Abstract
Further to reports of a reciprocal relationship between sugar and fat intakes, this review aimed to provide an in-depth analysis and to determine the likely influence of this relationship on the achievement of population dietary guidelines. Using systematic methods, relevant literature was selected according to preset criteria.
A strong and consistent inverse association was found between total sugars and total fat intakes expressed as percentage energy. Fewer studies considered absolute intakes and these reported a positive relationship, which may be influenced by confounding with energy intakes. Evidence for an inverse relationship between percentage energy from fat and extrinsic sugars was weaker and less consistent than for fat and total sugars. Reciprocal relationships were also observed for sugar-saturated fat, sugar−protein, sugar−alcohol, and sugar−starch expressed as percentage energy. Under-reporting of dietary intakes had no major influence on the findings.
This review confirms the existence of the sugar−fat seesaw on a percentage energy basis and concludes that it is most likely explained by a combination of mathematical and food compositional effects. This finding is relevant because dietary guidelines are expressed as percentage energy and implies that at the population level multiple guidelines may be difficult to achieve in practice.
Keywords: dietary guidelines, sugars, sucrose, alcohol, protein, starch
INTRODUCTION
A reciprocal relationship between the intake of sugars and fat has been previously reported from observational data (Committee on Medical Aspects of Food Policy, 1989; Gibney et al., 1995), an association termed the ‘‘sugar−fat seesaw.’’ It has been hypothesized that in freely chosen diets, reducing intake of energy from both fat and sugars to comply with dietary guidelines may be difficult to achieve at the population level (Gibney, 1990). Some data from intervention trials are consistent with this conclusion (West and De Looy, 2001).
The reciprocal relationship between sugars and fat has been mainly observed when nutrient intake is expressed as percentage of dietary energy (% E) and it is unclear whether the relationship holds on the basis of absolute intakes of sugars and fat. It is also unclear whether the relationship is influenced by any particular type of dietary sugars, e.g., extrinsic sugars. Other limitations to the current state of knowledge are whether the relationship is applicable across the general population, if there is any evidence of a threshold effect, if the relationship is influenced by any particular type of fatty acid, if there is a relationship between sugars and other macronutrients, and if there is any influence of particular food groups. The current review aimed to systematically examine these issues to help determine whether, in practice, simultaneous population reductions in sugar and fat intakes are likely to be successful.
In this review, ‘‘total sugars’’ refer to all monosaccharides (glucose, fructose, galactose) and disaccharides (sucrose, maltose, lactose). ‘‘Extrinsic sugars’’ refers to the terms ‘‘added sugars,’’ ‘‘refined sugars,’’ sucrose, sugar, and nonmilk extrinsic sugars (NMES). ‘‘Intrinsic sugars’’ refer to the sugars present in unprocessed foodstuffs such as fruit and vegetables and those termed ‘‘natural sugars.’’ ‘‘Milk sugar’’ refers to lactose, and the term ‘‘other sugars’’ invariably refers to a particular assessment of total sugars minus lactose. The lack of analytical data to determine intake of sugar subtypes means that the accuracy of such distinctions is poor.
METHODS
Research Question
This review addressed the following research question: “What is the evidence for an inverse relationship between dietary fat and sugars intake in populations freely selecting food and drink?” The scope of the review was observational studies of dietary intakes of healthy population groups (including children through to elderly people but excluding patient groups or people living in institutional care), from countries with cultural similarities to the UK (including the UK, Ireland, other European countries, USA, and Australasia). The key relationships investigated were total sugars and total fat, subtypes of sugars and total fat, sugars (all types) and fatty acids, and sugars (all types) and other macronutrients. The review also explored potential threshold effects, and whether the relationships were particular to any population groups or were influenced by underreporting or by specific food groups. Eligible data from intervention studies were considered in a separate analysis. Potential mechanisms were also considered.
Data Sources and Literature Search Strategy
Medline, Embase, and the Cochrane database of systematic reviews were searched for observational data and intervention studies using text terms with appropriate truncation and relevant indexing terms. The search was in the following form: ‘‘‘sugar terms’’ (e.g., sugars, sucrose) and fat and ‘‘relationship terms’’ (e.g., seesaw, reciprocal). Dates of publication were restricted to 1985 onward and only human studies in English language papers were eligible. Published government reports and reports of national survey data were included. The full list of search terms is detailed in Appendix A. One author (1) carried out the database searches in June 2010 together with a hand/internet search. Reference lists of retrieved studies were also scrutinized. Unpublished data were not included in the review.
