Abstract
Iodine is a micronutrient essential for the production of thyroid hormones. Iodine deficiency is the most common cause of preventable mental impairment worldwide. Universal salt iodization (USI) has been introduced in many countries as a cost-effective and sustainable way to eliminate iodine deficiency disorders for more than 25 years. Currently, the relationship between USI and iodine excess has attracted more attention. Iodine excess can lead to hypothyroidism and autoimmune thyroiditis, especially for susceptible populations with recurring thyroid disease, the elderly, fetuses, and neonates. Nationwide USI was introduced in China in 1996. This review focused on the effects of iodine excess worldwide and particularly in China.
Keywords: Iodine excess, Universal salt iodization, Thyroid diseases
INTRODUCTION
Iodine is not only a major component of thyroid hormones but is also a microenvironment for thyroid cells to thrive. A U curve has been proved by a number of studies, indicating that both iodine deficiency and iodine excess can result in an increased prevalence of thyroid disorders. Over the past 25 years, universal salt iodization (USI) has been introduced in many countries as a safe, cost-effective and sustainable method to eliminate iodine deficiency disorders (IDDs), resulting in a greatly improved iodine status. However, an increased iodine intake has brought new challenges in long-term iodine sufficient regions covered by USI for more than 10 years. This review focused on the effect of iodine excess on thyroid disorders in China and other regions in the world.
THE HISTORY AND CURRENT STATUS OF USI IN CHINA
China was an iodine-deficient country with 370 million people living in iodine-deficient areas prior to 1970. As a result, a program of local iodine supplementation was introduced in iodine-deficient areas in 1979 [1]. A national survey in 1995, when household salt iodine was 16.2 mg/kg, showed that iodine status was adequate with a median urinary iodine concentration (UIC) of 164.8 µg/L, yet goiter prevalence was high, at 20.4% [2].
From 1996, a mandatory USI program was introduced nationwide; the recommended standard for the concentration of iodine in iodized salt was 50 mg/kg at the production level, 30 mg/kg at the retail level and 20 mg/kg at the household level [3]. USI resulted in a sharp increase in the median UIC in school children to 330 µg/L in 1997 and 306 µg/L in 1999, when the iodine concentration of household salt was measured at 37 to 42 mg/kg, which was much higher than the recommended standard. For this reason, in 2002, national standards for iodized salt were revised to reduce the iodine concentration from 50 to 35±15 mg/kg at the production level resulting in a downward shift to a more than adequate iodine intake that lasted for another 8 years (2003 to 2011) (Table 1). In 2012, World Health Organization (WHO) identified China as a region with more than adequate iodine intake [4] and in that year, China's USI program was again revised with two key policy changes: first, a new set of national criteria for salt iodization was launched whereby the concentration of iodine in salt was decreased to 20 to 30 mg/kg at the production level; second, provincial governments were authorized to set the local iodine concentration in salt to within ±30% of the recommended level to account for the natural background iodine level [3]. Consequently, the Chinese population has been exposed to excessive iodine intake for 6 years and to more than adequate iodine intake for 11 years since the introduction of USI.
Table 1.
Iodine Status in China after Introduction of USI
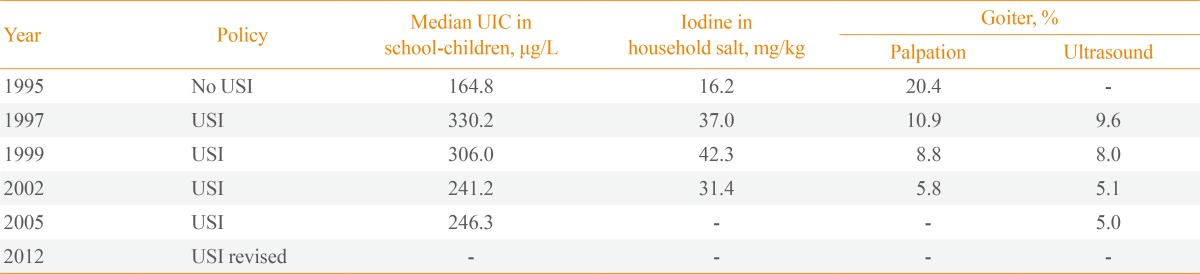
USI, universal salt iodization; UIC, urinary iodine concentration.
IODINE ASSESSMENT
Iodine status of the population
Assessment of iodine status of the population >12 years of age is based on the UIC of school-age children (Table 2) [5].
Table 2.
