Abstract
In this issue of Blood, Fan et al discover the presence of a new receptor-ligand pair that inhibits platelet activation.1
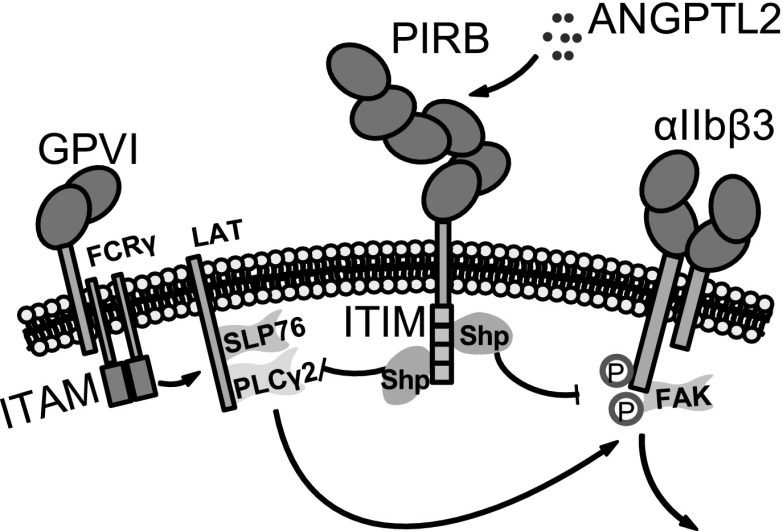
Outside-in signaling of integrins requires additional molecules not depicted in the schematic.3 See Figure 7E in the article by Fan et al that begins on page 2421.
Platelets play important roles in maintaining the integrity of blood vessels and preventing bleeding. However, the activities of platelets are tightly regulated to avoid thrombosis and allow vascular permeability. The classical mechanism for the negative regulation of platelets includes prostacyclin binding to its receptor, which inhibits platelet activation by activating Gs protein, elevation of cyclic adenosine monophosphate (cAMP), and activation of cAMP-dependent protein kinase. P2Y12 adenosine 5′-diphosphate (ADP), receptor antagonists such as clopidogrel block ADP-induced activation of Gi protein (which inhibits cAMP elevation), thus indirectly elevating cAMP levels and contributing to their antiplatelet effect. The effect of another often-mentioned inhibitor, nitric oxide (NO) is debatable. NO plays biphasic roles of stimulating and negatively regulating platelet activation mainly by activating cyclic guanosine monophosphate (cGMP)- and cAMP-dependent protein kinases.2,3 Possibly due to its biphasic roles, no NO- or cGMP-based antiplatelet agents have been successfully developed.
Recent studies in platelets indicate the importance of a family of receptors containing an immunoreceptor tyrosine-based activation motif (ITAM), which includes the collagen receptor GPVI/Fc receptor γ chain complex and podoplanin receptor Clec-2. They are important not only in thrombosis but also in regulating vascular permeability during inflammation and maintaining hemostasis at the junction of blood and lymphatic vessels.4,5 The signaling of ITAM-containing receptors requires phosphorylation of ITAM by Src family kinases and subsequent Syk binding to ITAM and activation. Syk induces formation of the complex of linkers for activated T cells (LATs), the Src homology 2 domain-containing leukocyte phosphoprotein of 76-kDa (SLP76) and phospholipase Cγ2, which induces calcium elevation and activation of protein kinase C.6 The ITAM signaling pathway is important not only in transducing GPVI and Clec-2 signals but also in amplifying signals induced by adhesion receptors, the glycoprotein Ib-IX complex, and integrins.7,8 Similar to that found in other immune cells, the counterbalance for the ITAM receptors are the receptors containing an immunoreceptor tyrosine-based inhibition motif (ITIM), which inhibits ITAM signaling by recruiting phosphatases. The currently known platelet ITIM receptor includes platelet endothelial cell adhesion molecule-1 (PECAM-1), which is an adhesion receptor that binds to PECAM-1 receptors in other cells and plays an inhibitory role in collagen- and von Willebrand factor-induced platelet activation.9 G6B, another ITIM receptor expressed in platelets, appears to be important in normal platelet production by megakaryocytes.10
Fan et al demonstrate that platelets express the leukocyte immunoglobulin-like receptor subfamily B (LILRB), and its murine homolog, the paired immunoglobulin-like receptors B (PIRB). LILRB/PIRB are members of the immunoglobulin superfamily containing the ITIM motif. As expected for a functional ITIM receptor, platelet PIRB is important in inducing activation of protein tyrosine phosphatase Shp1/2 and inhibition of ITAM signaling. The authors further report that a ligand for the LILRB/PIRB, angiopoietin-like-protein 2 (ANGPTL2), inhibits platelet activation induced by GPVI agonists and integrin outside-in signaling. Importantly, mouse platelets expressing a functionally deficient mutant of PIRB showed enhanced platelet activation. These results suggest a new ligand-ITIM receptor pair that inhibits platelet activation. Different from previously identified ITIM receptor ligands, ANGPTL2 is a soluble protein that is stored in platelet granules and released during platelet activation, which raises interesting questions: whether ANGPTL2 serves as a self-control mechanism that negatively regulates ITAM signaling during platelet activation and inflammation and whether ANGPTL2 or its analogs can be developed as a new type of platelet inhibitor.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Fan X, Shi P, Dai J, et al. Paired immunoglobulin-like receptor B regulates platelet activation. Blood. doi: 10.1182/blood-2014-03-557645. 2014;124(15):2421-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Xi X, Gu M, et al. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003;112(1):77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30(12):2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog BH, Fu J, Wilson SJ, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502(7469):105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulaftali Y, Hess PR, Getz TM, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123(2):908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmeier W, Stefanini L. Platelet ITAM signaling. Curr Opin Hematol. 2013;20(5):445–450. doi: 10.1097/MOH.0b013e3283642267. [DOI] [PubMed] [Google Scholar]
- 7.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112(7):2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullam PM, Hyun WC, Szöllösi J, Dong J, Foss WM, López JA. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcgammaRIIA on the platelet plasma membrane. J Biol Chem. 1998;273(9):5331–5336. doi: 10.1074/jbc.273.9.5331. [DOI] [PubMed] [Google Scholar]
- 9.Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97(6):1727–1732. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- 10.Mazharian A, Wang YJ, Mori J, et al. Mice lacking the ITIM-containing receptor G6b-B exhibit macrothrombocytopenia and aberrant platelet function. Sci Signal. 2012;5(248):ra78. doi: 10.1126/scisignal.2002936. [DOI] [PMC free article] [PubMed] [Google Scholar]