Study Selection and Quality
Inclusion and exclusion criteria (Online Table 1) were agreed by the authors prior to reviewing the search results. Since valuable data on sugar−fat intake relationships were known to have been published in abstracts and letters, including analyses based on good quality data sets, these sources of data were included in the review, recognizing that they would not be accorded the same credibility as findings from full papers. To reduce confounding, eligible data were restricted to correlational analyses and analyses based on categorization (e.g., quartiles, quintiles) of either sugars or fat intakes. Hence, studies analyzed according to clusters or dietary indices based on food groups, studies with total carbohydrate rather than sugar categorization, and studies categorized on the basis of energy intake or energy density were excluded. Papers were also excluded if the fat or sugar intake data were not represented as total daily intakes (e.g., intake was only measured for certain meals or only for high-fat-sweetened food groups). Relevant intervention studies were considered separately and included those investigating dietary manipulation or advice to alter intake of sugars or fat, which was thus distinct from the primary research question relating to the free selection of food and drink. Though such studies may have been far smaller than many observational studies, they were important for understanding cause and effect, provided they were carefully controlled. Intervention studies that aimed to test manipulations in single foods or food groups, that did not allow subjects to freely select any final intake of macronutrients, or that followed a before-and-after design (with only one study group), were excluded.
One author (1) reviewed titles and abstracts from the search results and excluded clearly irrelevant records. Two investigators (1 and 3) each independently reviewed eligibility of 50% of the remaining potentially relevant records from the title, abstract, and as necessary the full paper. To assess consistency of decisions 20% of records were cross-checked by both reviewers and good agreement was found. Discrepancies were resolved by discussion with the third author (2). One investigator (1) extracted the key data from the qualifying papers using predefined data fields. The data were analyzed on the basis of statistical significance for sugar/fat intake relationships and were interpreted with regard to the study quality, and strength and consistency of the data. As proposed by Ryan (2007) quantitation of study quality was not considered feasible and was thus considered qualitatively within the tables and text by reference to the number of subjects, response rates, and proportion of drop outs (where reported), method of statistical analysis, method of dietary intake assessment and assessment of under-reporting. For intervention studies, other relevant quality markers included randomization and length of follow-up.
SEARCH RESULTS
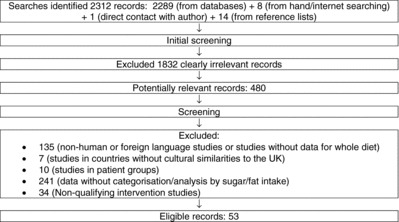
Papers reporting relationships between intakes of sugars and fat were potentially difficult to identify due to use of different terminologies. The search terms were thus deliberately comprehensive and identified a large number of potential records (2312). Initial screening identified 480 potentially relevant records, and of these 427 were excluded (Fig. 1) resulting in a final list of 53 eligible papers. This included 16 for the total sugar analysis, 24 additional papers for the sugar subtypes and fatty acids analyses, and 12 reports of intervention studies (11 intervention studies overall).
Figure 1 . Search results and study selection.
Risk of Bias
Assessment of dietary intake is prone to random and systematic sources of error whatever the method of data collection (Miller et al., 2008). The data are thus prone to a number of sources of bias including recall bias, responder bias, reporting bias, and social approval bias (Miller et al., 2008). Attrition bias may occur in prospective studies and interventions due to high participant dropout rates. Detection bias is possible in intervention studies due to the difficulty of blinding dietary studies. Reporting bias is possible where authors have only reported significant findings for sugar−fat intake relationships. Potentially, there are many data sets for which sugar−fat intake relationships have not been explored, though this does not mean that such relationships did not exist. Publication bias is unlikely to be a key factor as investigation of the relationship was not always the primary focus of studies.