Epidemiological Criteria for Assessing Iodine Nutrition Based on MUI Concentrations in School-Age Children
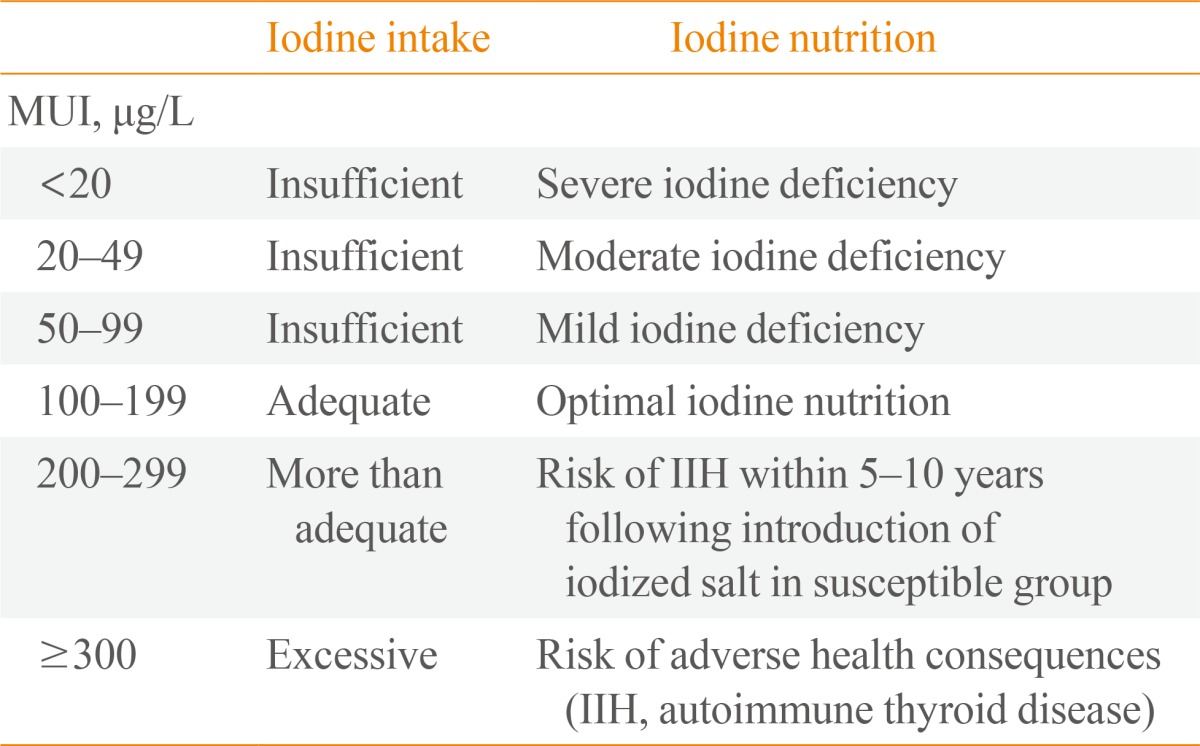
MUI, median urinary iodine; IIH, iodine-induced hyperthyroidism.
Iodine status of pregnant and lactating women
In 2007, WHO Technical Consultation first recommended both a low (250 µg/day) and upper (500 µg/day) limit of iodine intake for pregnant and lactating females. Meanwhile, a standard to assess the iodine nutrition status for pregnant and lactating females based on UIC was proposed and included insufficient, adequate, more than adequate and excessive iodine status categories (Table 3). UIC <150 µg/L was defined as iodine deficiency during pregnancy, compared with the classical definition of iodine deficiency in the general population, which was UIC <100 µg/L [6].
Table 3.
The Range of MUI Concentrations Used for the Associated Iodine Intake Category of Pregnant Females, Lactating Females and Children Less than 2 Years of Age
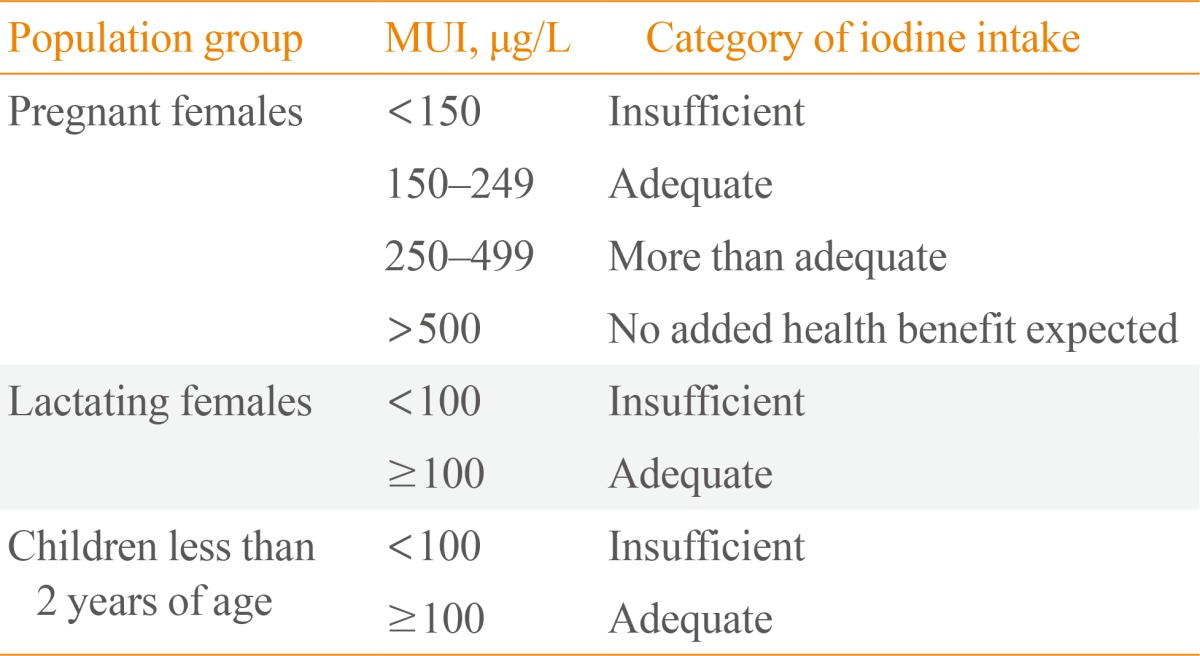
MUI, median urinary iodine.
