Key Points
Adherence rates were significantly lower in African Americans (87%) and Asian Americans (90%), as compared with non-Hispanic whites (95%).
Adherence to 6MP at <90% was associated with a 3.9-fold increased risk of relapse in a multiracial cohort of children with ALL.
Abstract
Durable remissions in children with acute lymphoblastic leukemia (ALL) require a 2-year maintenance phase that includes daily oral 6-mercaptopurine (6MP). Adherence to oral 6MP among Asian-American and African-American children with ALL is unknown. We enrolled 298 children with ALL (71 Asian Americans, 68 African Americans, and 159 non-Hispanic whites) receiving oral 6MP for the maintenance phase. Adherence was measured electronically for 39 803 person-days. Adherence declined from 95.0% (month 1) to 91.8% (month 5, P < .0001). Adherence rates were significantly (P < .0001) lower in Asian Americans (90.0% ± 4.9%) and African Americans (87.1% ± 4.4%), as compared with non-Hispanic whites (95.2% ± 1.3%). Race-specific sociodemographic characteristics helped explain poor adherence (African Americans: low maternal education [less than a college degree: 78.9%, vs at least college degree: 94.6%; P < .0001]; Asian Americans: low-income households [<$50 000: 84.5%, vs ≥$50 000: 96.7%; P = .04]; households without mothers as full-time caregivers [85.6%] vs households with mothers as full-time caregivers [97.2%; P = .05]). Adherence rate below 90% was associated with increased relapse risk (hazard ratio, 3.9; P = .01). Using an adherence rate <90% to define nonadherence, 20.5% of the participants were nonadherers. We identify race-specific determinants of adherence, and define a clinically relevant level of adherence needed to minimize relapse risk in a multiracial cohort of children with ALL. This trial was registered at www.clinicaltrials.gov as #NCT00268528.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy.1 Although over 95% of children with ALL enter remission after the first 28 days of induction therapy, up to 20% suffer a relapse.2 Prognosticators have included age and white blood cell count at diagnosis,3 specific blast chromosomal abnormalities,4 and minimal residual disease (MRD).5 Durable remissions require a 2-year maintenance phase that includes daily oral 6-mercaptopurine (6MP).6,7 Low erythrocyte levels of 6MP metabolites (thioguanine nucleotide [TGN]) have been reported to be associated with relapse.8,9 Significant interpatient variability in erythrocyte TGN levels, observed even among individuals with thiopurine methyltransferase (TPMT) wild-type genotype,10 could be due to failure on the part of patient or parent to adhere to prescribed therapy.11
Lack of adherence to oral chemotherapy has been reported in children with ALL.11-16 We have described significantly lower adherence to oral 6MP in Hispanic children compared with non-Hispanic white children with ALL receiving maintenance therapy.17 African Americans and Asian Americans constitute a significant proportion of the US population,18 and face unique challenges in accessing health care. Yet, little is known regarding adherence to oral 6MP among Asian-American and African-American children with ALL. The current study addresses this gap, by describing the prevalence and predictors of adherence to oral 6MP unique to African Americans and Asian Americans drawn from a geographically diverse cohort of children with ALL, and treated according to Children’s Oncology Group (COG) protocols. We also describe the impact of adherence to oral 6MP on the risk of relapse in this population, and define a critical level of adherence needed to minimize relapse risk.
Methods
Study participants
Participating COG institutions (n = 77; supplemental Table 1, see supplemental Data available at the Blood Web site) contributed patients after obtaining approval for the study (COG-AALL03N1) from local institutional review boards. Written informed consent/assent was obtained from patients and/or parents/legal guardians. The study was conducted in accordance with the Declaration of Helsinki. Eligibility criteria included: diagnosis of ALL at age 21 years or younger, in first continuous remission, belonging to 1 of 3 self-reported racial groups (Asian American, African American, non-Hispanic white [referent group]), and receiving maintenance chemotherapy that included oral 6MP. The non-Hispanic white patients had contributed to a previous report describing adherence in Hispanic and non-Hispanic white children with ALL.17 Table 1 details the inclusion/exclusion criteria with rationale.
Table 1.
Inclusion and exclusion criteria with rationale
| Criteria* | Rationale |
|---|---|
| Inclusion | |
| Diagnosis of ALL at <21 y of age, in first remission | The study aimed to explore the underlying causes of racial differences in outcomes in children with ALL diagnosed at <21 y of age. |
| This age cutoff was used because the current study was attempting to understand the causes of racial differences in survival that had been observed by us and others in previous studies that had also used age at diagnosis of 21 y as the cutoff. | |
| Belongs to the following self-reported racial categories: non-Hispanic white, African American, or Asian American† | The study aimed to explore the prevalence and predictors of nonadherence in children from these three racial groups. |
| Has completed the first 6 mo block of maintenance therapy for ALL | This allows patients to become accustomed to the routine of maintenance therapy. It also increases the probability that >90 d have elapsed since last red cell transfusion, avoiding contamination of TGN and TPMT activity by transfusion (the majority of transfusions administered during maintenance therapy are within the first 6 mo). |
| ≥6 mo of maintenance therapy remain at enrollment | The last 6-mo block of maintenance therapy is needed to ensure adequate time for adherence monitoring during study enrollment. |
| Receiving oral 6MP during maintenance therapy for ALL | The study is designed to measure adherence to oral 6MP. |
| Signed assent/consent by the patient/parent | To ensure that all human subjects’ protection issues are addressed. |
| Exclusion | |
| Patients of multiethnic/multiracial backgrounds | The study aimed to explore systemic exposure to 6MP among patients belonging to the 3 self-reported racial groups: Non-Hispanic white, Asian American, African American. Patients of multiethnic/racial backgrounds were not included in this study; this will be the focus of future study. |
| Patients of Hispanic ethnicity | A study with the same study design has reported the adherence to oral 6MP among Hispanics. |
| Patients who are unable to use MEMS TrackCap (eg, using pillbox or liquid 6MP) | MEMS TrackCap was the primary method of assessing adherence; use of a pillbox or liquid 6MP would have prevented the use of MEMS TrackCap. |
Eligibility is not affected by the patient or parent/caregiver’s ability to speak English.