STUDY RESULTS AND DATA REVIEW
The eligible data addressed many relationships between sugars or sugar subtypes and fat, other macronutrients or alcohol, though not always on a consistent basis. The quality of the data was also variable. Most analyses were expressed as %E with fewer analyses for absolute intakes. Other analyses were based on a mixed comparison between %E or energy adjusted intake of one nutrient and absolute intake of the other. Most analyses were for total or extrinsic sugars and only a few addressed intrinsic or milk sugars (Online Tables 2 and 3). Some intake relationships were investigated in only one or two studies and it was not possible to draw firm conclusions about all potential relationships (Table 1).
Table 1 . Overview of results of observational studies.
| Percentage energy |
Absolute intake |
Mixed measurement comparison |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Analysis | Inverse | NS | Positive | Inverse | NS | Positive | Inverse | NS | Positive |
| Total sugars vs. fat | |||||||||
| Correlation coefficient | 6 | 1 | |||||||
| Sugars/fat categorization | 7 | 1 | 2 | 1 | |||||
| Extrinsic sugars vs. fat | |||||||||
| Correlation coefficient | 5 | 2 | 1 | ||||||
| Sugars/fat categorization | 11 | 4 | 1 | 3 | 3 | 2 | |||
| Intrinsic sugars vs. fat | |||||||||
| Correlation coefficient | 2 | 1 | |||||||
| Sugar/fat categorization | 1 | 1 | |||||||
| Milk sugar vs. fat | |||||||||
| Correlation coefficient | 1 | 1 | |||||||
| Sugars/fat categorization | 2 | ||||||||
| ‘Other sugars’ type vs. fat | |||||||||
| Sugars/fat categorization | 1 | ||||||||
| Fatty acids vs. sugars categorization | |||||||||
| Saturated/Total sugars | 3 | ||||||||
| Saturated/Extrinsic | 2 | 2 | 1 | 1 | |||||
| Monounsaturated/Extrinsic | 5 | 1 | |||||||
| Polyunsaturated/Extrinsic | 5 | 1 | |||||||
| Macronutrients other than fat vs. sugars categorization (for studies showing inverse sugars/fat relationship) | |||||||||
| Protein/Total sugars | 2 | 2 | 2 | 1 | |||||
| Protein/Extrinsic sugars | 7 | 1 | 3 | 2 | |||||
| Protein/Intrinsic sugars | 1 | ||||||||
| Protein/Other sugars | 1 | ||||||||
| Carbohydrate/Total sugars | 1 | 1 | 1 | ||||||
| Carbohydrate/Extrinsic | 6 | 2 | 1 | 4 | |||||
| Carbohydrate/Other sugars | 1 | ||||||||
| Starch or CC/ Total sugars | 2 | ||||||||
| Starch or CC/Extrinsic | 2 | 1 | 1 | 1 | |||||
| Starch or CC/Intrinsic | 1 | 1 | |||||||
| Fibre/Total sugars | 1 | ||||||||
| Fibre/Extrinsic sugars | 3 | 1 | |||||||
| Fibre/Other sugars | 1 | 1 | 1 | ||||||
| Alcohol vs. sugars categorization (for studies showing inverse sugars/fat relationship) | |||||||||
| Alcohol/Total sugars | 1 | ||||||||
| Alcohol/Extrinsic sugars | 3 | 1 | 1 | 4 | |||||
| Alcohol/Intrinsic sugars | 1 | ||||||||
NS: Nonsignificant; CC: complex carbohydrate; (1): value is number of studies in category.
Associations Between Total Sugars and Total Fat Intakes
Percentage Energy Analyses
Studies that measured a correlation between total sugars and total fat intakes provided strong and consistent evidence for an inverse association when assessed on the basis of %E (Table 2). One result, published only as an abstract, provided an unrealistically high-correlation coefficient (Baghurst et al., 1988), hence the conclusion does not rely on this value. The evidence base included analyses of data from large surveys (Bolton Smith and Woodward, 1994; Macdiarmid et al., 1995) and studies using seven-day weighed intakes (Macdiarmid et al., 1995; Ruxton et al., 1996). The relationship held across all population groups studied including children, adolescents, and adults. Data presented by fat or sugars categorization (e.g., quartiles, quintiles) and expressed as %E (Table 3) also provided strong and consistent evidence for an inverse relationship between total sugars and total fat intakes, across all population groups studied (adults and children). The evidence included data from large surveys (Gibson, 1993; Baghurst et al., 1994) and studies using a seven-day weighed dietary intake (Gibson, 1993; Boulton and Magarey, 1995).