HYPERTHYROIDISM
Since the initial description by Coindet in 1821 and the subsequent definition by Breuer and Kocher in 1904, iodine-induced hyperthyroidism (IIH) has been reported in patients with a variety of underlying thyroid diseases [7]. The prevalence of IIH was partly related to the previous level of iodine deficiency in the region and was transient. After USI was started in moderate to severe iodine deficiency areas, the incidence of hyperthyroidism increased significantly. USI came into effect in 1995 in Zimbabwe. The retrospective survey regarding the incidence of hyperthyroidism in Zimbabwe performed by WHO and International Council for the Control of Iodine Deficiency Disorders showed that the incidence of hyperthyroidism ranged from 2.8/100,000 to 7.4/100,000 per year [8], with a similar result found in Zaire [9]. The incidence of hyperthyroidism is associated with the increased amount of iodine intake. Another Austrian multi-center retrospective study including more than 400,000 participants was conducted in 19 regions in 1998 and showed that the incidence of clinical hyperthyroidism and subclinical hyperthyroidism increased by 36% and 64%, respectively, following an increase of iodine in salt from 10 to 20 mg/kg [10]. In Denmark, Ahlberg is a moderately iodine-deficient area (median urinary iodine, MUI 53 ug/L) where the incidence of hyperthyroidism increased every year after USI was introduced (iodine concentration in salt was 13 mg/kg) [11].
The increase of IIH was partly due to the effects of iodine on the thyroid autoimmunity. After the supply of iodized bread in Tasmania, the incidence of hyperthyroidism increased from 0.018% to 0.059% and 54% patients were positive for thyroid stimulating antibodies [12,13]. Another 494-case study was performed by Solomon et al. [14] investigating relationships between iodine intake and remission rate of Graves disease during a 20-year period. They found remission rates of Graves disease were 60% to 80% in 1963, decreased to 13% to 20% in 1973 and then increased again to 50.6% in 1987. The change of Graves disease remission rates was associated with iodine in the diet during the same period [14]. However, in two follow-up studies conducted by our group in China, the prevalence and cumulative incidence of Graves disease were found to be not related to iodine intake, which had no effect on the outcomes of patients with Graves disease following drug treatment [15,16].
HYPOTHYROIDISM
Currently, the reports of iodine-induced hypothyroidism are not considered important. In 1997, Szabolcs et al. [17] investigated the effect of different iodine levels on the prevalence of thyroid dysfunctions in Hungry. The survey was performed in Northern Hungry (MUI 72 µg/g creatinine), Slovakia (MUI 100 µg/g creatinine), and Eastern Hungry (MUI 513 µg/g creatinine). It showed that the prevalence of hypothyroidism was an increasing trend, accompanied by iodine intake, and was 0.8%, 1.5%, and 7.6%. The prevalence of subclinical hypothyroidism was 4.2%, 10.4%, and 23.9%. They also concluded that occurrence of hypothyroidism was mainly due to autoimmune thyroiditis [17]. In 1998, in Denmark, Laurberg et al. [18] reported that an elderly population living in a relatively high urinary iodine region (MUI 150 µg/L) had a higher serum thyrotropin (TSH) than those in a lower urinary iodine region (MUI 38 µg/L). Notably, two Danish cities were identified as moderate and mild iodine deficiency areas (Aalborg, MUI 45 µg/L and Copenhagen, MUI 61 µg/L). Although the MUI were relatively similar for Aalborg and Copenhagen, the differences in clinical hypothyroidism incidence were large (26.5/100,000 and 40.1/100,000 per year, respectively) [19]. Even 6 years after USI was introduced in Denmark, the overall incidence rate of hypothyroidism increased during the study period (38.3/100,000 to 47.2/100,000 per year), occurring primarily in young and middle-aged subjects with previous moderate iodine deficiency [20]. In addition, the people with autoimmune thyroiditis were more susceptible to excessive iodine. In 1998, Reinhardt et al. [21] reported that when patients with Hashimoto's thyroiditis took 250 µg iodine supplement daily (MUI increased from 72 µg/g creatinine to 268 µg/g creatinine), the occurrence of thyroid dysfunction in the thyroiditis group was significantly higher than in the control group.
