to the downstream transcriptional activation of the C/EBP homologous protein (CHOP/growth-arrest-and DNA-damage-inducible gene 153 (GADD153)). PERK regulated ATF4 expression directly mediates CHOP expression and apoptotic cell death. ER stress induced ATF6 expression may play a role in the determination of cell fate and the transition from anti- to pro-apoptotic pathways, depending on the level of expression. Morishima et al found that ATF6 regulated increased expression of WW domain binding protein 1 (WBP1) resulted in myoblast apoptosis [23]. Although ATF6 can induce CHOP mRNA, reports that directly link ATF6-mediated CHOP expression to ER stress-induced apoptosis are lacking. CHOP sensitizes cells to ER stress through the down-regulation of the anti-apoptotic protein Bcl-2 and the activation of growth-arrest and DNA-damage-inducible gene 34 (GADD34) and the redox-altering ER oxidase, ERO1α. CHOP expression also leads to mitochondrial initiated apoptotic pathways via Bax mitochondrial translocation and caspase activation. Murine caspase-12 and human caspase-4 pathways have been directly implicated in ER stress-induced cell death.
Apoptosis is additionally activated through IRE1 recruitment of TNF-receptor-associated factor 2 (TRAF2) and apoptosis-signal-regulating kinase 1 (ASK1), and through the activation of the mitogen-activated protein kinase (MAPK) signaling pathways of p38 and c-Jun NH2-terminal kinase (JNK). The importance of ASK1 in ER stress mediated apoptotic pathways is exemplified by either over-expression or knockout studies. In lung epithelial cells and mouse embryonic fibroblasts (MEFs), ASK1 over-expression induced apoptosis through mitochondria-dependent caspase-3 and −9 activation; while the neuronal cells of ASK1−/− mice showed resistance to prolonged ER stress [24,25]. IRE1 mediated TRAF2 recruitment also permits TRAF2 dissociation from procaspase-12 (pCP12), allowing for the activation of pCP12 and downstream caspases. JNK mediated phosphorylation of Bcl-2 and Bim induces apoptosis both by suppressing the anti-apoptotic activity of Bcl-2 and enhancing additional pro-apoptotic pathways [26]. Regulation through IRE1 association/dissociation with Jun activation domain binding protein 1 (JAB1) may play a role in determining the choice between the induction of cell survival or death responses [27]. The complete in vivo signaling pathways involved in ER stress-associated apoptosis have not been entirely elucidated and are further confounded by research that suggests that cellular responses may be tissue, insult, and/or redox dependent [28–30].
Protein disulfide Isomerase
The ER lumen is a highly organized cellular compartment that contains numerous chaperones and oxidoreductases that assist in protein folding. Protein disulfide isomerases (PDI) compose a superfamily of more than 20 members reported to date that have diverse sequences and multiple functions including, acting as molecular chaperones, protein-binding partners, and hormone reservoirs [31,32]. The highly abundant redox-active enzyme PDI (P4HB, PDIA1) was one of the initial ER chaperones to be reported and plays a crucial role in catalyzing disulfide bond formation, reduction, and isomerization. PDI may also act as an intracellular anti-infammatory molecule through negative regulation of nuclear factor-kappa-B (NF-κB), inhibiting lipopolysaccharide (LPS)-induced pro-inflammatory cytokine production in macrophages [33].
The majority of PDI family members are characterized by at least one thioredoxin (Trx)-like Cys-Xaa-Xaa-Cys catalytic domain responsible for regulating PDI redox activity and a KDEL-like ER retention signal sequence. While PDI is primarily an ER protein, PDI expression has been documented on the cell surface as well as in the mitochondria, nucleus, and cytosol [34–37]. The ability to bypass KDEL-mediated ER anchoring has also been reported in the chaperone proteins GRP78 and GRP94, which can similarly display cell surface expression and extracellular secretion [38,39]. Furthermore, ER stress can induce leakage of PDI into the cytoplasm [40]. The functional consequence of KDEL-containing PDI in non-ER compartments is poorly understood.
Oxidative Protein Folding and the UPR
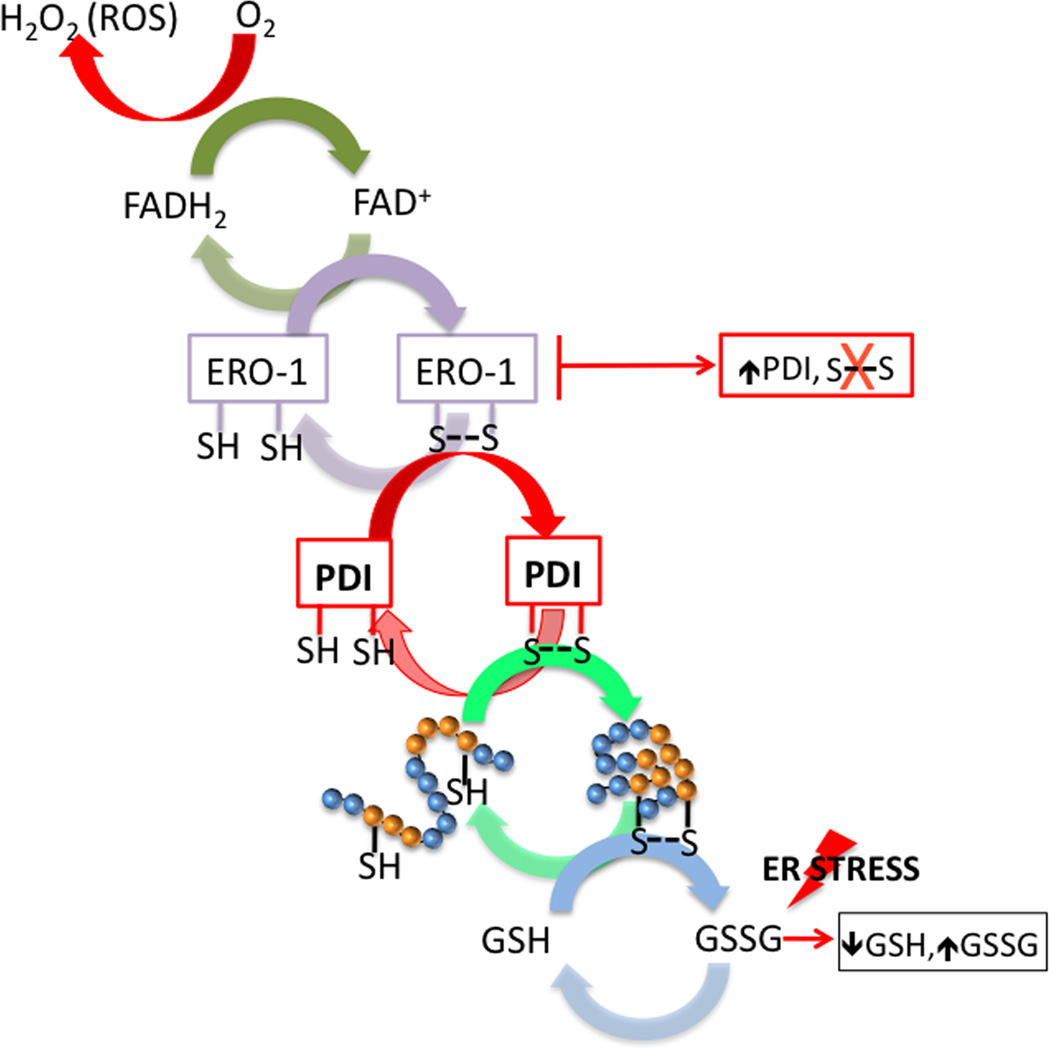
