Abstract
Background:
The aim was to compare the intensity of pain caused by suprapubic aspiration (SPA) and urethral catheterization for urine sampling in premature infants.
Methods:
A prospective randomized controlled design with 80 premature infants in Alzahra University Hospital, Isfahan, Iran was conducted. Premature newborns who needed urine samples for microbiologic analysis were randomly assigned into two groups: SPA group and urethral catheterization group. Newborn faces and upper parts of the body were videotaped during the study and the pain was assessed during urine collection using Premature Infant Pain Profile (PIPP) score. Furthermore, crying time compared between groups.
Results:
The mean crying time was significantly higher in SPA than urethral catheterization group (77 vs. 34.4 s) (P < 0.001). The PIPP score was significantly lower in urethral catheterization group (13.4) than SPA group (11.5) P < 0.001. The success rate of SPA was 53% compared with 71% success rate of urethral catheterization.
Conclusions:
SPA is more painful than urethral catheterization in premature male infants as assessed by PIPP score and is more likely leads to procedure failure.
Keywords: Premature infants, suprapubic aspiration, urethral catheterization, urine sampling
INTRODUCTION
Urinary tract infection (UTI) is a common problem, especially in male and premature infants, which can lead to major problems, including sepsis and renal scarring. The only way to make a definitive UTI diagnosis is an analysis of urine sample. There are two methods for sterile urine collection in newborn infants, including urinary catheterization (UC) (passing a catheter through the urethra into the bladder) and suprapubic aspiration (SPA) (inserting a needle into the bladder through the abdominal wall).[1] Both of these methods are supposed to be invasive and each has benefits and limitations. However, the best way for a urine sample collection is SPA because of lower rate of contamination.[1] The results of several studies have shown different rates of successful urine sampling by SPA and UC. For successful sterile urine sampling especially by SPA, skill and expertise are important factors.[2,3,4] The main drawbacks of SPA are its limited success rate and the associated pain. Premature infants are subjected to an average of 10-16 painful invasive procedures per day.[5,6] Untreated pain caused by invasive procedures is associated with important undesired behavioral and physiological consequences in many organ systems, which could be life threatening.[7] Moreover, there is increasing evidence of short and long term adverse neurodevelopmental consequences of pain in the newborn period.[8,9] Thereby, finding reliable methods with lesser pain for painful procedures including collection of urine samples is very important.
The aim of this study was to compare the intensity of pain during SPA with the pain during UC in male premature infants.
METHODS
This prospective randomized controlled study was conducted at the Neonatal Intensive Care Unit (NICU) of Alzahra University Hospital, affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. From May 2011 to April 2012, 80 preterm male infants born between 28 and 34 weeks of gestation were studied. Due to different anatomy of external genitalia in male and female, we conducted this study only on male newborns to increase the accuracy of results.
Uncircumcised male newborn infants with postnatal ages of 1-28 days who needed urine cultures for sepsis workup were entered the study. Infants were excluded if they had urogenital anomaly, neurological problem, abdominal distension, abdominal wall anomaly, perinatal asphyxia, urination during the procedure and if they had received analgesics or sedatives within previous 72 h. We also excluded newborn with one unsuccessful attempt for SPA or UC.
The research ethics board of Isfahan University of Medical Sciences approved the study and written informed consent was obtained from the parents.
The infants were randomly assigned to one of two groups using a system of sealed envelopes. Urine samples were obtained by SPA in the first group and by urethral catheter in the second group.
After enrollment patients were taken to the study room and placed on a preheated radiant warmer and connected to a Masimo pulse oximeter (Irvine, CA, USA) during the study. Newborn faces, upper parts of the body and also pulse oximeter were videotaped by two camera from 5 min before the procedure until 5 min after it.