To understand the effect of different iodine intakes, we investigated iodine-induced thyroid disease in three regions of China with different iodine levels in nutrition (iodine-induced thyroid disorders, IITD). In the three communities, MUI was 88, 214, and 634 µg/L. According to WHO criteria these levels were considered as mild iodine deficiency, more than adequate iodine and iodine excess, respectively. A total of 3,761 random volunteers were enrolled at baseline and 3,018 subjects (80.2%) were included in a 5-year follow-up study. Except for the different levels of iodine, other factors such as the age, gender, economic status, and health care received in the three regions were similar. The iodine levels were almost the same in the three communities during the 5-year period showing the prevalence of overt hypothyroidism was 0.3%, 0.9%, and 2% and the cumulative incidence was 0.2%, 0.5%, and 0.3%. The prevalence of subclinical hypothyroidism was 0.9%, 2.9%, and 6.1% and the cumulative incidence was 0.2%, 2.6%, and 2.9% [22]. We conducted another population-based cross-sectional study in two regions of China with different iodine levels in nutrition. The study included 3,813 subjects in Rongxing (MUI 261 µg/L) and Chengshan (MUI 145 µg/L). The prevalence of subclinical hypothyroidism was significantly higher for individuals living in Rongxing than in Chengshan [23].
AUTOIMMUNE THYROIDITIS
There is increasing evidence that the occurrence of thyroiditis is related to iodine supplementation. When mice of an autoimmunity-prone strain were first fed an iodine-deficient diet followed by an iodine-excessive diet, they developed ultrastructural thyroid epithelial cell damage in a dose-dependent manner suggestive of autoimmune disease. The incidence of thyroiditis as well as the degree of lymphocytic infiltration in the thyroid increased gradually, dose-dependently [24]. Analysis of 3,500 fine-needle aspiration biopsy cases in Poland between 1985 and 1999 showed that the frequency of cytologically-diagnosed chronic thyroiditis increased from 1.5% in 1992 to 5.7% in 1999 due to USI in 1997 [25]. Kahaly et al. [26] performed a double-blind trial evaluating efficacy and tolerability of low-dose iodine supplementation in adults with euthyroid, diffuse, endemic goiter. Sixty-two participants were randomly assigned an iodine supplement (0.2 mg/day) or placebo for 12 months. After trial completion, thyroid dysfunctions and autoimmunity were observed in nearly 10% of the subjects [26]. Similar results were observed in Greek and Sri Lankan children. After USI, the prevalence of autoimmune thyroiditis in Greek children was three times greater than it was 7 years prior [27]. The prevalence of positive thyroglobulin antibody in Sri Lankan young females was markedly increased, ranging from 14.3% (11 years of age) to 69.7% (16 years of age) [28].
In our ITTD study, the prevalence of autoimmune thyroiditis in the three regions was 0.5%, 1.7%, and 2.8% and the cumulative incidence was 0.2%, 1.0%, and 1.3% [22]. Beginning in 2002, we evaluated whether excessive intake of iodine in postpartum females caused postpartum thyroiditis (PPT). Of 488 pregnant females, the prevalence of overt PPT and subclinical PPT was 7.2% (35/488) and 4.7% (23/488), respectively. When the 488 subjects were divided into three subgroups according to urinary iodine levels, the prevalence of PPT in the high iodine intake group was significantly higher than in the other groups. Pregnant females with high iodine intake were at greater risk of developing PPT than were those with low iodine intake (relative risk, 2.92; 95% confidence interval [CI], 1.31 to 6.50) [29].
GOITER
Iodine-induced goiter was first discovered in Hokkaido, Japan, in 1966. The incidence of goiter in the region is 6% to 12% and urinary iodine of local people is up to 8 to 24 mg/day [30]. In 1987, Li et al. [31] reported that the prevalence of goiter in children living in areas of Shanxi Province in China with high iodine levels in water (water iodine content 462.5 µg/L) was as high as 65%; the prevalence of goiter in the control area (water iodine content 54 µg/L) was 15.4%. Zimmermann et al. [32] also found that chronic iodine intakes over 500 µg/day in children can increase thyroid volume.
THYROID NODULES
Recently, controversy that iodized salt may increase the risk of thyroid nodules has emerged in China. In our study, the prevalence of a single nodule was 8.8%, 8.3%, and 4.1% in Panshan (MUI 88 µg/L), Zhangwu (MUI 214 µg/L), and Huanghua (MUI 634 µg/L), respectively, and the prevalence of multiple nodules was 3.8%, 1.9%, and 6.7%, respectively. In Panshan, Zhangwu, and Huanghua the cumulative incidence of a single nodule was 4.0%, 5.7%, and 5.6% and multiple nodules was 0.4%, 1.2%, and 1.0%, respectively. The incidence of single and multiple nodules was similar between males and females in all three regions [33]. A cross-sectional study was conducted in Hangzhou, China, in 2010. After adjustment for potential covariates, as compared to those with normal urinary iodine, the subjects with low UICs had an increased risk of thyroid nodules (odds ratio [OR], 1.25; 95% CI, 1.07 to 1.45); however, subjects with a high or excessive UIC did not have an increased risk of thyroid nodules [34].