The oxidative environment of the ER is primed for the formation of disulfide bonds where glutathione (GSH) is the main redox buffer. PDI mediated folding of nascent proteins in the ER lumen involves repeated cycles of thiol oxidation and reduction; processes which become up-regulated during ER stress. During disulfide bond formation, PDI is reduced following the acceptance of electrons from cysteine residues (Figure 2). During this process PDI interacts with flavin adenine dinucleotide (FAD) -dependent ER oxidoreductin (Ero1) enabling the passage of electrons to molecular oxygen and the restoration of oxidized PDI [41]. Inhibition of Ero1 activity results in the accumulation of PDI and the cessation of disulfide bond formation [42]. PDI also has molecular chaperone activity that can include non-Cys containing proteins [43]. In vivo, it is possible that this chaperone activity prevents nonspecific interactions. In an elegant study using the yeast UPR model system, Delic et al introduce the importance of inter-compartmental redox homeostasis between the ER and cytosol during the UPR and a role for cytosolic glutathione peroxidase in the oxidative protein folding pathway [44]. Under conditions of ER stress and activation of the UPR, the redox potential within ER environment becomes increasingly reduced [45]. PDI may provide a redox-dependent switch by acting as a chaperone rather than a disulfide isomerase in its reduced state. In vitro studies show that reduced PDI binds and unfolds cholera toxin in the ER lumen whereas PDI oxidation results in toxin release and cytosolic translocation [46,47]. In the context of enhanced UPR-mediated protein folding, a more reduced ER environment may enable PDI-mediated disulfide reshuffling of mis-oxidized proteins, a prerequisite for ERAD [48].
Figure 2.
The formation of disulphide bonds in the ER is driven by PDI and ERO1. During disulfide bond formation, ERO1 operates in association with the flavin, FAD. ERO1 uses a FAD-dependent reaction to transfer electrons from PDI to molecular oxygen (O2), which results in the production of reactive oxygen species (ROS) in the from of H2O2 and the oxidation of PDI. PDI is reduced following the acceptance of electrons from protein-folding substrates, thereby oxidizing thiol (SH) groups in the target protein’s cysteine residues and forming disulphide bonds. Reduced GSH assists in disulphide-bond reduction, resulting in the production of oxidized glutathione (GSSG). Under conditions of ER stress and the UPR, GSH is consumed and the ER environment becomes increasingly reduced. Inhibition of Ero1 activity results in the accumulation of PDI and the cessation of disulfide bond formation. ER, endoplasmic reticulum; ER, endoplasmic reticulum; ERO1, ER oxidoreductin; FAD, flavin adenine dinucleotide; GHS, glutathione, PDI, protein disulfide isomerase.
Oxidative stress pathways have been linked with the pathology and etiology of diseases. For example, Nardai et al. found a selective reductive shift in the redox status of PDI and Erp57 in the liver of diabetic rats when compared to healthy animals [49]. This shift was accompanied by an increase in levels of oxidized Ero1 and a significant decrease in PDI enzymatic activity. The redox state of other proteins, including calnexin, Grp78 and Grp94 and the inactive PDI-homolog Erp28, remained unchanged. Systemic ER redox stress in diabetic animals may influence hepatic PDI redox state and enzymatic activity, ultimately disrupting hepatic protein secretion.
It is broadly accepted that redox regulation through the reversible oxidation of susceptible cysteine residues influences the activity of proteins such as enzymes, transcription factors or transporters [50–52]. Given that PDI’s active site cysteines are targets for redox defined post-translational modifications, such as S-glutathionylation and S-nitrosylation, and that these modifications regulate the chaperone and isomerase activities of PDI, it is likely that an interplay between cytoplasmic and ER redox environment govern the role of PDI in ER stress pathways [8,53–55]. The importance of PDI post-translational modifications and their role in ER-stress mediated apoptosis will be discussed in more detail in following sections.
ER stress, PDI and disease
Aging, genetic mutations, and environmental stressors may collectively and/ or independently lead to pathologically chronic ER stress and the accumulation of misfolded proteins. Diseases that are a direct result of this protein aggregation are collectively known as ‘conformational’ or ‘folding diseases’ [56]. These include atherosclerosis, ischemia, liver and heart disease, diabetes, inflammation and neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease and bipolar disorders. ER stress signaling is also associated with the pathology in cancer (Table 1).