Racial categories: non-Hispanic white: European, North African, or Middle Eastern ancestry; Asian Americans: Asian Indian subcontinent, Chinese, Japanese, Korean, Native Hawaiian, Guamanian or Chamorro, Pacific Islander, Filipino, Vietnamese, Samoan, Hmong, Cambodian, Thai, Laotian, or other Asian-American races; African Americans: African American or of sub-Saharan black African ancestry.
Measurement of adherence
We recorded adherence for up to 5 months per patient, using an electronic monitoring device (MEMS TrackCap; Aprex Corporation). The MEMS cap utilizes microelectronic technology to record date/time of each pill bottle opening (see supplemental Figure 1A). Patients/parents were informed about the purpose of MEMS, and were instructed to take all doses of 6MP from the MEMS bottle. At the end of the adherence-monitoring period, the MEMS data were downloaded (see supplemental Figure 1B).
Monthly erythrocyte TGN levels were measured19 for each participant to determine the association between MEMS bottle opening and 6MP ingestion. Erythrocyte TGN levels reflect chronic (prior 1-4 weeks) systemic exposure to 6MP, and are a function of the prescribed dose (dose-intensity), adherence, and inherited variability in TPMT activity. TPMT genotype was determined in all patients using leukocyte DNA and polymerase chain reaction–based methods specific for the TPMT *2, *3A, *3B, and *3C variant alleles as previously described.20 A TPMT wild-type genotype was assigned when no mutations were detected.
Demographic questionnaire
The demographic questionnaire was administered at study entry, and elicited self-reported information regarding annual household income, household structure, parents’ education, and patient’s race.
Health care provider reports
Participating institutions submitted monthly reports for each patient, detailing prescribed 6MP dose for each day of the preceding month and dates when 6MP was held for toxicity or illness. This information was used to calculate dose-intensity. After completion of adherence monitoring, participating institutions submitted clinical status reports (every 6 months for the first 5 years and annually for the subsequent 5 years), detailing dates of last visit, relapse, or death and cause of death (if applicable) during the interim period.
Adherence questionnaire
The adherence questionnaire was administered at 4 time points during the study (day 29, 57, 113, and 141) and elicited self-reported reasons for missing 6MP doses.
Statistical analyses
Adherence to oral 6MP.
Adherence rate was defined as the ratio of the number of days with MEMS cap openings (X) to the number of days 6MP was prescribed (N), reported as a percentage (X/N × 100). Days when 6MP was withheld by the prescriber were removed from the denominator (N). Adherence rate was computed for each month of adherence monitoring. Dose-intensity was defined as the ratio of 6MP dose actually prescribed, to the planned protocol dose (75 mg/m2 per day).
Longitudinal binomial regression was conducted using generalized estimating equation (GEE) methods by modeling monthly adherence rate as an unstructured mean model using 4 indicator variables of time for the 5 study months. Compound symmetry was assumed as the working correlation matrix over time.21 Covariates hypothesized to be predictors of adherence included sex, age at study participation, race (non-Hispanic white vs Asian American vs African American), household structure (single parent/single child [single parent with a single child] vs nuclear family [2 parents with >1 child and with or without other adults in the household] vs single parent/multiple children [single parent with >1 child]), mother as a full-time caregiver (yes vs no), annual household income (≥$50 000 vs <$50 000), paternal and maternal education, National Cancer Institute (NCI) risk group (high vs standard risk), blast chromosomal abnormalities (normal, unfavorable, and favorable), 6MP dose-intensity, and time since start of maintenance. The analysis was conducted for the entire cohort and then individually for each racial group. Relation between MEMS-based adherence and erythrocyte TGN levels was sought using GEE for normally distributed data, using the square root of 6MP dose-intensity–adjusted TGN levels and MEMS-based adherence rate.
Adherence to oral 6MP and risk of relapse.
All patients were in first complete remission at study entry. A proportional subdistribution hazards (PSH) model was used to evaluate the impact of adherence on relapse, while treating death due to nonmalignant causes and development of second malignancies as competing risks.22,23 The following variables were examined univariately, and were included in the multivariable model, if their association with relapse was significant at P < .1: TPMT genotype, 6MP dose-intensity, sex, race, parental education, annual household income, and time from initiation of maintenance to entry into adherence study. NCI risk and blast chromosomal abnormalities were retained in the multivariable model, irrespective of the significance of association. Adherence rate was treated as a time-varying covariate, updating the rate each month by cumulating the values of X and N. Overall 5-month adherence rate was used for the remaining weeks of maintenance after completion of adherence study.