For SPA, we cleansed the suprapubic area with a 0.02% chlorhexidin solution and performed the procedure on the basis of the standard method. We inserted a needle (21 gauge) which was attached to a 5 ml syringe in to the bladder in the midline, 1-2 cm superior to the pubic symphysis with a slightly cephalad direction approximately 20° from vertical and applied negative pressure to the syringe as the needle was advanced. To improve the success rate of SPA, a urine bag was attached to the genitalia and we tried to aspirate the bladder if there was not any urine in the bag at least 30 min before the procedure. UC was also performed using standard methods. Using a 0.02 chlorhexidin solution we cleansed the external genitalia and then entered a feeding tube (5-fr), which was lubricated with a water soluble jelly in to the urethral meatus. Failure was defined as obtaining <1 ml of urine for each attempt. The personnel who performed SPA and UC was specifically trained for these procedures.
All videotapes were scored by one investigator who was blinded to the assignment of infants to the study groups. We measured pain response using Premature Infant Pain Profile (PIPP) score. PIPP score is a seven indicator composite scale assigning points for three behavioral (facial action, brow bulge, eye squeeze, and nasolabial furrow), two physiological (heart rate and oxygen saturation) and two contextual (gestational age, behavioral state) indicators of pain. Each indicator scored on a 4-point score (0-3) to give a maximum total score of 21.[10]
On the basis of these videos, newborns were evaluated for PIPP score by expert investigators. The primary outcome of the study was pain caused by urine sampling evaluated by PIPP score and the secondary outcomes of the study were duration of crying and success rate of the sampling method to obtain at least 1 ml of urine. We calculated that a sample size of 40 infants per group would be required to achieve a statistically significant difference in pain score with a power of 80% and a P < 0.05. The subject characteristics were compared by the Student's t-test for numerical data and Chi-square or Fisher exact test for qualitative variables. We used SPSS software (version 21, SPSS Inc., Chicago, IL, USA) for data analysis.
RESULTS
A total of 89 newborn infants needed urine culture during the study period. One patient was excluded because of urogenital anomaly, five infants excluded because of parenteral consent withdrawal and three infants excluded because of urination during the procedure. As a result 80 newborns enrolled, 40 of whom were randomly allocated to SPA and 40 to UC groups [Figure 1]. Baseline characteristics of the study population are shown in Table 1. There were no significant differences between groups with regard to birth weight, gestational age and postnatal age.
Figure 1.
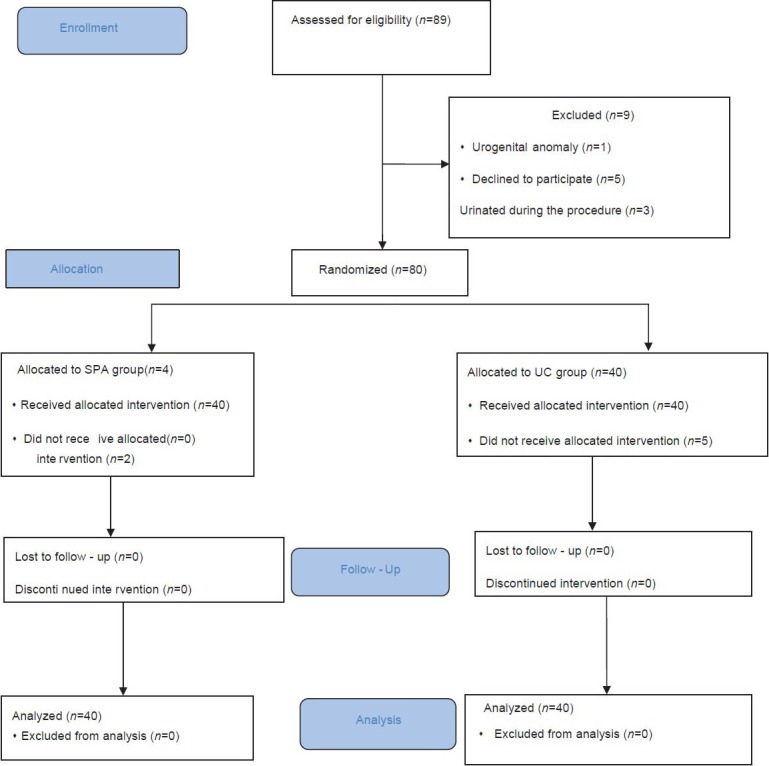
The study flow
Table 1.