Neurodegenerative diseases
While neurodegenerative diseases are linked with aging and/or have an underlying genetic cause, disease pathogenesis and cell cytotoxicity have been directly associated with an accumulation misfolded, aggregated proteins that form plaques [57,58]. In Alzheimer’s disease patients, the formation of senile plaques, composed of fibrillar β-amyloid (Aβ), is associated with disease progression. In vitro, the addition of high concentrations of Aβ to mature neurons results in dendritic and axonal retraction followed by neuronal death [59]. In Parkinson’s disease patients, there is an accumulation of α-synuclein in plaques referred to as Lewy Bodies. In vitro models showed that α-synuclein accumulation was toxic to cultured dopamine-dependent human dopaminergic neurons, but exhibited neuroprotective activity in non-dopaminergic cortical neurons [60]. In diseases that involve aberrant protein conformational changes, ER stress responses frequently result in chaperone up-regulation, particularly PDI, to protect against misfolded protein aggregation. Using cell-free in vitro assays, Cheng et al. showed that PDI prevents α-synuclein aggregation [61]. PDI expression is upregulated in the cerebrospinal fluid of amyotrophic lateral sclerosis patients manifesting intracellular aggregates of mutant superoxide dismutase (mtSOD1), as well as in the dopaminergic neurons and Lewy bodies of Parkinson’s disease patients. PDI also co-localizes with ubiquitin-positive neuronal inclusions of torsin-A in a transgenic mouse model of dystonia as well as with neurofbrillary tangles in Alzheimer’s patients and is up-regulated in glial cells in response to brain ischemia [62–66]. In cellular prion disease models, the up-regulation of the PDI family member Erp57 (PDIA3; GRP58) correlates with the accumulation of misfolded prion protein [67]. Wang et al. have recently reported a role for PDI as a pleiotropic regulator in the cellular management of misfolded prion protein [7].
PDI: neuroprotective or proapoptotic?
ER stress-mediated PDI up-regulation in neurodegenerative diseases attenuates misfolded protein accumulation and prevents neurotoxicity. In neuronal cell models of Parkinson’s disease and amyotrophic lateral sclerosis, PDI overexpression decreases synphilin-1 protein and mtSOD1, respectively [53,68]. The protective role for PDI in glial cells in response to brain ischemia has similarly been attributed to roles in decreasing protein misfolding [66]. It is worth noting however, that PDI does not decrease the number of inclusions formed by the variant of α1-antitrypsin linked with liver disease [69]. This suggests that PDI-mediated cellular protection may be disease and protein-specifc.
Discovery of S-nitrosylated PDI in brains manifesting sporadic Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis suggest that redox-dependent posttranslational modifications may impact PDI chaperone and isomerase activity and contribute to neurodegenerative conditions. Uerara et al. reported nitric oxide (NO) -induced S-nitrosylation of the active-site thiols of PDI (PDI-SNO) and showed that this modification abrogates activity, thus triggering activation of the UPR and ultimately inducing neuronal cell death [53]. Interestingly, in transgenic mouse models of amyotrophic lateral sclerosis, treatment with reticulon-4A (Nogo-A) induced cellular redistribution of PDI. Deletion of Nogo expression exacerbated disease progression and prevented PDI translocation, thus emphasizing the importance of PDI subcellular localization in these pathways [70].
PDIs can have similar anti-apoptotic/neuroprotective roles in transmissible neurodegenerative prion disorders. PDI overexpression protects against prion-induced neurotoxicity. Furthermore, infected animals exhibit S-nitrosylation of PDI, while blocking cellular NOS inhibits PDI-SNO formation and associated cell cytoxicity [7]. Prion disease-induced ER stress responses are also associated with Erp57 up-regulation. Erp57 overexpression in a neuroblasotoma cell line model protected against caspase-12 activation and misfolded prion protein aggregation induced neuronal apoptosis [67]. Given that many neurodegenerative diseases, including prion diseases, are characterized by excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), it is likely that PDI-SNO or redox-dependent post-translational modifications of other chaperone molecules may influence the pathophysiology of such disorders.
Interestingly, in a rat brain cell-based model for Huntington’s disease, expression of mutant Huntington exon 1 resulted in PDI concentration at ER-mitochondrial junctions and the subsequent induction of apoptotic pathways that lead to mitochondrial outer-membrane permeablization (MOMP). Selective small-molecule PDI inhibitors or shRNAs that inhibited the enzymatic activity of PDI suppressed the toxicity of the misfolded Huntingtin and of Aβ peptides processed from the amyloid precursor protein. PDI inhibition did not protect against other apoptotic stimuli, suggesting specificity for misfolded protein-mediated response [9]. GRP78 knockdown did not influence PDI-mediated MOMP induction nor anti-apoptotic pathways resulting from PDI knockdown. These data imply a role for non-UPR mediated pathways. The authors suggest that cellular death pathways are initiated when the accumulation of PDI in response to misfolded proteins reaches a threshold, similar to p53 expression, where cell apoptosis is induced at extreme thresholds of DNA damage. The concept of PDI as a pleiotropic apoptotic regulator is further supported by a recent study examining apoptotic pathways in a prion disease [7]. ER-targeted PDI overexpression in hamster brain cells expressing prion protein caused attenuation, but did not reverse caspase-12 mediated cellular apoptosis by reversing increases in Bax/Bcl-2 ratios. These events may represent early-stage infection. However, knockdown of endogenous PDI preferentially amended cytoxicity associated with a mutant prion protein with an extra octa-repeat insertion [7]. Wang et al., suggest that in late stages of prion infection, disruptions in oxidative homeostasis induce formation of PDI-SNO and facilitate apoptotic pathways. Both the cellular location and the mutant type of prion influenced PDI-mediated cell death.
