Abstract
Background
The liver is an important organ for its ability to transform xenobiotics, making the liver tissue a prime target for toxic substances. The carotenoid bixin present in annatto is an antioxidant that can protect cells and tissues against the deleterious effects of free radicals. In this study, we evaluated the protective effect of bixin on liver damage induced by carbon tetrachloride (CCl4) in rats.
Results
The animals were divided into four groups with six rats in each group. CCl4 (0.125 mL kg-1 body wt.) was injected intraperitoneally, and bixin (5.0 mg kg-1 body wt.) was given by gavage 7 days before the CCl4 injection. Bixin prevented the liver damage caused by CCl4, as noted by the significant decrease in serum aminotransferases release. Bixin protected the liver against the oxidizing effects of CCl4 by preventing a decrease in glutathione reductase activity and the levels of reduced glutathione and NADPH. The peroxidation of membrane lipids and histopathological damage of the liver was significantly prevented by bixin treatment.
Conclusion
Therefore, we can conclude that the protective effect of bixin against hepatotoxicity induced by CCl4 is related to the antioxidant activity of the compound.
Keywords: Bixin, Carbon tetrachloride, Hepatotoxicity, Oxidative stress, Protective activity
Background
The liver is the main organ involved in the biotransformation of exogenous substances (xenobiotics) and has the ability to convert hydrophobic compounds into water-soluble ones that are more easily eliminated by the body [1]. The activity of the liver in the metabolism of xenobiotics is mediated primarily by cytochrome P450 (CYP 450). Although biotransformation reactions are associated with the detoxification process, in some cases, the metabolism of xenobiotics is detrimental to cells due to the production of highly reactive metabolites that are more toxic than the parent compound, such as electrophiles, radicals and reactive oxygen species (ROS), that can react directly with cellular macromolecules or initiate chain reactions [2].
Carbon tetrachloride (CCl4) is a small, lipophilic molecule that spreads easily in the lipid compartments of the body and is metabolized in the liver. Its mechanism of toxicity requires a CYP 450-mediated bioactivation step that produces free radicals, such as trichloromethyl (CCl3.) [3], and induces the peroxidation of lipids. These lipids then damage the membranes of organelles and liver cells, causing the swelling and necrosis of hepatocytes and resulting in the release of cytosolic enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) into the circulating blood (Singh et al., 1998; [4, 5]). Due to these properties, CCl4 is a chemical that is widely used to induce liver damage in experimental studies [6–10].
The species Bixa orellana L. belongs to the family Bixaceae and is popularly known as annatto. The main use of annatto is as a dye. Among the natural colors, annatto is most used by the food industry, especially in the preparation of butter, cheeses, bakery products, oils, ice cream, cereals and meats [11, 12]. In addition to its use in coloring, annatto is also used in folk medicine for the treatment of coronary diseases, disorders of the stomach and intestine, respiratory disorders, burns, and as an aphrodisiac. Annatto leaves are used to fight kidney disease and fever [13, 14], and the tincture prepared from the leaves, immature fruit and flower organs have been shown to present antimicrobial activity [15]. Recently, [16] demonstrated that the aqueous extract of the seeds of Bixa orellana was capable of reversing the hypertriglyceridemia induced by Triton, fructose and ethanol, demonstrating a hypolipidemic effect.
The main product of annatto is the seed, from which bixin, a dye of group of carotenoids of great interest in national and international markets, is extracted. Bixin is one of the most effective suppressors of biological molecular oxygen and can protect cells and tissues against the harmful effects of free radicals; additionally, bixin is also an effective inhibitor of lipid peroxidation [17–19]. In the present study, we aimed to investigate the potential effects of bixin in reducing damage and oxidative stress and in improving histopathological abnormalities in the liver of rats treated with CCl4 in order to determine the potential of this compound for the treatment or prevention of liver disease.
Results
Analysis of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzyme activities
Figure 1 shows the effect of bixin on ALT (A) and AST (B) activities in serum. Hepatotoxicity was verified by a significant increase in ALT and AST activities in the CCl4–treated group compared with the control group. Pretreatment with bixin significantly prevented the release of these enzymes compared with the CCl4–only treated group in serum. The prevention was only partial since the activities of the enzymes from rats treated with CCl4 and bixin are significantly different from control. Bixin alone did not have any effect on enzyme activity.