A relation between adherence (treated as a continuous variable) and risk of relapse was sought. However, nonadherence forms a continuum from the occasional missed dose to total refusal, creating a need to identify a clinically relevant level of adherence below which the risk of relapse is unacceptable. Accordingly, we fitted PSH models, where adherence rate was included as a dichotomized variable with cutpoints, in 5% increments (<75% vs ≥75%, <80% vs ≥80%, <85% vs ≥85%, <90% vs ≥90%, and <95% vs ≥95%).
All missing data were addressed with multiple imputation (see supplemental Methods).24 PROCs GENMOD, LIFETEST, PHREG, MI, MIANALYZE of SAS 9.3 (SAS Institute), and the SAS macro PSHREG were used for analysis (see supplemental Methods). Two-sided tests with P < .05 were considered statistically significant.
Results
Patient characteristics
Two hundred ninety-eight participants (159 non-Hispanic whites, 71 Asian Americans, and 68 African Americans) provided 39 803 person-days of 6MP adherence data. The disease and sociodemographic characteristics of the cohort are summarized in Table 2. The median age at diagnosis for the cohort was 5 years (range, 1-19 years) and at study participation was 6 years (2-20 years). Forty-three percent of the cohort presented with NCI high-risk disease, 11.8% had T-cell disease, and 5.6% presented with unfavorable cytogenetics.
Table 2.
Demographic and clinical characteristics of the study participants
| Patient characteristics | Entire cohort, N = 298 | Non-Hispanic whites, n = 159 | Asian Americans, n = 71 | African Americans, n = 68 | P* |
|---|---|---|---|---|---|
| Age, median (range), y | |||||
| Age at diagnosis | 5.0 (1-19) | 5.0 (1-19) | 4.0 (1-18) | 6.0 (1-15) | .1 |
| Age at study participation | 6.0 (2-20) | 6.0 (2-20) | 6.0 (2-20) | 7.0 (3-16) | .1 |
| Gender, n (%) | |||||
| Male | 198/298 (66.4) | 104/159 (65.4) | 46/71 (64.8) | 48/68 (70.6) | .7 |
| Disease characteristics, n (%) | |||||
| High-risk disease (NCI criteria) | 127/297 (42.8)† | 64/159 (40.3) | 33/71 (46.5) | 30/67 (44.8)† | .6 |
| Unfavorable cytogenetics‡ | 16/285 (5.6)† | 9/151 (6.0)† | 3/70 (4.3)† | 4/64 (6.3)† | .9 |
| Favorable cytogenetics‡ | 146/285 (51.2)† | 78/151 (51.7)† | 33/70 (47.1)† | 35/64 (54.7)† | |
| T-cell ALL | 35/ 297 (11.8)† | 15/159 (9.4) | 8/71 (11.3) | 12/67 (17.9)† | .2 |
| Indices of 6MP metabolism | |||||
| Daily 6MP dose intensity, mean ± SD§ | 80.4% ± 25.8% | 80.7% ± 21.4% | 75.4% ± 24.7% | 84.8% ± 34.4% | .07 |
| TPMT wild-type genotype n (%) | 282/298 (94.6) | 149/159 (93.7) | 69/71 (97.2) | 64/68 (94.1) | .5 |
| Average TGN, median (range), pmol/8 × 108 RBC | 146.8 (2.1-714.1) | 150.7 (42.9-714.1) | 145.4 (2.1-459.1) | 126.3 (38.9-414.5) | .2 |
| Sociodemographic characteristics | |||||
| Annual household income <$50K, n (%) | 130/282 (46.1)† | 68/154 (44.2)† | 21/64 (32.8)† | 41/64 (64.1)† | .001 |
| No. of people in household, median (range) | 3 (1-10) | 3 (1-9) | 3 (1-10) | 3 (1-7) | .9 |
| Stay-at-home mother, n (%) | 113/289 (39.1)† | 60/157 (38.2)† | 36/69 (52.2)† | 17/63 (27.0)† | .01 |
| Paternal education, n (%) | |||||
| Less than college degree/vocational training | 139/291 (47.8)† | 76/159 (47.8) | 21/68 (30.9)† | 42/64 (65.6)† | .0009 |
| College degree/vocational training | 103/291 (35.4)† | 58/159 (36.5) | 28/68 (41.2)† | 17/64 (26.6)† | |
| Postgraduate degree | 49/291 (16.8)† | 25/159 (15.7) | 19/68 (27.9)† | 5/64 (7.8)† | |
| Maternal education, n (%) | |||||
| Less than college degree/vocational training | 135/293 (46.1)† | 73/159 (45.9) | 22/68 (32.4)† | 40/66 (60.6)† | .03 |
| College degree/vocational training | 110/293 (37.5)† | 61/159 (38.4) | 31/68 (45.6)† | 18/66 (27.3)† | |
| Postgraduate degree | 48/293 (16.4)† | 25/159 (15.7) | 15/68 (22.1)† | 8/66 (12.1)† | |
| Household structure, n (%) | |||||
| Nuclear family | 254/296 (85.8)† | 145/159 (91.2) | 67/70 (95.7)† | 42/67 (62.7)† | <.0001 |
| Single-parent/single-child | 18/296 (6.1)† | 6/159 (3.8) | 3/70 (4.3)† | 9/67 (13.4)† | |
| Single-parent/multiple children | 24/296 (8.1)† | 8/159 (5.0) | 0/70 (0.0) | 16/67 (23.9)† | |
| Length of follow-up, median (range), y | |||||
| Time from start of maintenance to study entry | 0.8 (0.2-2.3) | 0.8 (0.2-2.1) | 0.8 (0.2-2.3) | 0.8 (0.2-2.2) | 1.0 |
| Time from diagnosis to study entry | 1.6 (0.9-2.9) | 1.6 (0.9-2.9) | 1.6 (1.0-2.8) | 1.6 (0.9-2.7) | .5 |
| Time from diagnosis to date of last contact | 6.4 (1.3-10.6) | 7.1 (1.3-10.6) | 5.4 (2.5-9.6) | 5.3 (1.8-10.1) | <.0001 |
| Time from study entry to date of last contact | 5.0 (0.07-9.1) | 6.4 (0.07-9.1) | 3.8 (0.07-7.7) | 3.7 (0.4-8.7) | <.0001 |
| Time from study exit to date of last contact | 4.6 (0-9.0) | 6.0 (0-9.0) | 3.6 (0-7.6) | 3.3 (0.04-8.6) | <.0001 |
| Adherence | |||||
| Person-days of adherence monitoring | 39 803 | 21 258 | 9375 | 9170 | __ |
| Adherence ≥85%, n (%) | 253/298 (84.9) | 148/159 (93.1) | 62/71 (87.3) | 43/68 (63.2) | <.0001 |
| Adherence ≥90%, n (%) | 237/298 (79.5) | 139/159 (87.4) | 60/71 (84.5) | 38/68 (55.9) | <.0001 |
TGN, erythrocyte thioguanine nucleotide concentrations; RBC, erythrocyte. — indicates not applicable.