THYROID CANCER
Thyroid cancer is a common endocrine tumor, and the annual incidence of clinical thyroid cancer is (4 to 10)/100,000 per year. Some reports have shown that increased incidence of thyroid cancer is related to iodine intake. After the USI program was introduced in Australia in 1963, the incidence of thyroid cancer increased from 3.07/100,000 to 7.8/100,000 per year [35]. However, in some cases the total incidence of thyroid cancer was unchanged [36]; therefore, more focus was directed to the change of tumor type after USI. Harach and Williams [37] analyzed thyroid cancer cases over a 31-year period. The incidence of thyroid cancer increased from 0.78/100,000 to 0.84/100,000 per year after USI. They suggested a high intake of iodine may be related to a high incidence of papillary carcinomas and thyroiditis, the latter more commonly associated with papillary carcinomas than other thyroid tumors [37]. USI was started in 1936 in Sweden and was enhanced in 1966. A survey in Sweden also showed that high incidence of papillary carcinomas was observed in areas with higher levels of iodine [38]. A study analyzing the incidence of thyroid carcinoma (TC) before and after USI was conducted in Shenyang, a city in Northeastern China. The pathology reports were collected from patients with thyroid diseases who underwent surgery in the First Hospital of China Medical University from January 1, 1992, to December 31, 2009. The prevalence of TC, papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma increased, follicular thyroid carcinoma decreased and undifferentiated thyroid carcinoma (DTC) showed no change after USI. The incidence of PTC complicated with either a nodular goiter or chronic lymphocytic thyroiditis also increased after USI [39].
MUI in Korean subjects (n=540, 2012) was 267.6 µg/L and was significantly higher in the older group (383.9 µg/L) than in the younger group (191.8 µg/L). The results also indicated the MUI in the Korean urban population was in a more than adequate iodine nutritional state [40]. Similar to worldwide trends, the incidence of thyroid cancer in South Korea has increased steadily in recent decades from 1.9 in 1996 to 27.0 in 2010 in males and from 10.6 to 111.3 in females, using a hypothetical world standard population. The proportion of papillary-type thyroid cancer increased from 74.2% and 75.4% in 1996 to 97.9% and 98.3% in 2010 for males and females, respectively. Thyroid cancer is the leading cancer in females since 2003 and is now the fifth most common cancer in males in Gwangju and Jeonnam, South Korea [41].
A meta-analysis proved higher serum TSH concentration was associated with an increased risk of thyroid cancer [42]. In 2010, we initiated a new population-based cross-sectional study (IITD-4) in 10 cities including 15,177 individuals in China. Six of 10 cities were adequate iodine intake regions and four cities were more than adequate iodine intake regions. The median TSH was 2.38 mIU/L (reference, 0.71 to 6.25) in China. The National Health and Nutrition Examination Survey III was a similar survey in the USA, but the median TSH was 1.40 mIU/L (reference, 0.39 to 4.6). We also found TSH levels in the population increased from 1999 to 2010 in China. The median TSH of a Chinese population in 1999, 2007 and 2010 was 1.22, 1.52, and 2.38 mIU/L, respectively. The reference TSH levels also increased from 0.3 to 4.8 mU/L to 0.71 to 6.25 mIU/L (data currently unpublished). We hypothesize that this change was related to iodine intake, and the increased TSH level could lead to thyroid abnormalities. We have found a similar relationship between chronic iodine excess and elevated serum TSH levels in animals [43]. We also found that as TSH increased, the prevalence of DTC rose clearly. The OR in favor of having DTC with a serum TSH 1.9 to 4.8 mIU/L and >4.8 mIU/L, compared with having a serum TSH 1.0 to 1.9 mIU/L were 1.57 (95% CI, 1.03 to 2.40) and 5.71 (95% CI, 2.31 to 14.14), respectively [44].
IODINE EXCESS DURING PREGNANCY
The upper limit of acceptable iodine intake in pregnancy is controversial. Iodine excess during pregnancy existed in Somalia [45], Japan and other countries. The fetal thyroid does not acquire the capacity to suppress the acute Wolff-Chaikoff effect until approximately 36 weeks of gestation [46]. Therefore, a maternal iodine excess could potentially cause fetal congenital hypothyroidism [47,48]. Nohr found iodine supplementation during pregnancy could cause TSH of cord blood to be 27.3% higher than that of those with no artificial iodine supplementation. He suggested the fetal thyroid is more sensitive to the inhibitory effect of iodine than is usually anticipated [49]. A study of pregnant females by Rebagliato demonstrated increased risk of TSH levels above 3 mIU/L in females who consumed 200 µg or more of iodine supplements daily compared with those who consumed less than 100 µg/day (adjusted OR, 2.5; 95% CI, 1.2 to 5.4). Pregnant females from a region with the highest MUI and highest supplement coverage showed the lowest levels of serum-free thyroxin [50]. According to another survey, a maternal intake of more than 150 µg/day compared with <100 µg/day of iodine from supplements was associated with a 5.2-point decrease in psychomotor development index (PDI) and a 1.8-fold increase in the odds of PDI <85 [51]. Currently, numerous studies have focused on avoiding an iodine deficiency during pregnancy, but monitoring methods for iodine excess in pregnancy is lacking.