Baseline characteristics of two groups
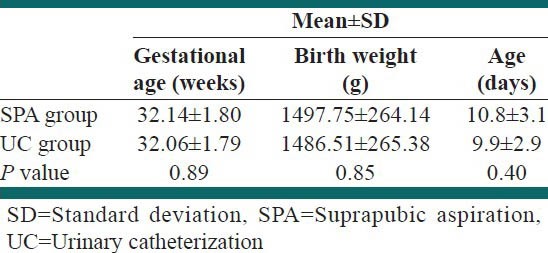
Figure 2.
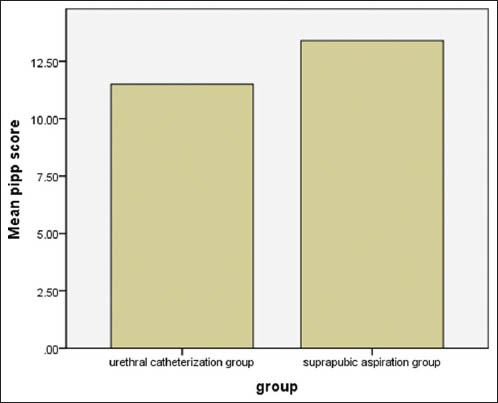
comparison of mean PIPP score between two study groups
Four urine cultures were positive in SPA group and two urine cultures were positive in UC group. There was no complication in groups due to the procedure. Table 2 shows the mean ± SD of crying time, PIPP score, baseline and maximum heart rate and baseline and minimum sPO2 in UC and SPA groups. The mean crying times during the procedure was 77.8 ± 18.4 s in the SPA group and 34.4 ± 15.7 s in the UC group (P < 0.001).
Table 2.
The mean±SD of crying time, PIPP score, baseline and maximum heart rate and baseline and minimum SPO2 in UC and SPA groups
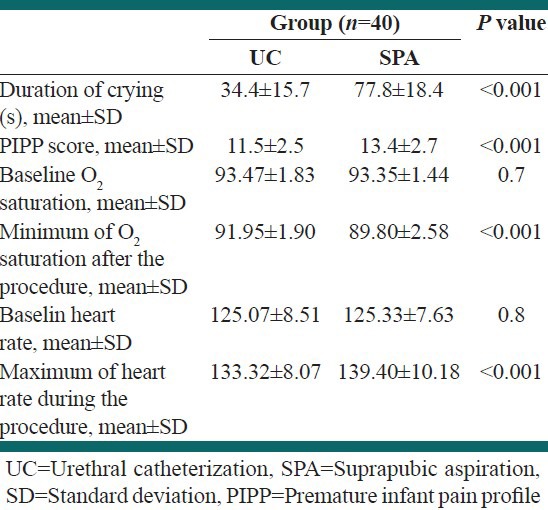
The PIPP score was significantly lower in UC group (11.5 ± 2.5) than SPA group (13.4 ± 2.7) P < 0.001. The maximum PIPP score was 17 in SPA group and 15 in UC group. The success rate of SPA was 53% compared with 71% success rate of UC.
DISCUSSION
In this study, we compared the pain intensity of SPA and UC in uncircumcised male premature infants. We found that using PIPP score, SPA is more painful than UC in uncircumcised male preterm infants. In addition, duration of crying in the UC group was about one half of SPA group. Moreover, the success rate of UC was more than SPA.
With the increasing awareness among health care providers about pain experiences in newborn infants, special attention has been paid to the sequential later effects of invasive painful procedures in newborn infants.[11,12]
Newborn infants admitted to NICU undergo multiple painful procedures. To evaluate infants with suspected UTI we should take sterile urine sample using SPA or UC, which are both painful procedures. PIPP score is a pain measurement tool with good reliability in premature and term infants.[10] In this study, we used PIPP score to measure the pain intensity of infants who needed SPA or UC for evaluation of UTI.