Figure 1.
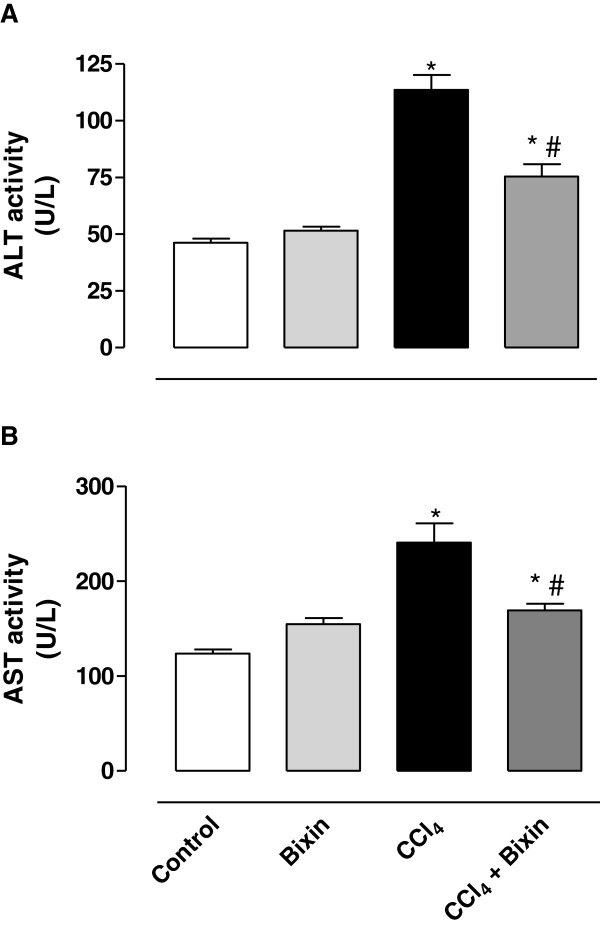
The effects of bixin on CCl 4-induced hepatotoxicity evaluated by ALT (A) and AST (B) activities in the serum. The results represent the mean ± SEM of six animals per group. * Significantly different from the control group (P < 0.05). # Significantly different from the CCl4-only group (P < 0.05).
Lipid peroxidation
A significant increase in the level of MDA, an end product of lipid peroxidation, was observed in the liver of CCl4–treated rats when compared with the control group (Figure 2). Pretreatment with bixin significantly prevented the MDA production in the liver when compared with that of rats administered CCl4. Bixin alone did not have any effect on this parameter.
Figure 2.
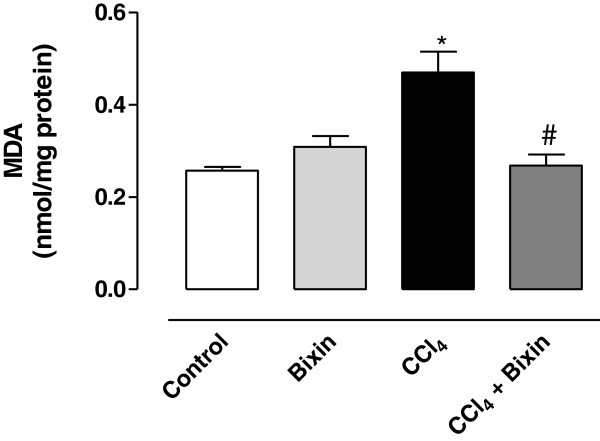
The effects of bixin on CCl 4 -induced malondialdehyde (MDA) generation in rat liver homogenate. The results represent the mean ± SEM of six animals per group. * Significantly different from the control group (P < 0.05). # Significantly different from the CCl4-only group (P < 0.05).
Determination of GSH and NADPH levels on liver homogenate
Treatment of rats with CCl4 significantly reduced the levels of GSH and NADPH in the liver when compared with the control group (Figures 3A and B, respectively). The pretreatment of animals with bixin significantly prevented these changes, maintaining levels of the compounds to the normal range. Bixin alone did not have any effect on these parameters.