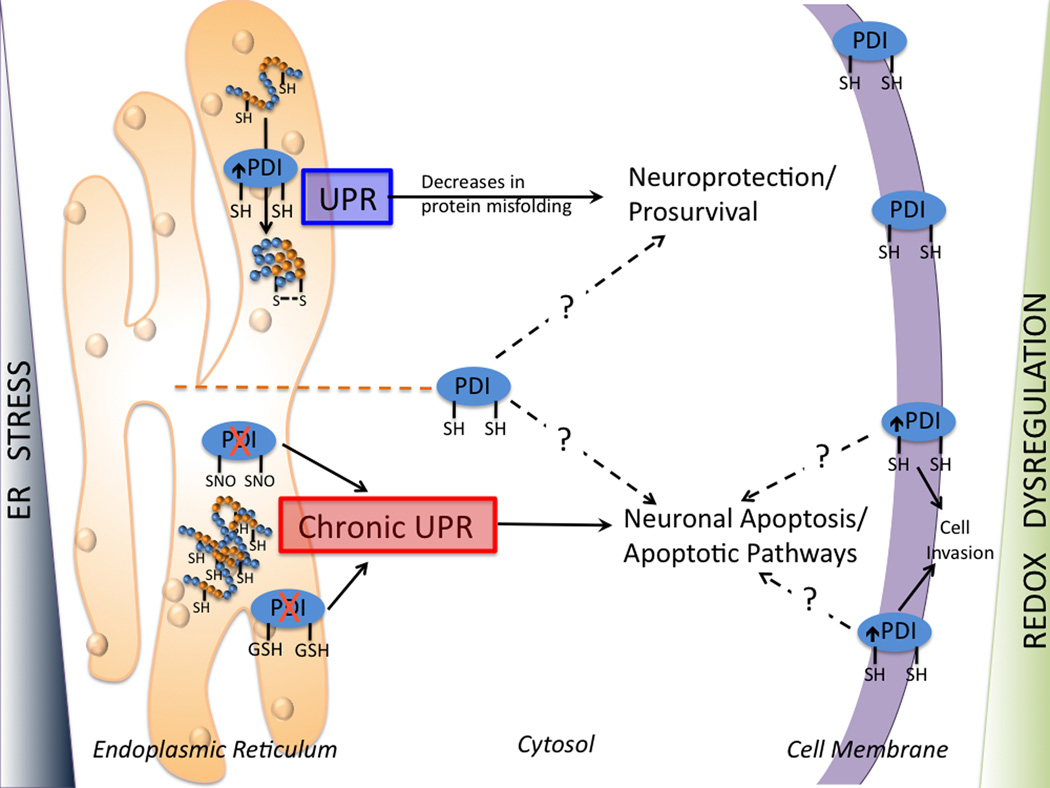
Collectively these studies suggest that PDI may participate in multiple phases of ER stress, where initial responses are protective and defined by attempts to process accumulated levels of unfolded proteins, while subsequent effects are pro-apoptotic, when protein repair has been deemed ineffective. It may be speculated that subcellular localization of PDI and/or protein aggregates, redox environment and post-translational modification of susceptible thiols, may contribute to defining and regulating the protective or apoptotic roles of PDI (Figure 3). Furthermore, important considerations of PDI binding partners in the neuroprotective roles of PDI are emphasized in studies that identify ubiquilin as an endoplasmic reticulum-associated protein in PDI-mediated acquisition of tolerance against ischemic stress in glial cells [71]. Indeed, additional investigation into how PDI-family members interact with each other and with other ER and non-ER resident proteins will further elucidate the apoptotic roles of PDI.
Figure 3.
The protective or apoptotic roles of PDI are regulated by the subcellular localization of PDI, redox environment and degree of ER stress. When unfolded/misfolded proteins accumulate in the ER lumen, ER stress triggers the UPR. Transient UPR induces cell survival pathways while a prolonged UPR elicits apoptotic pathways. PDI mediates proper protein folding. Under oxidative or nitrosative stress (as seen in neuronal disorders), the S-glutathionylation or S-nitrosylation of PDI inhibits normal protein folding and induces the activation of ER stresss. Prolonged UPR contributes to aberrant protein accumulation and cell apoptosis. ER luminal chaperones localized outside of the ER lumen may have proapoptotic roles. However, in some cell lines, overexpression of PDI constructs lacking the KDEL ER retention sequence increased cell survival following the induction of apoptotic stimuli. Elevated expression of cell surface PDI is associated with high-generation invasive tumors, suggesting that cell-surface PDI may have function in mediating apoptotic pathways. See text for additional discussion of these pathways. ER, endoplasmic reticulum; PDI, protein disulfide isomerase; UPR, unfolded protein response.
PDI-mediated apoptotic signaling in other disorders
Diabetes
ER stress pathways may have a role in the predisposition and onset of diabetes mellitus. Due to perpetual, fluctuating demands on insulin and glucagon synthesis and folding, β-cells are highly affected by deregulation of the UPR. PERK and eIF2α targeted knockout mice individually manifest pathologies associated with Type 1 diabetes mellitus, while CHOP knockout animals exhibit delayed onset [72–74]. Similar to PERK−/− mice, P58IPK deficient mice showed increased apoptosis of pancreatic islet cells and develop diabetes [75]. Enhanced expression of both ATF4 and CHOP following P58IPK gene silencing suggest the induction of apoptotic pathways most likely resulting from prolonged PERK activity, and the translational attenuation of pro-survival proteins. The up-regulation of chaperone proteins, including GRP78 and GRP94, in the insulin-secreting β-cells of diabetic mouse models further implies a role for additional ER stress-mediated pathways. In Type 2 diabetes models, ectopic overexpression of the molecular chaperones oxygen-regulated protein 150 (ORP-150) and GRP78 in β-cells improves insulin resistance, most likely by improving ER folding capabilities and protecting cells from ER stress-induced apoptosis [76,77]. However, PDI overexpression induced the opposite effect; resulting in elevated levels of misfolded pro-insulin and a reduction in glucose-stimulated insulin secretion and additional ER stress induction. Such data suggest that the regulation of PDI isomerase and associated chaperone activities may be essential in maintaining β-cell function and viability, whereas PDI overexpression may be lethal.
Cardiovascular Disease
A number of recent studies indicate that ER stress and the UPR are chronically activated in atherosclerotic cells [78]. Atherosclerosis associated ER stress has been linked to the accumulation of homocysteine, an intermediate amino acid in the metabolism of methionine and cysteine. Homocysteine accrual results in the up-regulation of ER stress pathways, including those involving BIP, GRP94, and CHOP-mediated apoptosis of macrophages [79,80].
Additional reports indicate a role for oxidized low-density lipoproteins (oxLDLs) in sustained ER stress, UPR pathways and the apoptosis of vascular endothelial cells and macrophages. A series of siRNA studies confirmed apoptosis mediation through IRE1α/JNK mediated XBP-1s and CHOP expression [81]. The pro-apoptotic and pro-atherogenic role of prolonged ER stress is exemplifed in studies with CHOP −/− mice where knockout animals exhibit a reduction in atherosclerotic plaque necrosis and apoptosis [82]. PDI overexpression counteracts ER stress and oxLDL-induced apoptosis. A recent study found that electrophilic aldehydes present in oxLDLs disrupt PDI redox state and induce formation of carbonyl-PDI. PDI modification resulted in enzymatic inactivation and cellular apoptosis, through the activation of CHOP and XBP-1s. Furthermore, carbonylated-PDI was detected in lipid rich areas of advanced human atherosclerotic lesions, suggesting that PDI modification directly contributes to ER-stress-induced apoptotic events in vivo [83].
