Abstract
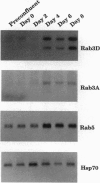
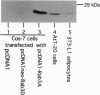
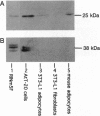
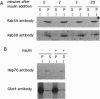
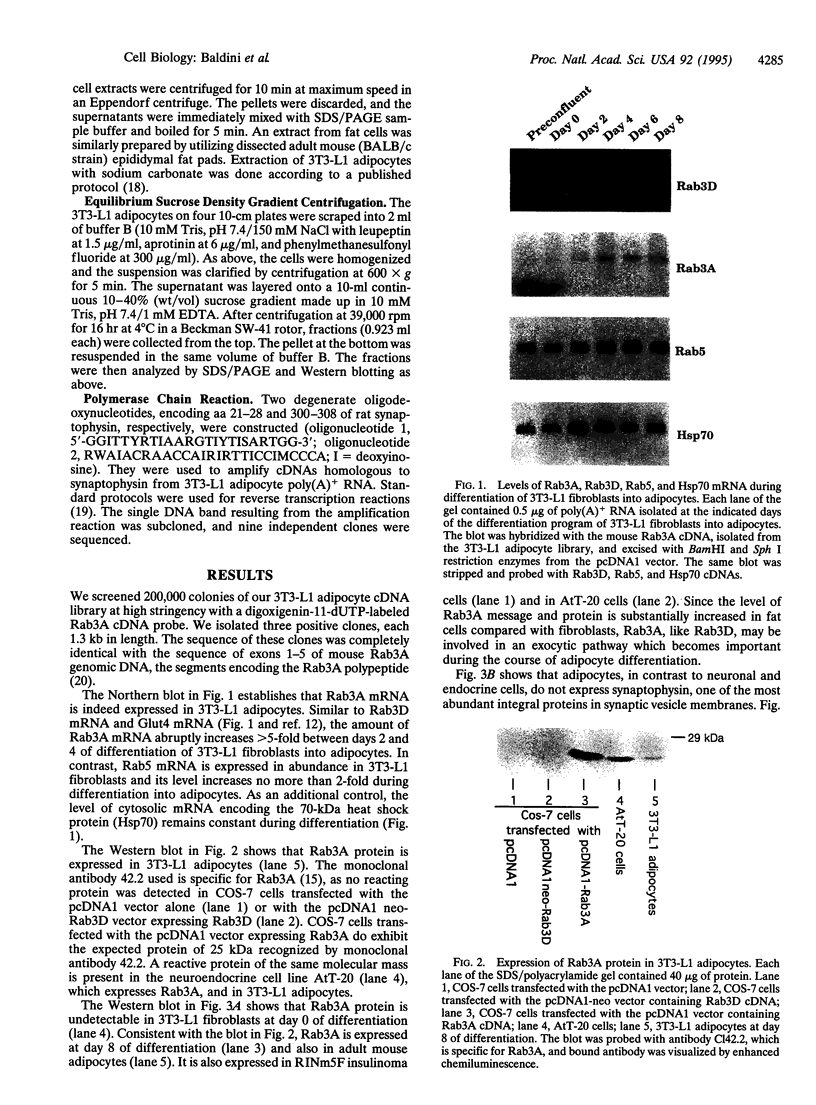
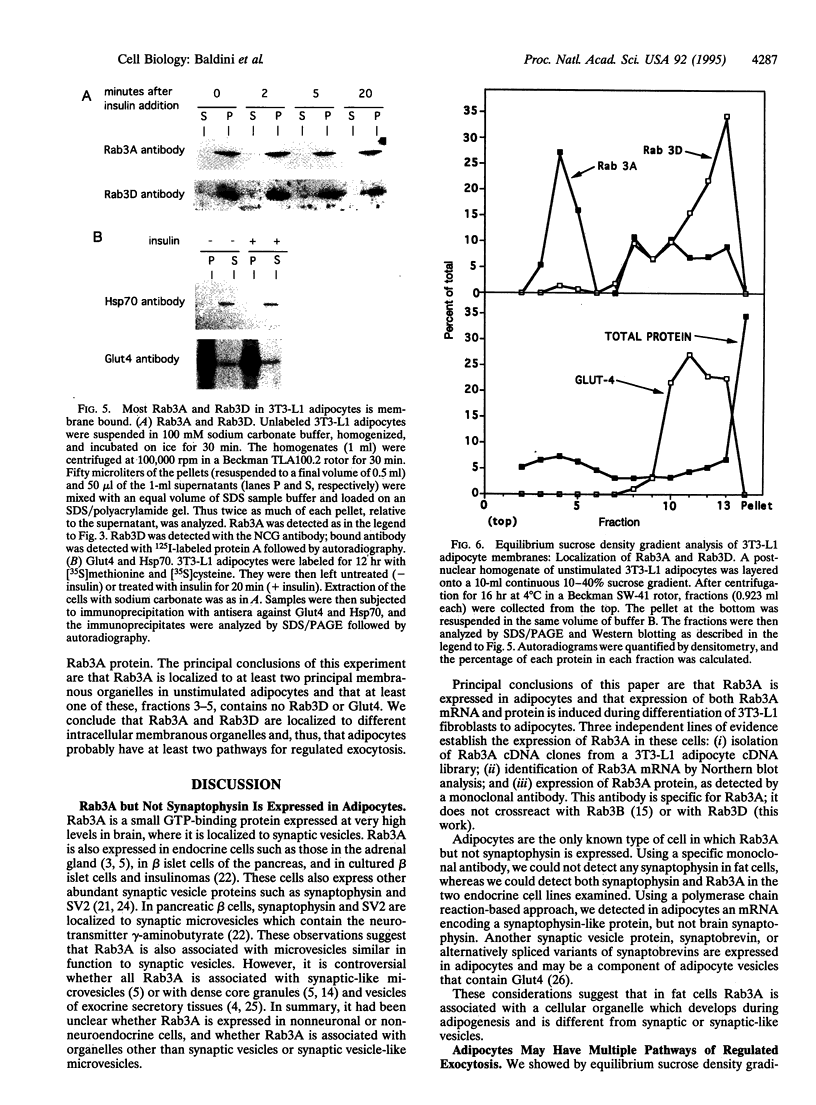
Rab3A is a small GTP-binding protein expressed predominantly in brain and neuroendocrine cells, in which it is associated with synaptic and synaptic-like vesicles, respectively. Here we report that adult mouse fat cells and 3T3-L1 adipocytes also express Rab3A mRNA and protein. They do not express synaptophysin, an abundant protein in synaptic vesicles or synaptic-like vesicles. The amount of Rab3A mRNA and protein, like that of the highly homologous isoform Rab3D, increases severalfold during differentiation of 3T3-L1 fibroblasts into mature adipocytes. In fat cells, most Rab3D and Rab3A protein is bound to membrane, irrespective of insulin addition. Rab3A and Rab3D are localized in different subcellular compartments, since about half of the Rab3A, but none of the Rab3D, is associated with a low-density organelle(s). Rab3D and Rab3A may be involved in different pathways of regulated exocytosis in adipocytes. Moreover, in adipocytes Rab3A may define an exocytic organelle that is different from synaptic vesicles or synaptic-like microvesicles found in neuronal and endocrine cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini G., Hohl T., Lin H. Y., Lodish H. F. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini G., Hohman R., Charron M. J., Lodish H. F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991 Mar 5;266(7):4037–4040. [PubMed] [Google Scholar]
- Baumert M., Fischer von Mollard G., Jahn R., Südhof T. C. Structure of the murine rab3A gene: correlation of genomic organization with antibody epitopes. Biochem J. 1993 Jul 1;293(Pt 1):157–163. doi: 10.1042/bj2930157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormont M., Tanti J. F., Zahraoui A., Van Obberghen E., Tavitian A., Le Marchand-Brustel Y. Insulin and okadaic acid induce Rab4 redistribution in adipocytes. J Biol Chem. 1993 Sep 15;268(26):19491–19497. [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Khokhlatchev A., Südhof T. C., Jahn R. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicles after stimulation of exocytosis. J Biol Chem. 1994 Apr 15;269(15):10971–10974. [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Bolshakov V. Y., Siegelbaum S. A., Takei K., De Camilli P., Hammer R. E., Südhof T. C. The role of Rab3A in neurotransmitter release. Nature. 