Figure 3.
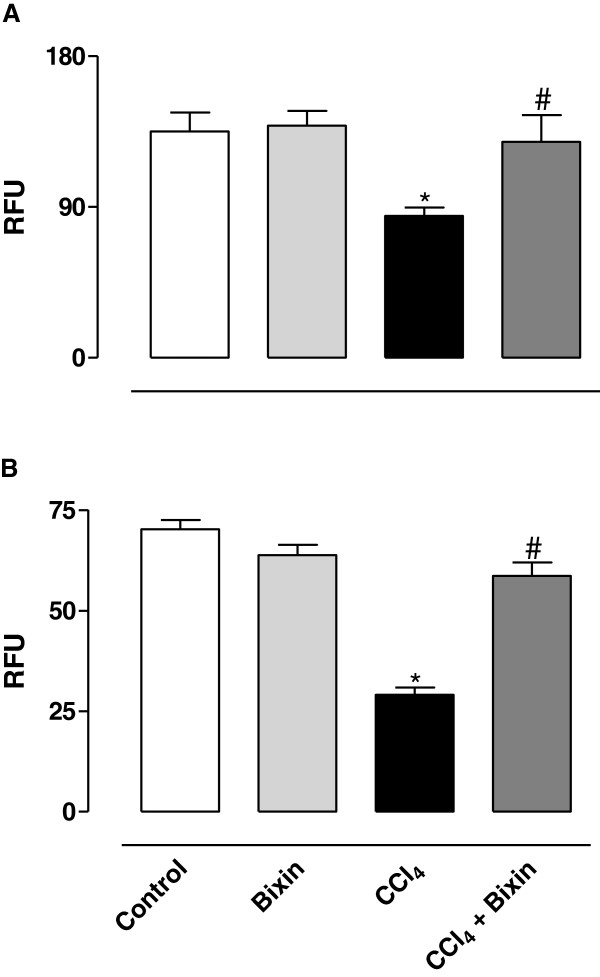
The effects of bixin on CCl 4 -induced GSH (A) and NADPH (B) oxidation in rat liver homogenate. The results represent the mean ± SEM of six animals per group. * Significantly different from the control group (P < 0.05). # Significantly different from the CCl4-only group (P < 0.05). RFU: Relative Fluorescence Unit.
Analysis of glutathione reductase activity
Analysis of glutathione reductase (GR) activity showed a significant reduction in activity in the CCl4–treated group when compared to the control group (Figure 4). Pretreatment of animals with bixin prevented the change in GR activity, showing a protection against the effects of CCl4. Bixin alone did not have any effect on enzyme activity.
Figure 4.
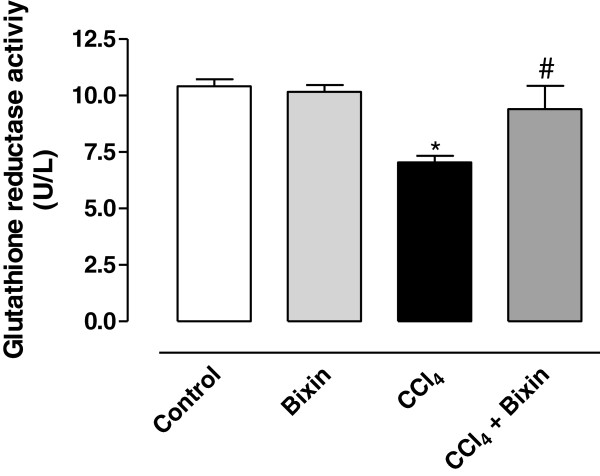
The effects of bixin on CCl 4 -induced reduction of GR activity in rat liver homogenate. The results represent the mean ± SEM of six animals per group. * Significantly different from the control group (P < 0.05). # Significantly different from the CCl4-only group (P < 0.05).