PDI also has a protective role in ischemic cardiomyopathy and in the inhibition of apoptosis during post-infarction remodeling. PDI expression is upregulated in hypoxia-treated myocardial capillary endothelial cells in viable peri-infarct and infarct regions of mouse hearts. Increased PDI expression was correlated with protection against endothelial cell apoptosis as well as in cellular migration, adhesion, and tubular formation. siRNA-mediated PDI inhibition negates these effects while induced PDI expression confers additional protection against hypoxia-induced apoptosis [84,85]. While this study did not evaluate subcellular localization of PDI, cell-surface PDI may act as a reductase on the disulfide bond of cell surface proteins or interact with integrins to mediate endothelial cell adhesion and migration [86]. Additional discussion of cell-surface PDI in cancers is included in the following section. The PDI-mediated signaling pathways involved in endothelial cell survival and in angiogenesis have yet to be clearly defined, but may involve the enzymatic rather than chaperone functions of PDI, since point-mutations in PDIs active sites blocked apoptotic protection. Severino et al, reported the increased expression of Ero1 and IRE1 in cardiomyocytes following hypoxic stress as well as PDI-mediated cellular protection from superoxide through increased SOD1 activity [85]. There are indications that systemic disease conditions may impact the redox state of PDI and its importance in protecting cardiomyocytes from apoptosis [87]. In a mouse model of diabetes, diabetic mice exhibited increases in the reduced form of PDI and decreased post-infarction remodeling as compared to healthy animals. The authors suggest that while diabetic animals showed elevated PDI expression, these increased levels of PDI were confounded by redox-mediated inactivation in diabetic hearts.
The type of cell stress or injury impacts which PDI family member might participate in the regulation of apoptosis. While PDI up-regulation has a protective role in ischemic cardiomyopathy, hyperoxia-induced down-regulation of ERP57 in neonatal rat lungs and in cultured endothelial cells conferred similar cellular protection. Furthermore, siRNA-mediated ERP57 knockdown inhibited caspase-3 activation and further protected against hyperoxia-induced apoptosis, while ERP57 overexpression exacerbated hyperoxia- or tunicamycin-induced apoptosis and augmented caspase-3 activation, while reducing GRP78 induction [88].
Cancer
Many cancer-related pathologies are associated with deregulation of ER homeostasis and the induction of ER stress and UPR pathways. ER stress may be the result of high protein folding demands associated with increased rates of cancer cell proliferation or may be a consequence of tumor microenvironment. Hypoxia, pH, nutrient limitation, or therapeutic intervention strategies can all lead to perturbation of ER function and ER stress in cancer cells. A number of ER-resident proteins are upregulated in cancer and have diverse functions. Elevated ER stress chaperone protein expression has been correlated with tumor initiation, malignancy and metastasis. Chaperone proteins GRP78 and GRP94 are involved in anti-apoptotic pathways and tumor progression, and may serve as prognostic as well as diagnostic biomarkers [89,90]. ORP-150 influences tumor angiogenesis through the mediation of extracellular vascular endothlelial growth factor-A (VEGF-A) release [91]. PDI upregulation has been documented in a number of human cancer cells including ovarian, prostate, lung, lymphoma, glioma, acute myeloid leukemia, and melanoma [92–97]. Increases in GRP94 and PDI in multiple myeloma cell lines have been correlated with upregulated secretory demands. Targeting these cells with mitochondrial inhibitors overwhelmed existing ER stress responses, exacerbated Ca2+ leakage, and induced CHOP associated apoptosis [98].
Cell surface PDI is associated with high-generation invasive glial tumors. Targeting PDI with PDI monoclonal antibodies and the PDI-inhibitor, bacitracin, inhibited tumor cell migration and invasion, implying that cell-surface PDI may be involved in mediating apoptotic pathways [97]. Several PDI family members are associated with non-ER locations and have been assigned a broad array of functions [35]. For example, ER luminal chaperones localized outside of the ER lumen may have pro-apoptotic roles. Cell surface expression of the ER chaperone calreticulin is involved in antigen processing as a recognition ligand in phagocytotic processes and is involved in determining cancer cell immunogenicity during apoptosis [99,100]. ERP57-calreticulin interactions are necessary for calreticulin membrane translocation. Furthermore, ER-stress in prostate cancer cells induces GRP78 translocation to the cell surface where it serves to initiate FADD/caspase8/caspase3 apoptotic signaling via interaction with extracellular prostate apoptosis response 4 (Par-4) [101]. Bcl-2 proteins may have a role in increasing ER permeability and facilitating luminal protein translocation during ER-stress induced apoptosis [102]. Interestingly, cytosolic PDI acts as a substrate for caspase-3 and 7, and overexpression of PDI constructs lacking the KDEL ER retention sequence increased cell survival following the induction of apoptotic stimuli in premyeloid HL-60 cells [103]. PDI may also modulate apoptosis through the reversal of iron(III) mediated caspase-3 inhibition through the formation of iron-sulfur complexes at active site thiols [104].
Cell-surface redox environment defines thiol content, disulfide bond formation and chaperone function. PDI-mediated reduction of β-integrin disulfide bonds mediates cell migration and adhesion, while its redox-sensitive interaction with other surface proteins serves to facilitate viral entry [37,86,105,106]. Furthermore, S-glutathionylation of cell surface proteins by oxidized glutathione impacts intracellular and extracellular redox homeostasis [107]. The cell-surface redox environment may have a pleiotropic impact on PDI-mediated apoptotic pathways, acting directly at the cell membrane as well as intracellularly. Indeed, human cancer cells treated with redox-modulating agents that modify cell surface thiols show increases in the S-glutathionylation of PDI (PDI-SSG) which leads to inhibition of PDI activity and the induction of UPR–mediated cell death [8,107]. Recently, S-glutathionylation of the tumor necrosis factor (TNF) receptor family member, Fas (CD95, Apo-1), was found to be defined by subcellular localization and interactions with ERP57 and glutathione S-transferase pi (GSTP), influencing pathways that determine the strength of apoptosis. Inhibition of ERP57 or GSTP decreased Fas-ligand dependent Fas S-glutathionylation (Fas-SSG) and enhanced cell survival, where increased expression of ERP57 and GSTP resulted in translocation of Fas-SSG from the ER and eventually caspase-mediated cell death [10]. This study exemplifes the importance of the subcellular redox environment and protein-protein interactions in the complex pathways that determine cell survival.