The P value describes statistical significance of difference in the various characteristics of patients in the 3 racial groups.
Statistics were calculated for this table by excluding patients with missing values for the characteristics.
Unfavorable chromosomal abnormalities included t(9;22), t(4;11), hypodiploidy, or extreme hypodiploidy. Favorable cytogenetics included 1 or more of the following: t(12;21); hyperdiploidy; trisomy 4 and 10; or trisomy 4, 10, and 17.
6MP dose intensity: ratio of 6MP dose actually prescribed (mg/m2 body surface area), to the planned protocol dose (75 mg/m2 per day).
Although disease characteristics were comparable across racial groups (Table 2 and supplemental Table 2), sociodemographic characteristics differed significantly (Table 2). Thus, 64% of African-American families reported annual household income <$50 000 as compared with 44% of non-Hispanic white, and 33% of Asian-American families (P = .001). Furthermore, African-American families were significantly (P = .0009) more likely to report lower levels of paternal education (less than a college degree, 66%) as compared with non-Hispanic white (48%) and Asian-American (31%) families, as well as a significantly (P = .03) lower level of maternal education (less than a college degree, 61%) as compared with non-Hispanic white (46%) and Asian-American (32%) families. Also, single-parent households were significantly (P < .0001) more common among African Americans (37%) as compared with non-Hispanic whites (9%) and Asian Americans (4%). Finally, African-American children were least likely (P = .01) to have mothers as full-time caregivers (27%) as compared with non-Hispanic white children (38%) and Asian-American children (52%).
Adherence to oral 6MP
Entire cohort.
Adherence to oral 6MP declined from 95.0% ± 0.7% at the end of month 1, to 91.8% ± 1.0% at the end of month 5 (linear trend, P < .0001; Figure 1A). Patients with lower adherence rates demonstrated a steeper decline (interaction between adherence and time, P = .0001). Multivariable longitudinal analysis (Table 3) revealed adherence rates to be significantly lower in: (1) Asian Americans (90.0% ± 2.5%) and African Americans (87.1% ± 2.2%) as compared with non-Hispanic whites (95.2% ± 0.6%, P < .0001, Figure 1B) and (2) patients from low-income households (<$50 000: 89.7% ± 1.8% vs ≥$50 000: 95.3% ± 0.8%, P = .005, Figure 1C). Adherence rates were significantly higher among patients from: (1) single-parent/single-child households (96.6% ± 1.4%,) when compared with patients from nuclear families (92.3% ± 0.9%, P = .04, Figure 1D) and (2) households where mothers were full-time caregivers (94.9% ± 0.9%,) as compared with households with other caregiver configurations (91.0% ± 1.3%, P = .02, Figure 1E).
Figure 1.
Adherence rates. (A) Adherence rate for the entire cohort over the 5 months of observation. (B) Adherence rate over time according to race. Test for heterogeneity: P < .0001; Asian Americans vs non-Hispanic whites: P = .01; African Americans vs non-Hispanic whites: P < .0001; African Americans vs Asian Americans: P = .41. (C) Adherence rate over time according to annual household income (<$50 000 vs ≥$50 000). (D) Adherence rate over time according to household structure (single parent/single child; nuclear family; single parent/multiple children). Test for heterogeneity: P = .05; single parent/single child vs single parent/multiple children: P = .1; nuclear family vs single parent/single child: P = .04; nuclear family vs single parent/multiple children: P = .6. (E) Adherence rate over time according to presence/absence of mother as the full-time caregiver. (A-E) Presented on the plots are the 95% CIs of model estimates.
Table 3.