Currently, we are conducting a new epidemiological study on iodine and thyroid diseases (IITD-5) in China including 80,600 subjects from 31 provinces. This large survey investigates thyroid disorders and iodine status in China. Hopefully, this study will summarize iodine nutritional status and thyroid diseases in China systematically. The results may not only benefit China in the prevention and treatment of thyroid disease but may also benefit populations worldwide.
CONCLUSIONS
Iodine is required for the production of thyroid hormones. Although iodine supplementation should be implemented to prevent and treat IDDs, iodine intake must be maintained at a safe level. The majority of excessive iodine exposure cases does not generally result in apparent clinically fatal consequences but could be harmful. More than adequate or excessive iodine levels are unsafe and may lead to hypothyroidism and autoimmune thyroiditis, especially for susceptible populations with recurring thyroid disease, the elderly, fetuses, and neonates. TSH levels are increasing in the Chinese population and the consequences of excessive iodine should be closely investigated.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ma T, Guo J, Wang F. The epidemiology of iodine-deficiency diseases in China. Am J Clin Nutr. 1993;57(2 Suppl):264S–266S. doi: 10.1093/ajcn/57.2.264S. [DOI] [PubMed] [Google Scholar]
- 2.Delange F, Burgi H, Chen ZP, Dunn JT. World status of monitoring iodine deficiency disorders control programs. Thyroid. 2002;12:915–924. doi: 10.1089/105072502761016557. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health of the People's Republic of China. National guideline for food safetyiodine concentration in table salt [Internet] Beijing: Ministry of Health of the People's Republic of China; c2011. [cited 2013 Aug 8]. Available from: http://wenku.baidu.com/view/7db4f3de6f1aff00bed51eae.html. [Google Scholar]
- 4.Zimmermann MB, Andersson M. Update on iodine status worldwide. Curr Opin Endocrinol Diabetes Obes. 2012;19:382–387. doi: 10.1097/MED.0b013e328357271a. [DOI] [PubMed] [Google Scholar]
- 5.International Council for Control of Iodine Deficiency Disorders; UNICEF; World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 2nd ed. Geneva: World Health Organization; 2001. [Google Scholar]
- 6.WHO Secretariat. Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 7.Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid. 2001;11:493–500. doi: 10.1089/105072501300176453. [DOI] [PubMed] [Google Scholar]
- 8.Todd CH, Allain T, Gomo ZA, Hasler JA, Ndiweni M, Oken E. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet. 1995;346:1563–1564. doi: 10.1016/s0140-6736(95)92095-1. [DOI] [PubMed] [Google Scholar]
- 9.Bourdoux PP, Ermans AM, Mukalay wa Mukalay A, Filetti S, Vigneri R. Iodine-induced thyrotoxicosis in Kivu, Zaire. Lancet. 1996;347:552–553. doi: 10.1016/s0140-6736(96)91188-5. [DOI] [PubMed] [Google Scholar]
- 10.Mostbeck A, Galvan G, Bauer P, Eber O, Atefie K, Dam K, Feichtinger H, Fritzsche H, Haydl H, Kohn H, König B, Koriska K, Kroiss A, Lind P, Markt B, Maschek W, Pesl H, Ramschak-Schwarzer S, Riccabona G, Stockhammer M, Zechmann W. The incidence of hyperthyroidism in Austria from 1987 to 1995 before and after an increase in salt iodization in 1990. Eur J Nucl Med. 1998;25:367–374. doi: 10.1007/s002590050234. [DOI] [PubMed] [Google Scholar]
- 11.Bulow Pedersen I, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB. Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark. J Clin Endocrinol Metab. 2006;91:3830–3834. doi: 10.1210/jc.2006-0652. [DOI] [PubMed] [Google Scholar]
- 12.Connolly RJ, Vidor GI, Stewart JC. Increase in thyrotoxicosis in endemic goitre area after iodation of bread. Lancet. 1970;1:500–502. doi: 10.1016/s0140-6736(70)91582-5. [DOI] [PubMed] [Google Scholar]
- 13.Adams DD, Kennedy TH, Stewart JC, Utiger RD, Vidor GI. Hyperthyroidism in Tasmania following iodide supplementation: measurements of thyroid-stimulating autoantibodies and thyrotropin. J Clin Endocrinol Metab. 1975;41:221–228. doi: 10.1210/jcem-41-2-221. [DOI] [PubMed] [Google Scholar]
- 14.Solomon BL, Evaul JE, Burman KD, Wartofsky L. Remission rates with antithyroid drug therapy: continuing influence of iodine intake? Ann Intern Med. 1987;107:510–512. doi: 10.7326/0003-4819-107-4-510. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Teng W, Fan B, Zhang Y, Yin C. Thyroid autoimmunity in members from multiplex families with Graves' disease and effect of iodine intake on its incidence. Chin J Endocrinol Metab. 2001;17:79–83. [Google Scholar]