PDI as Therapeutic Target
As outlined above, dysregulation of PDI plays a role in a number of human pathologies and as such presents a plausible target for therapeutic intervention. In particular, small molecules that mimic chaperone function or induce endogenous chaperone function may be viable targets in diseases characterized by protein aggregation, defective protein secretion or ischemia. Alternatively, agents that specifically target chaperone function would benefit in treating cancers that are defined by constitutive UPR activation and/or chemotherapy resistance, where chaperone inhibition sensitizes cancer cells to therapy-induced apoptosis.
Therapeutic application of PDI inhibitors in the treatment of cancers that are closely associated with poor prognosis and therapy resistance have shown promise in a number of studies, including those using melanoma, glioblastoma, breast cancer and ovarian cell lines/xenograft models [92,94,97,108]. PDI knockdown in melanoma and ovarian cell models inhibits xenograft formation and tumor growth and induces cellular apoptosis, in response to ER inducing agents and as a solo treatment, respectively [92,94]. However, therapeutic application of PDI inhibition may be confounded by studies that suggests that the UPR of different tumor cell lines differentially respond to various UPR stimuli and by differential regulation of ER chaperones. PDI induction was induced following tunicamycin but not ritonavir treatment in sarcoma cell lines [109]. Furthermore, in a single study, siRNA-mediated PDI knockdown induced varying degrees of cytoxicity in human breast cancer, neuroblastoma, and cervical cancer cell lines [108]. The therapeutic success of PDI-targeted therapies may depend on understanding how ER stress response pathways are integrated in individual cell types and in individual pathologies.
Drug resistant tumors and cancer cells have elevated levels of GSTP. The development of nitric oxide (NO) releasing prodrugs that are metabolized by GSTPs have recently shown preclinical therapeutic potential in treating malignancy through tumor- specific activation [110,111]. Treatment of premyloid HL60 and ovarian cancer cells with O(2)-[2,4-dinitro-5-(N-methyl-N-4-carboxyphenylamino)phenyl]1-(N,N-methylamino) diazen-1-ium-1,2-diolate (PABA/NO) resulted in elevated intracellular nitric oxide and the activation of UPR-mediated cytototoxic pathways [8,55,112]. PABA/NO-induced cytotoxicity is linked to the S-glutathionylation of cysteines within PDI’s active site, which blunts activity and leads to activation of the UPR and JNK activated apoptosis [55]. Recent studies suggest that nitrosative/oxidative induced S-glutathionylation of PDI also impacts the chaperone activity. Specifically it was shown that PDI-SSG mediates UPR activation and cell death through abrogation of estrogen receptor α (ERα) stability and signaling in ERα-positive breast cancer cells [54]. In a model of PABA/ NO acquired drug-resistance, HL60 cells were shown to have a constitutively activated UPR, including elevated levels of PDI, blunted cellular differentiation and decreased levels of S-glutathionylation caused by adaptive GSTP down-regulation [112]. Collectively, these data indicate the involvement of multiple extrinsic and intrinsic ER stress-mediated apoptotic cascades that may be harnessed individually or in concert to overcome drug resistant cancer pathologies.
Balancing the protein folding capacity within the cell is critical and the PDI superfamily plays a key role in both opposing pro- survival and death pathways. The emerging role of PDI in human pathologies remains poorly understood yet underscores the keystone role in normal physiology.
Acknowledgements
This work was supported by grants from the National Center for Research Resources (5P20RR024485-02) and the National Institute of General Medical Sciences (8 P20 GM103542-02) from the National Institutes of Health and by CA08660, CA117259, R56 ES017453 and support from the South Carolina Centers of Excellence program.
References
- 1.Gaut JR, Hendershot LM. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol. 1993;5:589–595. doi: 10.1016/0955-0674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 2.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 3.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 4.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 6.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang SB, Shi Q, Xu Y, Xie WL, Zhang J, Tian C, et al. Protein disulfide isomerase regulates endoplasmic reticulum stress and the apoptotic process during prion infection and PrP mutant-induced cytotoxicity. PLoS One. 2012;7:e38221. doi: 10.1371/journal.pone.0038221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Jr., Hutchens S, et al. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffstrom BG, Kaplan A, Letso R, Schmid RS, Turmel GJ, Lo DC, et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat Chem Biol. 2010;6:900–906. doi: 10.1038/nchembio.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, et al. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol. 2012;32:3464–3478. doi: 10.1128/MCB.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerin R, Arseneault G, Dumont S, Rokeach LA. Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol Biol Cell. 2008;19:4404–4420. doi: 10.1091/mbc.E08-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 13.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 14.Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- 18.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 19.Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol. 2007;27:1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morishima N, Nakanishi K, Nakano A. Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via induction of WW domain binding protein 1. J Biol Chem. 2011;286:35227–35235. doi: 10.1074/jbc.M111.233502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, et al. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 26.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 27.Oono K, Yoneda T, Manabe T, Yamagishi S, Matsuda S, Hitomi J, et al. JAB1 participates in unfolded protein responses by association and dissociation with IRE1. Neurochem Int. 2004;45:765–772. doi: 10.1016/j.neuint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7670. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 29.Gorman AM, Healy SJ, Jager R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 31.Benham AM. The protein disulfide isomerase family: key players in health and disease. Antioxid Redox Signal. 2012;16:781–789. doi: 10.1089/ars.2011.4439. [DOI] [PubMed] [Google Scholar]
- 32.Imaoka S. Chemical stress on protein disulfide isomerases and inhibition of their functions. Int Rev Cell Mol Biol. 2011;290:121–166. doi: 10.1016/B978-0-12-386037-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Jacob A, Ho N, Miksa M, Wu R, Maitra SR, et al. Downregulation of protein disulfide isomerase in sepsis and its role in tumor necrosis factor-alpha release. Crit Care. 2008;12:R100. doi: 10.1186/cc6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigobello MP, Donella-Deana A, Cesaro L, Bindoli A. Distribution of protein disulphide isomerase in rat liver mitochondria. Biochem J. 2001;356:567–570. doi: 10.1042/0264-6021:3560567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 36.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi S, Hong PW, Lee B, Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci U S A. 2011;108:10650–10655. doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabata Y, Takano K, Ito T, Iinuma M, Yoshimoto T, Miura H, et al. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am J Physiol Cell Physiol. 2007;293:C411–C418. doi: 10.1152/ajpcell.00095.2007. [DOI] [PubMed] [Google Scholar]