Variable associated with adherence to oral 6MP for the entire cohort and within racial subgroups
| Variables | Entire cohort, N = 298 |
Non-Hispanic whites, n = 159 |
Asian Americans, n = 71 |
African Americans, n = 68 |
||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimate* | Estimated adherence, % | Parameter estimate* | Estimated adherence, % | Parameter estimate* | Estimated adherence, % | Parameter estimate* | Estimated adherence, % | |
| Intercept | 5.60† | — | 4.91† | — | 3.50† | — | 5.06† | — |
| Time on study | ||||||||
| Month 1 | — | 95.0 | — | 96.9 | — | 93.1 | — | 89.5 |
| Month 2 | −0.208‡ | 94.0 | −0.228§ | 96.1 | −0.53‡ | 91.7 | −0.068 | 88.7 |
| Month 3 | −0.427|| | 92.6 | −0.369|| | 95.6 | −1.01† | 89.9 | −0.196 | 87.3 |
| Month 4 | −0.520† | 92.0 | −0.567|| | 94.7 | −0.86|| | 89.0 | −0.347§ | 85.7 |
| Month 5 | −0.568† | 91.8 | −0.579|| | 94.7 | −1.0† | 88.6 | −0.373|| | 86.6 |
| P for linear trend | <.0001 | .002 | .0001 | .05 | ||||
| Annual household income | ||||||||
| ≥$50 000¶ | — | 95.3 | — | 96.7 | ||||
| <$50 000 | −0.86‡ | 89.7 | −1.68§ | 84.5 | ||||
| P | .005 | .04 | ||||||
| Gender | ||||||||
| Male¶ | ||||||||
| Female | ||||||||
| P | .15 | |||||||
| Race | ||||||||
| Non-Hispanic white¶ | — | 95.2 | ||||||
| Asian American | −0.80† | 90.0 | ||||||
| P | .01 | |||||||
| African American | −1.1† | 87.1 | ||||||
| P | <.0001 | |||||||
| Paternal education | ||||||||
| Postgraduate degree | — | 97.3 | ||||||
| Less than a postgraduate degree | −0.553‡ | 95.3 | ||||||
| P | .04 | |||||||
| Maternal education | ||||||||
| College degree/postgraduate/vocational training¶ | — | 94.6 | ||||||
| Less than a college degree | −1.46† | 78.9 | ||||||
| P | <.0001 | |||||||
| Household structure | ||||||||
| Single parent/single child¶ | — | 96.6 | — | 97.8 | — | 93.9 | ||
| Nuclear family | −0.94† | 92.3 | −0.69 | 95.7 | −0.77‡ | 86.5 | ||
| P | .04 | .04 | .07 | |||||
| Single parent/multiple children | −0.73† | 93.7 | −1.03§ | 94.0 | −0.69† | 85.9 | ||
| P | .01 | .05 | .18 | |||||
| Mother at home | ||||||||
| Stay-at-home mother¶ | — | 94.9 | — | 97.2 | ||||
| Working mother/absent mother | −0.62§ | 91.0 | −1.75|| | 85.6 | ||||
| P | .02 | .05 | ||||||
Analysis was adjusted for time from initiation of maintenance to study entry, NCI risk, blast chromosomal abnormalities and 6MP dose intensity. Age at study participation was not included in the model because of a lack of association with adherence. Test of homogeneity from month 1 through month 5: entire cohort, P < .0001; non-Hispanic white, P = .0006; Asian Americans, P = .0006; African Americans, P = .1. The individual P values for the parameter estimates for months 2 to 5 represent P values for change in adherence for each month, using month 1 as baseline.
More negative values of the parameter estimates indicate worse adherence rate when compared with the referent level which is indicated by ¶.
P < .001.
.01 ≤ P < .05.
.05 < P < .1.
.001 < P < .01.
Among patients with a TPMT wild-type genotype, each 1% increase in MEMS-based adherence was accompanied by a 16.6 pmol/8 × 108 erythrocyte increase in 6MP dose-intensity–adjusted erythrocyte TGN levels (P = .005).
Racial subgroups.
Results of multivariable longitudinal analyses within each racial group are detailed in Table 3, and summarized here.
Asian Americans:
Adherence rates demonstrated a statistically significant decline of 4.5%, from 93.1% to 88.6% (P = .0001) over the 5-month study period. Multivariable longitudinal analysis (Table 3) revealed adherence to be significantly higher among households with mothers as full-time caregivers (97.2% ± 2.0%), as compared with all other caregiver configurations (85.6% ± 4.9%, P = .05). Adherence rates also tended to be higher among patients from high-income households (≥$50 000: 96.7% ± 2.0% vs <$50 000: 84.5% ± 6.0%, P = .04).
African Americans:
Adherence rates declined from 89.5% at the end of month 1 to 86.6% at the end of month 5 (linear trend, P = 05). Multivariable longitudinal analysis (Table 3) revealed adherence rates to be significantly lower in patients from households with low maternal education (less than a college degree: 78.9% ± 4.0% vs at least college degree: 94.6% ± 1.4%, P < .0001). On the other hand, adherence rates tended to be higher among children from single-parent/single-child households (93.9% ± 2.8%), as compared with those from nuclear families (86.5% ± 2.8%, P = .07).
Non-Hispanic whites:
Adherence rates declined from 96.9% ± 0.4% at the end of month 1 to 94.7% ± 0.8% at the end of month 5 (linear trend, P = .002). Multivariable longitudinal analysis (Table 3) revealed higher adherence in households with high paternal education (postgraduate degree: 97.3% ± 0.7% vs less than a postgraduate degree: 95.3% ± 0.5%, P = .04). Furthermore, adherence rates were significantly higher among children from single-parent/single-child households (97.8% ± 0.8%), as compared with those from single-parent/multiple-children households (94.0% ± 0.2%, P = .05) or from nuclear families (95.7% ± 0.5%, P = .04).