Histopathological analysis
Histopathological studies of the liver of control and bixin-only treated animals showed normal histology (Figures 5A and B, respectively). In animals treated with CCl4, inflammation, necrosis and hydropic degeneration of hepatic cells was observed (Figure 5C). The group that was pretreated with bixin showed that severe hepatic lesions induced by CCl4 were partially prevented (Figure 5D), which were in agreement with the results of the serum aminotransferases activities and lipid peroxidation.
Figure 5.
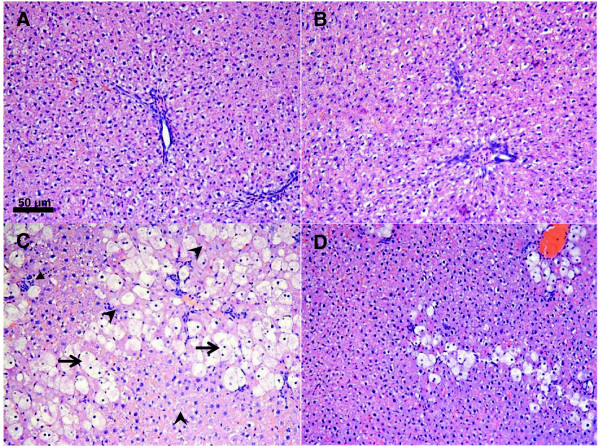
The effects of bixin on CC1 4 -induced liver damage in rat. (A) liver of control group showing intact liver structures; (B) liver of bixin group, showing normal structure; (C) liver of CCl4 group, showing hepatocytes necrosis (arrow head), hydropic degeneration (arrow) and infiltration of inflammatory cells (dashed arrow); and (D) liver of CCl4 + bixin showing prevented damage. H&E, original magnification 200 × .
Discussion and conclusion
The liver plays an important role in metabolism and biotransformation of xenobiotics. Due to its position between the digestive tract and circulatory system, it receives large amounts of nutrients and xenobiotics absorbed through the digestive tract and the portal vein becoming the target organ of several classes of toxins and toxicants, natural or synthetic [1].
Bixin, a carotenoid with one carboxylic acid and one methyl ester group, is the major pigment found in annatto and corresponds to approximately 80% of the carotenoids present in the plant seed [20, 21]. This compound has been demonstrated to protect cells and tissues against the deleterious effects of ROS and free radicals and also exhibits a cholesterol lowering effect [17, 18, 22–24]. In a previous study, [2] evaluated the role of bixin on cisplatin-induced oxidative stress in the kidneys of Wistar rats at doses of 2.5 or 5.0 mg kg-1 body wt. by gavage. Pretreatment with the highest dose of bixin resulted in a reduction in the total number of chromosome aberrations, inhibited increases in lipid peroxidation, and inhibited renal glutathione depletion induced by cisplatin. Therefore, in our study we decide to evaluate the potential hepatoprotective effects of bixin using this same dose.
CCl4 is a hepatotoxin that causes liver damage and has been used in several studies as a hepatotoxic agent [5, 8, 25–28]. The most striking pathological features of CCl4-induced hepatotoxicity are hepatic steatosis, cirrhosis and necrosis, which results from the formation of reactive radical intermediates such as trichloromethyl (CCl3.) formed by CYP 450-mediated biotransformation that induces membrane lipid peroxidation [3, 29, 30].
The serum activities of alanine transaminase (ALT) and aspartate transaminase (AST) are used as an indication of the extent of liver damage due to the release of large quantities of these enzymes into the bloodstream [31]. AST is distributed in body tissues, including muscle and the heart. In the liver, AST is mainly present in the mitochondria of hepatocytes while the ALT is found outside of the mitochondria. CCl4 induces the peroxidation of lipids that damage the membranes of liver cells and organelles and results in the release of ALT and AST into the circulating blood [5]. Accordingly, our results demonstrated that treatment with CCl4 promoted a significant increase in ALT and AST serum activities. Pretreatment with bixin protected the liver from damage by CCl4, as there was a significant decrease in the release of enzymes.