- 41.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–1574. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 42.Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- 43.Cai H, Wang CC, Tsou CL. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269:24550–24552. [PubMed] [Google Scholar]
- 44.Delic M, Rebnegger C, Wanka F, Puxbaum V, Haberhauer-Troyer C, Hann S, et al. Oxidative protein folding and unfolded protein response elicit differing redox regulation in endoplasmic reticulum and cytosol of yeast. Free Radic Biol Med. 2012;52:2000–2012. doi: 10.1016/j.freeradbiomed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 45.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 47.Moore P, Bernardi KM, Tsai B. The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin. Mol Biol Cell. 2010;21:1305–1313. doi: 10.1091/mbc.E09-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillece P, Luz JM, Lennarz WJ, de La Cruz FJ, Romisch K. Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol. 1999;147:1443–1456. doi: 10.1083/jcb.147.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nardai G, Stadler K, Papp E, Korcsmaros T, Jakus J, Csermely P. Diabetic changes in the redox status of the microsomal protein folding machinery. Biochem Biophys Res Commun. 2005;334:787–795. doi: 10.1016/j.bbrc.2005.06.172. [DOI] [PubMed] [Google Scholar]
- 50.Uys JD, Xiong Y, Townsend DM. Nitrosative stress-induced S-glutathionylation of protein disulfide isomerase. Methods Enzymol. 2011;490:321–332. doi: 10.1016/B978-0-12-385114-7.00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7:313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Manevich Y, Tew KD, Townsend DM. S-Glutathionylation of Protein disulfide Isomerase Regulates Estrogen Receptor alpha Stability and Function. Int J Cell Biol. 2012;2012:273549. doi: 10.1155/2012/273549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, et al. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 57.Forman MS, Lee VM, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003;26:407–410. doi: 10.1016/S0166-2236(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 58.Torres M, Castillo K, Armisen R, Stutzin A, Soto C, Hetz C. Prion protein misfolding affects calcium homeostasis and sensitizes cells to endoplasmic reticulum stress. PLoS One. 2010;5:e15658. doi: 10.1371/journal.pone.0015658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 61.Cheng H, Wang L, Wang CC. Domain a’ of protein disulfide isomerase plays key role in inhibiting alpha-synuclein fibril formation. Cell Stress Chaperones. 2010;15:415–421. doi: 10.1007/s12192-009-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, et al. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet. 2005;14:125–133. doi: 10.1093/hmg/ddi012. [DOI] [PubMed] [Google Scholar]
- 64.Honjo Y, Ito H, Horibe T, Takahashi R, Kawakami K. Protein disulfide isomerase-immunopositive inclusions in patients with Alzheimer disease. Brain Res. 2010;1349:90–96. doi: 10.1016/j.brainres.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 65.Conn KJ, Gao W, McKee A, Lan MS, Ullman MD, Eisenhauer PB, et al. Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson’s disease and Lewy body pathology. Brain Res. 2004;1022:164–172. doi: 10.1016/j.brainres.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka S, Uehara T, Nomura Y. Up-regulation of protein-disulfide isomerase in response to hypoxia/brain ischemia and its protective effect against apoptotic cell death. J Biol Chem. 2000;275:10388–10393. doi: 10.1074/jbc.275.14.10388. [DOI] [PubMed] [Google Scholar]
- 67.Hetz C, Russelakis-Carneiro M, Walchli S, Carboni S, Vial-Knecht E, Maundrell K, et al. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J Neurosci. 2005;25:2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker AK, Farg MA, Bye CR, McLean CA, Horne MK, Atkin JD. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2010;133:105–116. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- 69.Papp E, Szaraz P, Korcsmaros T, Csermely P. Changes of endoplasmic reticulum chaperone complexes, redox state, and impaired protein disulfide reductase activity in misfolding alpha1-antitrypsin transgenic mice. FASEB J. 2006;20:1018–1020. doi: 10.1096/fj.05-5065fje. [DOI] [PubMed] [Google Scholar]
- 70.Yang YS, Harel NY, Strittmatter SM. Reticulon-4A (Nogo-A) redistributes protein disulfide isomerase to protect mice from SOD1-dependent amyotrophic lateral sclerosis. J Neurosci. 2009;29:13850–13859. doi: 10.1523/JNEUROSCI.2312-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ko HS, Uehara T, Nomura Y. Role of ubiquilin associated with protein-disulfide isomerase in the endoplasmic reticulum in stress-induced apoptotic cell death. J Biol Chem. 2002;277:35386–35392. doi: 10.1074/jbc.M203412200. [DOI] [PubMed] [Google Scholar]
- 72.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 74.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 76.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but Not Protein-disulfide Isomerase, Partially Reverses Hyperglycemia-induced Inhibition of Insulin Synthesis and Secretion in Pancreatic {beta}-Cells. J Biol Chem. 2009;284:5289–5298. doi: 10.1074/jbc.M805477200. [DOI] [PubMed] [Google Scholar]
- 78.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, Hossain GS, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 80.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanson M, Auge N, Vindis C, Muller C, Bando Y, Thiers JC, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 82.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller C, Bandemer J, Vindis C, Camare C, Mucher E, Gueraud F, et al. Protein Disulfide Isomerase Modification and Inhibition potentiates ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4577. [DOI] [PubMed] [Google Scholar]
- 84.Tian F, Zhou X, Wikstrom J, Karlsson H, Sjoland H, Gan LM, et al. Protein disulfide isomerase increases in myocardial endothelial cells in mice exposed to chronic hypoxia: a stimulatory role in angiogenesis. Am J Physiol Heart Circ Physiol. 2009;297:H1078–H1086. doi: 10.1152/ajpheart.00937.2008. [DOI] [PubMed] [Google Scholar]
- 85.Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U, et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–1037. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 86.Swiatkowska M, Szymanski J, Padula G, Cierniewski CS. Interaction and functional association of protein disulfide isomerase with alphaVbeta3 integrin on endothelial cells. FEBS J. 2008;275:1813–1823. doi: 10.1111/j.1742-4658.2008.06339.x. [DOI] [PubMed] [Google Scholar]