Reasons for missing 6MP
Self-reported reasons for missing 6MP included forgetfulness, logistical barriers, and active refusal. Across all racial groups, forgetfulness was the most common reason for missing 6MP doses (non-Hispanic whites, 79%; Asian Americans, 90%; African Americans, 75%) and active refusal the least common reason (non-Hispanic whites, 0%; Asian Americans, 0%; African Americans, 5%).
Adherence to oral 6MP and risk of relapse
Entire cohort.
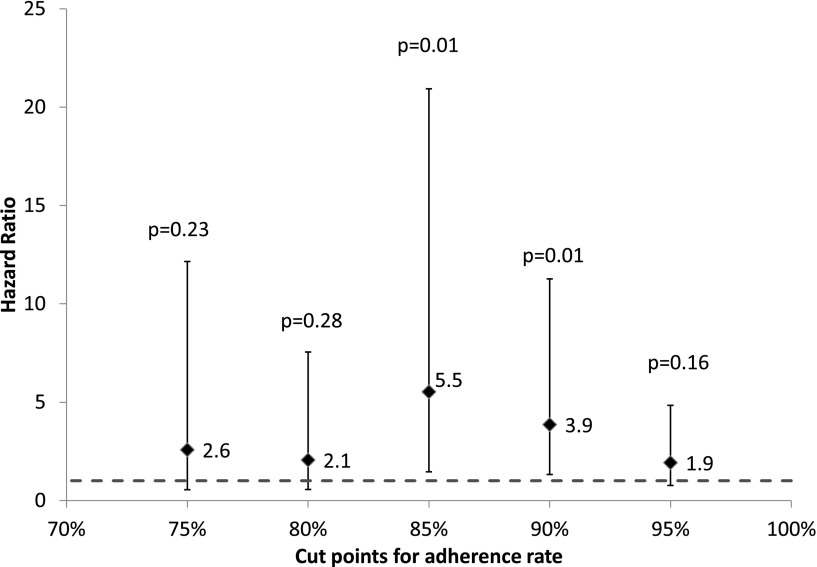
With a median follow-up of 6.4 years (range, 1.3-10.6 years) from diagnosis, 19 patients experienced relapse of their primary disease, 4 developed a second malignancy, and the remainder were alive and in complete remission at last contact. The proportion of patients with relapse increased with worsening adherence (supplemental Figure 2). However, neither a linear (P = .1) nor a quadratic (P = .16) relationship could be demonstrated between the risk of relapse and adherence treated as a continuous variable. We therefore created multivariable regression models with adherence rate dichotomized at 5 cutpoints (95%, 90%, 85%, 80%, 75%). Each of these models was adjusted for NCI risk group, 6MP dose-intensity, blast chromosomal abnormalities, race, and time from initiation of maintenance to study entry. The 5 multivariable regression models are summarized in Figure 2. The hazard ratio (HR) for relapse risk ranged from 1.9 to 5.5; however, 2 of the 5 cutpoints were associated with statistically significant differences in relapse risk (≥90% vs <90%: HR = 3.9, P = .01; and ≥85% vs <85%: HR = 5.5, P = .01). We chose to use 90% as a clinically relevant cutpoint because it was the first level below complete adherence (100%), where the risk of relapse became statistically significant (Figure 2). The final results of the multivariable regression analysis (Table 4) that adjusted for NCI risk, blast chromosomal abnormalities, 6MP dose-intensity, race, and time from initiation of maintenance to study entry demonstrated that adherence rate <90% was associated with a 3.9-fold increase in relapse risk (HR = 3.9, 95% confidence interval [CI], 1.3-11.3, P = .01) as compared with adherence rate ≥90%. Using this cutpoint, 20.5% of the entire cohort was nonadherent. Furthermore, 13% of the non-Hispanic whites, 15% of the Asian Americans, and 44% of the African Americans (P < .0001, for racial difference) were nonadherent to oral 6MP. Importantly, in this cohort that consisted of patients entering maintenance in first clinical remission, the adjusted risk of relapse attributable25 to nonadherence was 33%.
Figure 2.
Multivariable regression models with adherence rates dichotomized at 75%, 80%, 85%, 90%, 95%. Adjusted for NCI risk group, 6MP dose-intensity, blast chromosomal abnormalities, race, and for time from initiation of maintenance to study entry.
Table 4.
Multivariable analysis for role of adherence in relapse risk in African-American, Asian-American, and non-Hispanic white children with ALL
| Variables | HR (95% CI) | P* |
|---|---|---|
| NCI criteria for disease risk | ||
| Standard-risk disease | 1.0 | — |
| High-risk disease | 1.2 (0.5-3.2) | .7 |
| 6MP dose intensity | ||
| Per unit increase in dose intensity | 0.99 (0.97-1.01) | .3 |
| Blast chromosomal abnormalities | ||
| Normal/ none | 1.0 | — |
| Favorable | 0.3 (0.08-1.2) | .1 |
| Unfavorable | 2.1 (0.4-10.1) | .4 |
| Adherence to oral 6MP | ||
| ≥90% | 1.0 | — |
| <90% | 3.9 (1.3-11.3) | .01 |
Analysis was adjusted for time from initiation of maintenance to study entry, and race.
— indicates not applicable.
Discussion
To our knowledge, this is the first report to describe the significantly lower adherence to oral 6MP among African-American and Asian-American children with ALL, when compared with non-Hispanic white children. In this report, we identify a race-specific impact of sociodemographic variables on adherence. Finally, we describe the critical level of 6MP adherence that is needed to minimize the risk of relapse in this population.