As mentioned above, the hepatotoxic effects of the metabolism of CCl4 are mainly due to its active metabolite, the trichloromethyl radical, which in the presence of oxygen, is transformed into trichloromethyl peroxyl radical (CCl3OO.). These free radicals bind covalently to macromolecules and induce peroxidative degradation of membrane lipids that are rich in polyunsaturated fatty acids [23]. This leads to the formation of lipid peroxides that give rise to products such as malondialdehyde (MDA) that cause damage to the membranes [32, 33]. The increased MDA in the liver of CCl4-treated rats suggests that the natural antioxidant defense mechanism to scavenge excessive free radicals has been compromised. However, pretreatment with bixin significantly prevented the formation of MDA, indicating hepatoprotection by impairing initiation and propagation of the peroxidative process.
To protect itself from oxidation damage, the cell has a defense system that includes reduced glutathione (GSH), nicotinamide adenine dinucleotide phosphate in the reduced form (NADPH) and enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione reductase (GR). The imbalance between the formation and removal of free radicals in the body, due to the reduction of endogenous antioxidants or increased generation of oxidizing species, generates a pro-oxidant condition known as oxidative stress, which favors the occurrence of oxidative lesions in macromolecules and cellular structures, and possibly result in cell death [34]. Under conditions of oxidative stress, some of the endogenous protective factors decrease. Accordingly, treatment of animals with CCl4 caused a significant decrease in the levels of GSH and NADPH, and in the activity of the GR. However, we observed a protective effect of bixin on oxidative stress caused by CCl4 because the pretreatment of animals with the compound prevented the oxidation of GSH and NADPH and the reduction of the activity of GR, indicating an indirect antioxidant property of the bixin, probably by reacting with the free radicals arising from CCl4 as demonstrated by [35].
The histological observations in the liver samples strongly support the protective effect of bixin. CCl4 caused various histological changes to the liver, including cell necrosis, inflammation, and hydropic degeneration of hepatic cells. These alterations were significantly attenuated by bixin in livers, resulting in only minor hepatocellular necrosis and inflammatory cell infiltration. In conclusion, our results indicate that pretreatment with bixin attenuated liver injury produced by carbon tetrachloride while significantly reducing the alterations caused by ALT and AST activities, lipid peroxidation, and histopathological parameters. Additionally, bixin protected against reduction of GSH and NADPH levels and GR activity. These effects may be related to the antioxidant activity of bixin.
Methods
Chemicals
Annatto powder (containing 28% bixin, determined spectrophotometrically) was kindly supplied by Christian Hansen Indústria e Comércio Ltda (Valinhos, SP, Brazil). The amount of annatto supplied to the animals was adjusted to contain the desired dose of bixin. All other reagents were of the highest commercially available grade.
Animals
Male Wistar rats weighing approximately 200 g were used in this study. The animals were obtained from the Central Bioterium of UNESP – Univ Estadual Paulista, Campus de Botucatu, SP, Brazil, and were maintained with a maximum of 4 rats per cage under standard laboratory conditions with water and food provided ad libitum. The experimental protocols were approved by the Ethical Committee for the Use of Laboratory Animals of the UNESP – Univ Estadual Paulista, Campus de Dracena, SP, Brazil.
Treatment
The animals were divided into four groups, each with 6 animals. Group 1 was the control group, and received canola oil by gavage for 7 days and mineral oil intraperitoneally on the last day. Group 2 was treated with bixin suspended in canola oil (5.0 mg/kg body weight) by gavage for 7 days before mineral oil application. Group 3 received canola oil by gavage for 7 days and CCl4 dissolved in mineral oil (0.125 mL/kg body weight), administered intraperitoneally, on the last day. Group 4 received bixin suspended in canola oil (5.0 mg/kg body weight) by gavage for 7 days and carbon tetrachloride, administered intraperitoneally, on the last day. Twenty-four hours after the administration of the vehicle or CCl4, animals were euthanized by decapitation and biochemical and histopathological analyses were performed. The dose of bixin (5.0 mg/kg body weight) used in this study as pre-treatment, was based on data found in the literature as showing protective effects in the kidney [36].
Analysis of enzymes indicative of hepatic functions
Blood samples were collected and kept at room temperature for 15 minutes to allow for coagulation. Serum was separated by low-speed centrifugation (3000 g for 15 min), and the activity of the enzymes ALT and AST was measured using commercially available kits (Bioclin, Belo Horizonte, Brazil) according to the manufacturer's protocols.