1994 Jun 9;369(6480):493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Piper R. C. Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol. 1994 Sep;126(5):1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena B. P., Gumkowski F. D., Konieczko E. M., von Mollard G. F., Jahn R., Jamieson J. D. Redistribution of a rab3-like GTP-binding protein from secretory granules to the Golgi complex in pancreatic acinar cells during regulated exocytosis. J Cell Biol. 1994 Jan;124(1-2):43–53. doi: 10.1083/jcb.124.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M., Navone F., Haimann C., Cameron P. L., Solimena M., De Camilli P. Secretory organelles of neurons and their relationship to organelles of other cells. Cell Biol Int Rep. 1989 Dec;13(12):981–992. doi: 10.1016/0309-1651(89)90014-3. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Südhof T. C., Jahn R., De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991 Nov;115(3):625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Takai Y. Tissue distribution of smg p25A, a ras p21-like GTP-binding protein, studied by use of a specific monoclonal antibody. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1438–1445. doi: 10.1016/0006-291x(89)90835-8. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R., Vallar L., Ullrich S., Ravazzola M., Kikuchi A., Takai Y., Wollheim C. B. Characterization of small-molecular-mass guanine-nucleotide-binding regulatory proteins in insulin-secreting cells and PC12 cells. Eur J Biochem. 1992 Sep 15;208(3):729–737. doi: 10.1111/j.1432-1033.1992.tb17241.x. [DOI] [PubMed] [Google Scholar]
- Robinson L. J., Pang S., Harris D. S., Heuser J., James D. E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992 Jun;117(6):1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. S., Cook K. S., Yaglom J., Groves D. L., Volanakis J. E., Damm D., White T., Spiegelman B. M. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989 Jun 23;244(4911):1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Lisanti M. P., Baldini G., Sargiacomo M., Mastick C. C., Lodish H. F. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994 Dec;127(5):1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993 Nov;11(5):789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]