In the current cohort, sociodemographic characteristics differed significantly by race. Thus, African-American children were more likely to come from single-parent families with low annual household income and low parental education, whereas Asian-American children were more likely to come from high-income, high-education, nuclear families. Despite these differences in sociodemographics, and even after adjusting for them, the adherence rates were significantly lower in both Asian-American and African-American children than in non-Hispanic whites. This is possibly explained by the race-specific impact of sociodemographic characteristics on adherence. For example, maternal education had a significant impact on adherence rates in African-American children and paternal education played a significant role in adherence among non-Hispanic white children, but parental education had no impact on adherence in Asian-American children. Furthermore, adherence rates were high in African-American and non-Hispanic white children from single-parent/single-child households, and low in nuclear family households. On the other hand, adherence rates were significantly higher among Asian-American households with mothers as full-time caregivers, but the caregiver status did not influence adherence in non-Hispanic white or African-American children. Finally, adherence rates tended to be lower among Asian-American patients from low income households; household income played no role in adherence among African American or non-Hispanic white children.
Although these findings are unique and specific to the sociodemographic backgrounds of this cohort, they have some contextual similarities to previous reports. Low socioeconomic status, low health literacy, and racial/ethnic and cultural differences have been shown to play a role in nonadherence in several oncologic and nononcologic settings.26-31 Thus, low health literacy explained the racial disparities in medication adherence among African-American youth with diabetes.30 Furthermore, poor adherence among African Americans, Asian Americans, and Hispanics with hypertension improved by reducing patient copayments, improving access to medications, and optimizing choice of therapy.32 In the current study, parental education influenced adherence among African Americans (maternal education) and non-Hispanic whites (paternal education), identifying vulnerable subpopulations that could benefit from targeted interventions.
Previous studies have also demonstrated an association between having a caregiver and medication adherence.33-36 We have previously shown that Hispanic children with ALL from single-parent households are most vulnerable to lower adherence to oral 6MP,17 likely due to competing responsibilities and distractions that preclude undivided attention to the child with ALL. In the current study, adherence was significantly higher among Asian-American patients with a mother as a full-time caregiver, when compared with other caregiver configurations. Given that the most common reason for nonadherence in the current study was forgetting to take 6MP, we speculate that mothers as full-time caregivers likely provided the necessary supervision of medication administration.
There was a statistically significant decline in adherence over the study period, similar to the downward drift reported previously.14,17 This likely stems from either a loss of patient/parent vigilance as the novelty of the adherence study wears off (resulting in regression of behavior to the unobserved state), or the natural likelihood of decline in adherence over time. However, the decline in adherence over the 5-month study period varied by race, from 2.2% among non-Hispanic whites, to 2.9% among African Americans, and 4.5% among Asian Americans.
Our study found that 20.5% of the cohort was consuming <90% of the prescribed 6MP. Nonadherent patients were 3.9 times more likely to relapse when compared with adherent patients. Overall, 33% of relapses in this cohort were attributable to nonadherence. The current study found that 15.5% of Asian-American, 44.1% of African-American, and 12.6% of non-Hispanic white children with ALL were nonadherent to oral 6MP, and thus at increased risk of relapse. African Americans will constitute 15% of the US population by 2050.18 Asian Americans accounted for 5.6% of the US population in 2010, and are the fastest-growing racial group.18 African Americans trail in health insurance coverage, making them vulnerable to adverse health outcomes. Relapse-free survival rates for minorities are ∼10% lower than those seen in non-Hispanic whites.37-39 The current study identifies vulnerable subpopulations that could benefit from adherence-enhancing interventions aimed at improving long-term survival and thus eliminating disparities in cancer outcomes.
Findings in the current study need to be placed in the context of its limitations. Facilitators and barriers to adherence were not analyzed as part of the current study. Impact of anxiety and depression on adherence were not analyzed, nor did we examine the impact of parental distress. Furthermore, MRD status was not available, and was therefore not included in the analysis examining the association between adherence and relapse risk. These limitations notwithstanding, the strengths of the current study include reliance upon microelectronic technology in the MEMS device for monitoring,40 thus providing an objective measure of adherence over 39 803 person-days in a geographically and racially diverse patient population. Furthermore, the MEMS-based adherence in the current study correlated with TGN levels, indicating that opening the pill bottle was associated with 6MP exposure.
Of all medication-related hospitalizations in the United States, up to 69% are due to poor adherence, with a resultant annual cost of $100 billion.41 Furthermore, medication nonadherence is associated with an increase in all-cause mortality.31 Although outpatient oral 6MP treatment is relatively inexpensive, salvage treatment of relapsed ALL is expensive, associated with extensive morbidity, and largely unsuccessful, thus creating a critical need to develop measures to enhance adherence to oral 6MP.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Cancer Institute (R01 CA096670, U10 CA098543, U10 CA095861, R37 CA36401, CA 21765, GM 92666, CA 156449, and M01-RR00043); and American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.B., M.V.R., and W.L. conceived and designed the study; S.B., W.L., L.H., K.W.M., A.K.R., B.B., J.C., D.S.D., J.P.N., and Y.R. acquired data; S.B., Y.C., F.L.W., H.K., W.L., and M.V.R. analyzed and interpreted data; S.B., W.L., L.H., F.L.W., and M.V.R. drafted the manuscript; S.B., W.L., L.H., H.K., Y.C., W.E.E., K.R.C., A.K.R., B.B., K.W.M., J.P.N., Y.R., J.C., D.S.D., and M.V.R. revised the manuscript for important intellectual content; F.L.W., H.K., Y.C., and S.B. performed statistical analysis; S.B., W.L., L.H., and M.V.R. provided administrative, technical, or material support; and S.B., W.L., and L.H. supervised the study.