Preparation of rat liver homogenate
The liver was removed, sliced into 50 mL of medium (250 mM sucrose, 1 mM EGTA and 10 mM HEPES-KOH, pH 7.2) at 4°C, washed three times with the same medium and homogenized three times for 15 sec at 1 min intervals with a Potter-Elvehjem homogenizer. The protein concentration of the homogenate was determined by the biuret reaction with BSA as a standard [37].
Membrane lipid peroxidation (LPO) assay
The level of LPO was estimated by malondialdehyde (MDA) generation [38]. The liver homogenate (5 mg of protein) was added to a tube. Following the addition of 0.2 mL of 8.1% SDS, 1.5 mL of 20% acetic acid and 1.5 mL of 0.67% thiobarbituric acid (TBA, aqueous solution), glass-distilled deionized water was added to a final volume of 4 mL. The mixture was incubated for 60 min at 85°C. The MDA-TBA complex was extracted with 5 mL of n-butanol and the absorbance was measured at 535 nm. The MDA concentration was calculated with ϵ = 1.56 × 105 M-1 cm-1.
Determination of GSH level
Liver homogenate (1 mg of protein) was added to medium (125 mM sucrose, 65 mM KCl and 10 mM HEPES-KOH, pH 7.4) to a final volume of 1 mL and treated with 0.5 mL of 13% trichloroacetic acid. The mixture was stirred and then centrifuged at 9000 g for 3 min. Aliquots (100 μL) of the supernatant were mixed with 2 mL of 100 mM NaH2PO4 buffer at pH 8.0 containing 5 mM EGTA. One hundred microliters of an o-phthaldialdehyde solution (1 mg/mL) was added, and the fluorescence was measured 15 min later in a spectrofluorometer (Shimadzu-RFPC 5301, Tokyo, Japan) using 350/420 nm as the excitation/emission wavelength pair [39]. The data are expressed in relative units of fluorescence.
Determination of NADPH level
Liver homogenate (1.5 mg protein) was added to medium (125 mM sucrose, 65 mM KCl and 10 mM HEPES-KOH, pH 7.4) to a final volume of 1.5 mL and centrifuged at 8000 g for 3 min. The supernatant was collected, and the fluorescence was measured in a spectrofluorometer (Shimadzu-RFPC 5301, Tokyo, Japan) using 366/450 nm as the excitation/emission wavelength pair. The data are expressed in relative units of fluorescence.
Glutathione reductase activity
One milliliter of 0.1 mM sodium phosphate buffer, pH 7.6, with 0.5 mM EDTA, 10 μL of 10% Triton X-100, liver homogenate (1 mg of protein) and 10 μL of 100 mM GSSG was added to 4 mL quartz cuvettes. After incubating the samples at 30°C for 5 minutes, 10 μL of 10 mM NADPH was added, and the variation in absorbance was determined at a wavelength of 340 nm in a spectrophotometer (Beckman-Coulter model DU-800, Fullerton, CA, USA).
Histopathological analysis
Liver fragments were fixed in a 10% solution of formaldehyde, dehydrated in graduated ethanol (50-100%), cleared in xylene and embedded in paraffin. The hepatic sections (4–5 μm) were analyzed by light microscopy with a magnification of 200× after staining with hematoxylin and eosin (H&E) using standard techniques.
Statistical analysis
Significant differences were calculated by one-way analysis of variance (ANOVA) followed by the Tukey test using the GraphPad Prism software, version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). Values of P < 0.05 were considered significant.
Acknowledgement
The authors are grateful to Christian Hansen Indústria e Comércio Ltda (Valinhos, SP, Brazil) for providing the bixin.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PRM, MAM, HCDM and MG performed the experiments and analyzed the data. FTVP: performed the histopathological analysis. FEM: performed the experimental design, wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Priscila R Moreira, Email: priscilarodrigues1708@gmail.com.
Marcos A Maioli, Email: maioli_marcos@hotmail.com.
Hyllana CD Medeiros, Email: hyllanazootecnia@gmail.com.