El-Naggar et al.[9] compared the pain responses caused by SPA and UC in preterm infants who needed urine collection for urinalysis. For increasing the success rate of urine sampling, they used volumetric-bladder ultrasonography prior to SPA and UC. However, Instead of using a validated pain score for evaluation of pain in premature infants, they used brow bulging and facial grimacing to assess pain responses in male and female premature infants. They showed that brow bulging score was significantly lower in UC group than SPA group but facial grimacing score did not differ between groups. Unfortunately, they did not evaluate pain responses in male and female infants separately, which could be a confounding factor because the different anatomy of external genitalia in male and female infants could affect pain responses.
In contrast to aforementioned study, we did not use volumetric-bladder ultrasonography prior to the procedure. However, the success rate of urine sampling was very close to study of El-Naggar et al.[9] We speculated that relatively high rate of success in our study despite the lack of ultrasonographic guide is due to two factors. First, urine sampling in the female is more difficult than male newborns and we excluded female newborns from the study. Second, SPA and UC was performed by experience nurses which is a very important factor for successful urine sampling.
Kozer et al.[13] evaluated pain responses due to SPA and UC in female and circumcised male young infants (0-2 months) who required urine collection for culture. Although they used a local anesthetic cream 1 h prior to the procedure for attenuation of pain due to sampling, they also demonstrated that pain score was significantly higher during SPA compared with UC. Pain intensity was also significantly higher in SPA group compared with UC group in subgroup analysis of male and female infants.[13]
In contrary, Oswald et al.[14] evaluated pain score in children who underwent cystourethrography and showed that SPA was associated with lower pain score than UC in children. However, they used topical anesthetic cream for SPA and not for UC. Therefore the lower pain score in SPA group may be due to the anesthetic effects of local anesthetics. In addition the patients in their study was older (mean age of 33 months) and the procedure was longer than our study and researchers who measured the pain score were not blinded to the study.[14]
Some studies were used duration of crying as an index of pain intensity in newborn infants.[15,16] Our study showed that duration of crying was significantly lower in infants who were undergone UC compared to SPA group. Similarly, in the study of Kozer et al.[13] the duration of cry was shorter in UC group compared with SPA group, but that difference was not significant.
It is interesting that duration of crying in our study in UC group was 34.4 ± 15.7 s which is very close to the figure 49.7 ± 35.7 in the study of Kozer et al.[13] In the SPA group, crying time was 77.8 ± 18.4 s in our study and 62.9 ± 26 s in the Kozer's study.[13] We speculated that the longer crying time in our study is due to not using local anesthetic cream prior to the procedure.
The success rate of obtaining adequate urine samples by both methods revealed that we could use both methods when urine samples were needed. However, our findings indicated that SPA has a higher failure rate than UC, which is in agreement with the finding of some other studies.[9,17] Pollack et al.[3] conducted a study to compare the success rate and complications of SPA with UC in infants younger than 6 months of age who needed sterile urine sampling for evaluation of sepsis and UTI. They reported a higher rate of success with UC compared to SPA (100% instead of 46%). They supposed that successful urine sampling by SPA is dependent on urine volume in the bladder. Furthermore, the study of Austin et al.[17] showed a higher rate of successful urine sampling by UC compared to SPA (77% vs. 67%).
We speculated that differences in the success rate of SPA is results from differences in the experiences of nurses or physicians who do the procedure, the volume of urine in the bladder at the time of sampling and the presence or absence of prepuce. In addition, if the urethral catheter remains for some time in place, it can increase the success rate of urine sampling.