- 87.Toldo S, Boccellino M, Rinaldi B, Seropian IM, Mezzaroma E, Severino A, et al. Altered oxido-reductive state in the diabetic heart: loss of cardioprotection due to protein disulfide isomerase. Mol Med. 2011;17:1012–1021. doi: 10.2119/molmed.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu D, Perez RE, Rezaiekhaligh MH, Bourdi M, Truog WE. Knockdown of ERp57 increases BiP/GRP78 induction and protects against hyperoxia and tunicamycin-induced apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L44–L51. doi: 10.1152/ajplung.90626.2008. [DOI] [PubMed] [Google Scholar]
- 89.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 90.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Ozawa K, Tsukamoto Y, Hori O, Kitao Y, Yanagi H, Stern DM, et al. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 2001;61:4206–4213. [PubMed] [Google Scholar]
- 92.Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363–5369. doi: 10.1158/0008-5472.CAN-08-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haefliger S, Klebig C, Schaubitzer K, Schardt J, Timchenko N, Mueller BU, et al. Protein disulfide isomerase blocks CEBPA translation and is up-regulated during the unfolded protein response in AML. Blood. 2011;117:5931–5940. doi: 10.1182/blood-2010-08-304485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, et al. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci U S A. 2012;109:16348–16353. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, et al. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 96.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 97.Goplen D, Wang J, Enger PO, Tysnes BB, Terzis AJ, Laerum OD, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–9902. doi: 10.1158/0008-5472.CAN-05-4589. [DOI] [PubMed] [Google Scholar]
- 98.Kurtoglu M, Philips K, Liu H, Boise LH, Lampidis TJ. High endoplasmic reticulum activity renders multiple myeloma cells hypersensitive to mitochondrial inhibitors. Cancer Chemother Pharmacol. 2010;66:129–140. doi: 10.1007/s00280-009-1143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 100.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 101.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X, Olberding KE, White C, Li C. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 2011;18:38–47. doi: 10.1038/cdd.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Na KS, Park BC, Jang M, Cho S, Lee do H, Kang S, et al. Protein disulfide isomerase is cleaved by caspase-3 and −7 during apoptosis. Mol Cells. 2007;24:261–267. [PubMed] [Google Scholar]
- 104.Sliskovic I, Mutus B. Reversible inhibition of caspase-3 activity by iron(III): potential role in physiological control of apoptosis. FEBS Lett. 2006;580:2233–2237. doi: 10.1016/j.febslet.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 105.Fenouillet E, Barbouche R, Courageot J, Miquelis R. The catalytic activity of protein disulfide isomerase is involved in human immunodeficiency virus envelope-mediated membrane fusion after CD4 cell binding. J Infect Dis. 2001;183:744–752. doi: 10.1086/318823. [DOI] [PubMed] [Google Scholar]
- 106.Jain S, McGinnes LW, Morrison TG. Thiol/disulfide exchange is required for membrane fusion directed by the Newcastle disease virus fusion protein. J Virol. 2007;81:2328–2339. doi: 10.1128/JVI.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008;68:2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hashida T, Kotake Y, Ohta S. Protein disulfide isomerase knockdown-induced cell death is cell-line-dependent and involves apoptosis in MCF-7 cells. J Toxicol Sci. 2011;36:1–7. doi: 10.2131/jts.36.1. [DOI] [PubMed] [Google Scholar]
- 109.Kraus M, Malenke E, Gogel J, Muller H, Ruckrich T, Overkleeft H, et al. Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis. Mol Cancer Ther. 2008;7:1940–1948. doi: 10.1158/1535-7163.MCT-07-2375. [DOI] [PubMed] [Google Scholar]
- 110.Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, et al. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hutchens S, Manevich Y, He L, Tew KD, Townsend DM. Cellular resistance to a nitric oxide releasing glutathione S-transferase P-activated prodrug, PABA/NO. Invest New Drugs. 2011;29:719–729. doi: 10.1007/s10637-010-9407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 114.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 116.Sato N, Urano F, Yoon Leem J, Kim SH, Li M, Donoviel D, et al. Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol. 2000;2:863–870. doi: 10.1038/35046500. [DOI] [PubMed] [Google Scholar]
- 117.Terro F, Czech C, Esclaire F, Elyaman W, Yardin C, Baclet MC, et al. Neurons overexpressing mutant presenilin-1 are more sensitive to apoptosis induced by endoplasmic reticulum-Golgi stress. J Neurosci Res. 2002;69:530–539. doi: 10.1002/jnr.10312. [DOI] [PubMed] [Google Scholar]
- 118.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 119.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 120.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vidal R, Caballero B, Couve A, Hetz C. Converging pathways in the occurrence of endoplasmic reticulum (ER) stress in Huntington’s disease. Curr Mol Med. 2011;11:1–12. doi: 10.2174/156652411794474419. [DOI] [PubMed] [Google Scholar]
- 122.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23:239–252. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 126.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 127.Kakiuchi C, Iwamoto K, Ishiwata M, Bundo M, Kasahara T, Kusumi I, et al. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet. 2003;35:171–175. doi: 10.1038/ng1235. [DOI] [PubMed] [Google Scholar]
- 128.Shao L, Sun X, Xu L, Young LT, Wang JF. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 2006;78:1317–1323. doi: 10.1016/j.lfs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 129.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 130.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 131.Jeffrey KD, Alejandro EU, Luciani DS, Kalynyak TB, Hu X, Li H, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A. 2008;105:8452–8457. doi: 10.1073/pnas.0711232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic infammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 133.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasa-Nicotera M. The new kid on the block: the unfolded protein response in the pathogenesis of atherosclerosis. Cell Death Differ. 2004;11(Suppl 1):S10–S11. doi: 10.1038/sj.cdd.4401468. [DOI] [PubMed] [Google Scholar]
- 135.Zhang C, Kawauchi J, Adachi MT, Hashimoto Y, Oshiro S, Aso T, et al. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem Biophys Res Commun. 2001;289:718–724. doi: 10.1006/bbrc.2001.6044. [DOI] [PubMed] [Google Scholar]
- 136.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 137.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 138.Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–1498. doi: 10.4049/jimmunol.166.3.1492. [DOI] [PubMed] [Google Scholar]
- 139.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 140.Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–1605. doi: 10.1016/j.bbrc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 141.Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol. 2008;44:453–459. doi: 10.1016/j.yjmcc.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawless MW, Greene CM, Mulgrew A, Taggart CC, O’Neill SJ, McElvaney NG. Activation of endoplasmic reticulum-specifc stress responses associated with the conformational disease Z alpha 1-antitrypsin defciency. J Immunol. 2004;172:5722–5726. doi: 10.4049/jimmunol.172.9.5722. [DOI] [PubMed] [Google Scholar]