Marieli Guelfi, Email: guelf_marieli@hotmail.com.
Flávia TV Pereira, Email: fverechia@dracena.unesp.br.
Fábio E Mingatto, Email: fmingatto@dracena.unesp.br.
References
- 1.Guillouzo A. Liver cell models in vitro toxicology. Environ Health Perspect. 1998;106:511–532. doi: 10.1289/ehp.98106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Lechón MJ, Ponsoda X, Bort R, Castell JV. The use of cultured hepatocytes to investigate the metabolism of drugs and mechanisms of drug hepatotoxicity. Altern Lab Anim. 2001;29:225–231. doi: 10.1177/026119290102900307. [DOI] [PubMed] [Google Scholar]
- 3.Boelsterli UA. Mechanistic Toxicology: The molecular basis of how chemicals disrupt biological targets. Boca Raton: CRC Press: Boelsterli UA; 2007. Xenobiotic-induced oxidative stress: cell injury, signaling and gene regulation; pp. 117–175. [Google Scholar]
- 4.Singh B, Saxena AK, Chandan BK, Anand KK, Suri OP, Suri KA, Satti NK. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoter. 1998;69:135–140. [Google Scholar]
- 5.Shankar NLG, Manavalan R, Venkappayya D, Raj CD. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2008;46:3182–3185. doi: 10.1016/j.fct.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Moresco RN, Sperotto RL, Bernardi AS, Cardoso RF, Gomes P. Effect of the aqueous extract of Syzygium cumini on carbon tetrachloride-induced hepatotoxicity in rats. Phytother Res. 2007;21:793–795. doi: 10.1002/ptr.2158. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Li L, Wen T, Li YQ. Protective effects of echinacoside on carbon tetrachloride-induced hepatotoxicity in rats. Toxicology. 2007;232:50–56. doi: 10.1016/j.tox.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Ćebović T, Maksimović Z. Hepatoprotective effect of Filipendula hexapetala Gilib. (Rosaceae) in carbon tetrachloride-induced hepatotoxicity in rats. Phytother Res. 2012;26:1088–1091. doi: 10.1002/ptr.3703. [DOI] [PubMed] [Google Scholar]
- 9.Essawy AE, Abdel-Moneim AM, Khayyat LI, Elzergy AA. Nigella sativa seeds protect against hepatotoxicity and dyslipidemia induced by carbon tetrachloride in mice. J Appl Pharmac Sci. 2012;2:21–25. [Google Scholar]
- 10.Wafay H, El-Saeed G, El-Toukhy S, Youness E, Ellaithy N, Agaibi M, Eldaly S. Potential effect of garlic oil and silymarin on carbon tetrachloride-induced liver injury. Aust J Basic Appl Sci. 2012;6:409–414. [Google Scholar]
- 11.Jondiko IJ, Patterden G. Terpenoids and an apocarotenoid from seeds of Bixa orellana. Phytochemistry. 1989;28:3159–3162. doi: 10.1016/0031-9422(89)80298-5. [DOI] [Google Scholar]
- 12.Mercadante AZ. Chemistry and Physiology of Selected Food Colorants. Washington: ACS Symposium Series: AMES JM, Hofman TF; 2001. Composition of carotenoids from annatto; pp. 92–101. [Google Scholar]
- 13.Teske M, Trentini AMM. Herbarium: Compêndio de fitoterapia. Curitiba PR: Herbarium Laboratório Botânico; 1994. pp. 235–237. [Google Scholar]
- 14.Lorenzi H, Matos FJA. Plantas medicinais no Brasil: nativas e exóticas. Nova Odessa SP: Instituto Plantarum; 2002. pp. 95–96. [Google Scholar]
- 15.Coelho AMSP, Silva GA, Vieira OMC, Chavasco JK. Antimicrobial activity from Bixa orellana L. (Urucum) Rev Lecta. 2003;21:47–54. [Google Scholar]
- 16.Ferreira JM, Sousa DF, Dantas MB, Fonseca SGC, Menezes DB, Martins AMC, Queiroz MGR. Effects of Bixa orellana L. seeds on hyperlipidemia. Phytother Res. 2013;27:144–147. doi: 10.1002/ptr.4675. [DOI] [PubMed] [Google Scholar]
- 17.Mascio DIP, Devasagayam TP, Kaiser S, Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- 18.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10 T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