Our study has some limitations. First, blinding of nurses was not possible because they were responsible for urine sampling. Second, although we used a validated pain score for evaluation of pain intensity, we used only one pain score for pain assessment. Using more than one pain assessment tool can increase the reliability of results. However, this study has some important powers. First of all, we did not use any analgesic intervention such as local anesthetics or sucrose for pain reduction during the procedure to better evaluate the pain responses in newborn infants undergoing each procedure. Second, we included only uncircumcised male infant's into study to prevent effects of different anatomy of external genitalia on pain score.
CONCLUSIONS
Our study revealed that urine sampling using SPA is more painful than UC in premature male infants. Health care providers should contemplate these findings for selection of the method of urine sample collection from preterm infants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Practice parameter: The diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Pediatrics. 1999;103:843–52. doi: 10.1542/peds.103.4.843. [DOI] [PubMed] [Google Scholar]
- 2.Chu RW, Wong YC, Luk SH, Wong SN. Comparing suprapubic urine aspiration under real-time ultrasound guidance with conventional blind aspiration. Acta Paediatr. 2002;91:512–6. doi: 10.1080/080352502753711614. [DOI] [PubMed] [Google Scholar]
- 3.Pollack CV, Jr, Pollack ES, Andrew ME. Suprapubic bladder aspiration versus urethral catheterization in ill infants: Success, efficiency and complication rates. Ann Emerg Med. 1994;23:225–30. doi: 10.1016/s0196-0644(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 4.Tobiansky R, Evans N. A randomized controlled trial of two methods for collection of sterile urine in neonates. J Paediatr Child Health. 1998;34:460–2. doi: 10.1046/j.1440-1754.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- 5.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Lago P, Guadagni A, Merazzi D, Ancora G, Bellieni CV, Cavazza A, et al. Pain management in the neonatal intensive care unit: A national survey in Italy. Paediatr Anaesth. 2005;15:925–31. doi: 10.1111/j.1460-9592.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdulkader HM, Freer Y, Garry EM, Fleetwood-Walker SM, McIntosh N. Prematurity and neonatal noxious events exert lasting effects on infant pain behaviour. Early Hum Dev. 2008;84:351–5. doi: 10.1016/j.earlhumdev.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald M, Walker SM. Infant pain management: A developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 9.El-Naggar W, Yiu A, Mohamed A, Shah V, Manley J, McNamara P, et al. Comparison of pain during two methods of urine collection in preterm infants. Pediatrics. 2010;125:1224–9. doi: 10.1542/peds.2009-3284. [DOI] [PubMed] [Google Scholar]
- 10.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: Development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Grunau R. Early pain in preterm infants. A model of long-term effects. (vii-viii).Clin Perinatol. 2002;29:373–94. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald M, Beggs S. The neurobiology of pain: Developmental aspects. Neuroscientist. 2001;7:46–57. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 13.Kozer E, Rosenbloom E, Goldman D, Lavy G, Rosenfeld N, Goldman M. Pain in infants who are younger than 2 months during suprapubic aspiration and transurethral bladder catheterization: A randomized, controlled study. Pediatrics. 2006;118:e51–6. doi: 10.1542/peds.2005-2326. [DOI] [PubMed] [Google Scholar]
- 14.Oswald J, Riccabona M, Lusuardi L, Ulmer H, Bartsch G, Radmayr C. Voiding cystourethrography using the suprapubic versus transurethral route in infants and children: Results of a prospective pain scale oriented study. J Urol. 2002;168:2586–9. doi: 10.1016/S0022-5347(05)64222-X. [DOI] [PubMed] [Google Scholar]
- 15.Grunau RV, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 16.Lewindon PJ, Harkness L, Lewindon N. Randomised controlled trial of sucrose by mouth for the relief of infant crying after immunisation. Arch Dis Child. 1998;78:453–6. doi: 10.1136/adc.78.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin BJ, Bollard C, Gunn TR. Is urethral catheterization a successful alternative to suprapubic aspiration in neonates? J Paediatr Child Health. 1999;35:34–6. doi: 10.1046/j.1440-1754.1999.00305.x. [DOI] [PubMed] [Google Scholar]