Conflict-of-interest disclosure: St. Jude Children’s Research Hospital allocates a portion of the income it receives from licensing inventions and tangible research materials to those researchers responsible for creating the intellectual property; W.E.E. and M.V.R. receive a portion of the income St. Jude receives from licensing patent rights related to testing for TPMT genetic polymorphisms. The remaining authors declare no competing financial interests.
A complete list of the 77 Children’s Oncology Group institutions that participated in this study appears in the online data supplement.
Correspondence: Smita Bhatia, City of Hope, 1500 East Duarte Rd, Duarte, CA 91010-3000; e-mail: sbhatia@coh.org.
References
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18(2):115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen IM, Harvey RC, Mullighan CG, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(15):3512–3522. doi: 10.1182/blood-2011-11-394221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323(1):17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 7.Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93(9):2817–2823. [PubMed] [Google Scholar]
- 8.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7(12):1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- 9.Schmiegelow K, Schrøder H, Gustafsson G, et al. Nordic Society for Pediatric Hematology and Oncology. Risk of relapse in childhood acute lymphoblastic leukemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. J Clin Oncol. 1995;13(2):345–351. doi: 10.1200/JCO.1995.13.2.345. [DOI] [PubMed] [Google Scholar]
- 10.Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 11.Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance? Br J Cancer. 1995;72(4):1004–1006. doi: 10.1038/bjc.1995.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies HA, Lennard L, Lilleyman JS. Variable mercaptopurine metabolism in children with leukaemia: a problem of non-compliance? BMJ. 1993;306(6887):1239–1240. doi: 10.1136/bmj.306.6887.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau RC, Matsui D, Greenberg M, Koren G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30(2):85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Tebbi CK, Cummings KM, Zevon MA, Smith L, Richards M, Mallon J. Compliance of pediatric and adolescent cancer patients. Cancer. 1986;58(5):1179–1184. doi: 10.1002/1097-0142(19860901)58:5<1179::aid-cncr2820580534>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.MacDougall LG, Wilson TD, Cohn R, Shuenyane EN, McElligott SE. Compliance with chemotherapy in childhood leukaemia in Africa. S Afr Med J. 1989;75(10):481–484. [PubMed] [Google Scholar]
- 16.Smith SD, Rosen D, Trueworthy RC, Lowman JT. A reliable method for evaluating drug compliance in children with cancer. Cancer. 1979;43(1):169–173. doi: 10.1002/1097-0142(197901)43:1<169::aid-cncr2820430125>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30(17):2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humes KR, Jones NA, Ramirez RR. Overview of Race and Hispanic Origin: 2010. Washington, DC: US Department of Commerce; 2011. [Google Scholar]
- 19.Su Y, Hon YY, Chu Y, Van de Poll ME, Relling MV. Assay of 6-mercaptopurine and its metabolites in patient plasma by high-performance liquid chromatography with diode-array detection. J Chromatogr B Biomed Sci Appl. 1999;732(2):459–468. doi: 10.1016/s0378-4347(99)00311-4. [DOI] [PubMed] [Google Scholar]
- 20.Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126(8):608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Kohl M, Heinze G. PSHREG: A SAS® Macro for Proportional and Nonproportional Substribution Hazards Regression With Competing Risk Data. Technical Report 08/2012. Vienna, Austria: Medical University of Vienna, Center for Medical Statistics, Informatics and Intelligent Systems; 2012. [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 25.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Thumula V, Pace PF, Banahan BF, III, Wilkin NE, Lobb WB. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clin Ther. 2009;31(10):2178–2188, discussion 2150-2151. doi: 10.1016/j.clinthera.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv Res. 2009;9:24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR Adolescent Medicine HIV/AIDS Research Network. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 29.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129(6):e1404–e1410. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(suppl 3):268–278. doi: 10.1080/10810730.2011.604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egede LE, Lynch CP, Gebregziabher M, et al. Differential impact of longitudinal medication non-adherence on mortality by race/ethnicity among veterans with diabetes. J Gen Intern Med. 2013;28(2):208–215. doi: 10.1007/s11606-012-2200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams AS, Uratsu C, Dyer W, et al. Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users. JAMA Intern Med. 2013;173(1):54–61. doi: 10.1001/2013.jamainternmed.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredriksen-Goldsen KI, Shiu CS, Starks H, et al. “You must take the medications for you and for me”: family caregivers promoting HIV medication adherence in China. AIDS Patient Care STDS. 2011;25(12):735–741. doi: 10.1089/apc.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal B, Liao M, Mosca L. Medication adherence is associated with having a caregiver among cardiac patients. Ann Behav Med. 2013;46(2):237–242. doi: 10.1007/s12160-013-9492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowlton AR, Yang C, Bohnert A, Wissow L, Chander G, Arnsten JA. Informal care and reciprocity of support are associated with HAART adherence among men in Baltimore, MD, USA. AIDS Behav. 2011;15(7):1429–1436. doi: 10.1007/s10461-010-9749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beals KP, Wight RG, Aneshensel CS, Murphy DA, Miller-Martinez D. The role of family caregivers in HIV medication adherence. AIDS Care. 2006;18(6):589–596. doi: 10.1080/09540120500275627. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 38.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 39.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol. 2000;18(4):813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 40.Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32(5):345–356. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 41.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]