- 19.Mercadante AZ, Pfander H. Carotenoids from annatto: a review. Recent Res Dev Agric Food Chem. 1998;2:79–91. [Google Scholar]
- 20.Preston HD, Rickard MD. Extraction and chemistry of annatto. Food Chem. 1980;5:47–56. doi: 10.1016/0308-8146(80)90063-1. [DOI] [Google Scholar]
- 21.Scotter M. The chemistry and analysis of annatto food colouring: a review. Food Addit Contam Part A. 2009;26:1123–1145. doi: 10.1080/02652030902942873. [DOI] [Google Scholar]
- 22.Lima LRP, Oliveira TT, Nagem TJ, Pinto AS, Stringheta PC, Tinoco ALA, Silva JF. Bixin, norbixin and quercetin and lipid metabolism effects in rabbits. Braz J Vet Res An Sci. 2001;38:196–200. doi: 10.1590/S1413-95962001000400010. [DOI] [Google Scholar]
- 23.Barcelos GR, Grotto D, Serpeloni JM, Aissa AF, Antunes LM, Knasmüller S, Barbosa F., Jr Bixin and norbixin protect against DNA-damage and alterations of redox status induced by methylmercury exposure in vivo. Environ Mol Mutagen. 2012;53:535–541. doi: 10.1002/em.21715. [DOI] [PubMed] [Google Scholar]
- 24.Santos GC, Mendonça LM, Antonucci GA, Santos AC, Antunes LMG, Bianchi MLP. Protective effect of bixin on cisplatin-induced genotoxicity in PC12 cells. Food Chem Toxicol. 2012;50:335–340. doi: 10.1016/j.fct.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, Marroni N. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824–829. doi: 10.1023/A:1022869716643. [DOI] [PubMed] [Google Scholar]
- 26.Lee KJ, Choi JH, Jeong HG. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2007;45:2118–2125. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Quan J, Piao L, Wang X, Li T, Yin X. Rossicaside B protects against carbon tetrachloride-induced hepatotoxicity in mice. Basic Clin Pharmacol Toxicol. 2009;105:380–386. doi: 10.1111/j.1742-7843.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava A, Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010;118:411–417. doi: 10.1016/j.foodchem.2009.05.014. [DOI] [Google Scholar]
- 29.Recknagel RO, Glende JREA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–145. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez W, Clària J, Arroyo V, Rodés J. Carbon tetrachloride induced cirrhosis in rats: an useful tool for investigating the patogenesis in chronic liver disease. J Gastroenterol Hepatol. 1992;7:90–97. doi: 10.1111/j.1440-1746.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaplowitz N. Drug-induced liver disorders: Implications for drug development and regulation. Drug Saf. 2001;24:483–490. doi: 10.2165/00002018-200124070-00001. [DOI] [PubMed] [Google Scholar]
- 32.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 33.Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 34.Ross D, Moldeus P. Membrane lipid oxidation. Boca Raton: CRC Press: Vigo-Pelfrey C; 1991. Antioxidant defense systems and oxidative stress; pp. 151–170. [Google Scholar]
- 35.Zhao W, Yao S, Wang Q, Qian S, Wang W, Han Y. Reaction of carotenoids with CCl3OO by using pulse radiolysis. Sci China Ser B. 2003;46:57–63. [Google Scholar]
- 36.Silva CR, Antunes LMG, Bianchi MLP. Antioxidant action of bixin against cisplatin-induced chromosome aberrations and lipid peroxidation in rats. Pharmacol Res. 2001;43:561–567. doi: 10.1006/phrs.2001.0822. [DOI] [PubMed] [Google Scholar]
- 37.Cain K, Skilleter DN. Biochemical Toxicology. Oxford: IRL Press: Snell K, Mullock B; 1987. Preparation and use of mitochondria in toxicological research; pp. 217–254. [Google Scholar]
- 38.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 39.Hissin PJ, Hilf RA. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]