- 16.Hou X, Li Y, Li J, Wang W, Fan C, Wang H, Zhang H, Shan Z, Teng W. Development of thyroid dysfunction and autoantibodies in Graves' multiplex families: an eight-year follow-up study in Chinese Han pedigrees. Thyroid. 2011;21:1353–1358. doi: 10.1089/thy.2011.0035. [DOI] [PubMed] [Google Scholar]
- 17.Szabolcs I, Podoba J, Feldkamp J, Dohan O, Farkas I, Sajgo M, Takats KI, Goth M, Kovacs L, Kressinszky K, Hnilica P, Szilagyi G. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, long-term iodine prophylaxis and abundant iodine intake. Clin Endocrinol (Oxf) 1997;47:87–92. doi: 10.1046/j.1365-2265.1997.2271040.x. [DOI] [PubMed] [Google Scholar]
- 18.Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998;83:765–769. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]
- 19.Bulow Pedersen I, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey. J Clin Endocrinol Metab. 2002;87:4462–4469. doi: 10.1210/jc.2002-020750. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen IB, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007;92:3122–3127. doi: 10.1210/jc.2007-0732. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt W, Luster M, Rudorff KH, Heckmann C, Petrasch S, Lederbogen S, Haase R, Saller B, Reiners C, Reinwein D, Mann K. Effect of small doses of iodine on thyroid function in patients with Hashimoto's thyroiditis residing in an area of mild iodine deficiency. Eur J Endocrinol. 1998;139:23–28. doi: 10.1530/eje.0.1390023. [DOI] [PubMed] [Google Scholar]
- 22.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 23.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–950. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 24.Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med. 2009;9:51–59. doi: 10.1007/s10238-008-0014-0. [DOI] [PubMed] [Google Scholar]
- 25.Slowinska-Klencka D, Klencki M, Sporny S, Lewinski A. Fine-needle aspiration biopsy of the thyroid in an area of endemic goitre: influence of restored sufficient iodine supplementation on the clinical significance of cytological results. Eur J Endocrinol. 2002;146:19–26. doi: 10.1530/eje.0.1460019. [DOI] [PubMed] [Google Scholar]
- 26.Kahaly G, Dienes HP, Beyer J, Hommel G. Randomized, double blind, placebo-controlled trial of low dose iodide in endemic goiter. J Clin Endocrinol Metab. 1997;82:4049–4053. doi: 10.1210/jcem.82.12.4416. [DOI] [PubMed] [Google Scholar]
- 27.Zois C, Stavrou I, Kalogera C, Svarna E, Dimoliatis I, Seferiadis K, Tsatsoulis A. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid. 2003;13:485–489. doi: 10.1089/105072503322021151. [DOI] [PubMed] [Google Scholar]
- 28.Premawardhana LD, Parkes AB, Smyth PP, Wijeyaratne CN, Jayasinghe A, de Silva DG, Lazarus JH. Increased prevalence of thyroglobulin antibodies in Sri Lankan schoolgirls: is iodine the cause? Eur J Endocrinol. 2000;143:185–188. doi: 10.1530/eje.0.1430185. [DOI] [PubMed] [Google Scholar]
- 29.Guan H, Li C, Li Y, Fan C, Teng Y, Shan Z, Teng W. High iodine intake is a risk factor of post-partum thyroiditis: result of a survey from Shenyang, China. J Endocrinol Invest. 2005;28:876–881. doi: 10.1007/BF03345318. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Higuchi T, Sawa K, Ohtaki S, Horiuchi Y. "Endemic coast goitre" in Hokkaido, Japan. Acta Endocrinol (Copenh) 1965;50:161–176. [PubMed] [Google Scholar]
- 31.Li M, Liu DR, Qu CY, Zhang PY, Qian QD, Zhang CD, Jia QZ, Wang HX, Eastman CJ, Boyages SC, Jupp JJ, Maberly GF. Endemic goitre in central China caused by excessive iodine intake. Lancet. 1987;2:257–259. [PubMed] [Google Scholar]
- 32.Zimmermann MB, Ito Y, Hess SY, Fujieda K, Molinari L. High thyroid volume in children with excess dietary iodine intakes. Am J Clin Nutr. 2005;81:840–844. doi: 10.1093/ajcn/81.4.840. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Fan C, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C, Teng W. A five-year follow-up study of goiter and thyroid nodules in three regions with different iodine intakes in China. J Endocrinol Invest. 2008;31:243–250. doi: 10.1007/BF03345597. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Xu W, Huang Y, Jin X, Deng J, Zhu S, Liu H, Zhang S, Yu Y. Associations of noniodized salt and thyroid nodule among the Chinese population: a large cross-sectional study. Am J Clin Nutr. 2013;98:684–692. doi: 10.3945/ajcn.112.054353. [DOI] [PubMed] [Google Scholar]
- 35.Bacher-Stier C, Riccabona G, Tötsch M, Kemmler G, Oberaigner W, Moncayo R. Incidence and clinical characteristics of thyroid carcinoma after iodine prophylaxis in an endemic goiter country. Thyroid. 1997;7:733–741. doi: 10.1089/thy.1997.7.733. [DOI] [PubMed] [Google Scholar]
- 36.Sehestedt T, Knudsen N, Perrild H, Johansen C. Iodine intake and incidence of thyroid cancer in Denmark. Clin Endocrinol (Oxf) 2006;65:229–233. doi: 10.1111/j.1365-2265.2006.02580.x. [DOI] [PubMed] [Google Scholar]
- 37.Harach HR, Williams ED. Thyroid cancer and thyroiditis in the goitrous region of Salta, Argentina, before and after iodine prophylaxis. Clin Endocrinol (Oxf) 1995;43:701–706. doi: 10.1111/j.1365-2265.1995.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 38.Pettersson B, Coleman MP, Ron E, Adami HO. Iodine supplementation in Sweden and regional trends in thyroid cancer incidence by histopathologic type. Int J Cancer. 1996;65:13–19. doi: 10.1002/(SICI)1097-0215(19960103)65:1<13::AID-IJC3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, Liu Y. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit. 2013;19:49–53. doi: 10.12659/MSM.883736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi J, Kim HS, Hong DJ, Lim H, Kim JH. Urinary iodine and sodium status of urban Korean subjects: a pilot study. Clin Biochem. 2012;45:596–598. doi: 10.1016/j.clinbiochem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Kweon SS, Shin MH, Chung IJ, Kim YJ, Choi JS. Thyroid cancer is the most common cancer in women, based on the data from population-based cancer registries, South Korea. Jpn J Clin Oncol. 2013;43:1039–1046. doi: 10.1093/jjco/hyt102. [DOI] [PubMed] [Google Scholar]
- 42.McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012;97:2682–2692. doi: 10.1210/jc.2012-1083. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Jiang Y, Shan Z, Teng W. Prolonged high iodine intake is associated with inhibition of type 2 deiodinase activity in pituitary and elevation of serum thyrotropin levels. Br J Nutr. 2012;107:674–682. doi: 10.1017/S0007114511003552. [DOI] [PubMed] [Google Scholar]
- 44.Shi L, Li Y, Guan H, Li C, Shi L, Shan Z, Teng W. Usefulness of serum thyrotropin for risk prediction of differentiated thyroid cancers does not apply to microcarcinomas: results of 1,870 Chinese patients with thyroid nodules. Endocr J. 2012;59:973–980. doi: 10.1507/endocrj.ej12-0154. [DOI] [PubMed] [Google Scholar]
- 45.Kassim IA, Ruth LJ, Creeke PI, Gnat D, Abdalla F, Seal AJ. Excessive iodine intake during pregnancy in Somali refugees. Matern Child Nutr. 2012;8:49–56. doi: 10.1111/j.1740-8709.2010.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartalena L, Bogazzi F, Braverman LE, Martino E. Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinol Invest. 2001;24:116–130. doi: 10.1007/BF03343825. [DOI] [PubMed] [Google Scholar]
- 47.Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE, Pino S, LaFranchi SH. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161:760–762. doi: 10.1016/j.jpeds.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama S, Mikeda T, Okada T, Nakamura K, Kotani T, Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14:1077–1083. doi: 10.1089/thy.2004.14.1077. [DOI] [PubMed] [Google Scholar]
- 49.Nohr SB, Laurberg P. Opposite variations in maternal and neonatal thyroid function induced by iodine supplementation during pregnancy. J Clin Endocrinol Metab. 2000;85:623–627. doi: 10.1210/jcem.85.2.6391. [DOI] [PubMed] [Google Scholar]
- 50.Rebagliato M, Murcia M, Espada M, Alvarez-Pedrerol M, Bolumar F, Vioque J, Basterrechea M, Blarduni E, Ramon R, Guxens M, Foradada CM, Ballester F, Ibarluzea J, Sunyer J. Iodine intake and maternal thyroid function during pregnancy. Epidemiology. 2010;21:62–69. doi: 10.1097/EDE.0b013e3181c1592b. [DOI] [PubMed] [Google Scholar]
- 51.Murcia M, Rebagliato M, Iniguez C, Lopez-Espinosa MJ, Estarlich M, Plaza B, Barona-Vilar C, Espada M, Vioque J, Ballester F. Effect of iodine supplementation during pregnancy on infant neurodevelopment at 1 year of age. Am J Epidemiol. 2011;173:804–812. doi: 10.1093/aje/kwq424. [DOI] [PubMed] [Google Scholar]