Table 2 . Correlation coefficients for total sugars and total fat intakes.
| Reference | Analysis | Correlation coefficient (r) for population group (P-value): |
|---|---|---|
| Measurement: % energy | ||
| (Baghurst et al., 1988) | Fat vs. simple carbohydrates (% total energy) | Adults: −0.97 (no statistical analysis)* |
| (Bolton Smith and Woodward, 1994) | Total sugars vs. fat (% total energy) | Men: −0.34 P < 0.001 Women: −0.39 P < 0.001 |
| (Macdiarmid et al., 1995) | Fat vs. sugars (% food energy) | Adults: −0.57 P < 0.001 |
| (Payne and Belton, 1992) | Total sugars vs. fat (% total energy) | Children: −0.63 P< 0.001 |
| (Ruxton, Kirk et al., 1996) | Fat vs. total sugars (% total energy) | Children: −0.65 P< 0.001 |
| (Ziegler et al., 2001) | Sugars vs. fat (% total energy) (alcohol not consumed) | Male adolescents: −0.54 P < 0.01 Female adolescents: −0.41 P< 0.01 |
| Measurement: g/day | ||
| (Macdiarmid et al., 1995) | Fat vs. sugars (g/day) | Adults: 0.37 P < 0.001 |
*Conclusions do not rely on this value, which is unrealistically high
Table 3 . Association between fat or total sugars categorization and total sugars or fat intakes.
| Reference | Fat/total sugars categorization | Total sugars/fat measurement | Method of statistical analysis | Association for population group |
|---|---|---|---|---|
| Measurement: % energy | ||||
| (Baghurst et al., 1994) | Fat quintile (% total energy) | Sugars (% total energy) | Chi-squared test on one dietary factor for linear trend | Adults: inverse P < 0.001 |
| (Boulton and Magarey, 1995) | Fat: 3 groupings (% energy) | Sugars (% energy) | ANOVA | Children: inverse P < 0.0001 |
| (Cullen et al., 2004) | Fat tertile (% total energy) | Sugars (% total energy) | Two-way ANOVA | Adults: inverse P < 0.001 |
| (Farris et al., 1998) | Sugars quartiles (g/1000 kcal) | Fat (% energy) Fat (g/1000kcal) | ANOVA; coefficients of sums of squares for trend analysis | Children: inverse P < 0.0001 Children: inverse P < 0.0001 |
| (Flynn et al., 1996) | Fat quartile (% total energy) | Sugars (% total energy) | Student t-test | Women: inverse P < 0.0001 |
| (Gibney and Lee, 1991) | Fat quartiles (% food energy) | Sugars (g/10 MJ food energy) | N/A | No statistical analysis—data suggest an inverse relationship |
| (Gibson, 1993) | Sugars tertile (% energy) | Fat (% energy) | ANOVA & multiple range test | Children: inverse P < 0.001 |
| (Rogers and Emmett, 2002) | Fat quartile (% energy) | Sugars (% energy) | One-way ANOVA | Children: inverse P < 0.001 |
| Measurement: g/day | ||||
| (Lenders et al., 1994) | Sugars above/below 90th percentile (g/day) | Fat (g/day) | Student's t-test | Adolescents: positive P < 0.001 |
| (Lenders et al., 1994) | Sugars above/below 90th percentile (g/day) adjusted for energy | Fat (g/day) | Student's t-test | Adolescents: inverse P < 0.001 |
| Other measurement | ||||
| (Gibney and Lee, 1991) | Fat quartiles (g/day) | Sugars (g/10 MJ food energy) | N/A | No statistical analysis—data suggest an inverse relationship in women and no relationship in men |
| (Gibson, 1993) | Sugars tertiles (g/day) | Fat (% energy) | ANOVA & multiple range test | Boys & girls 10–11 years: inverse P < 0.001 Boys 14–15 years: inverse P < 0.001 Girls 14–15 years: NS |
| (Nicklas et al., 1992) | Fat: 4 groupings (% energy) | Sugars (g/day) | Pairwise comparisons | Children: NS |
Absolute Intake and Other Analyses
There were few analyses based on absolute intakes (Lenders et al., 1994; Macdiarmid, et al., 1995) and these reported a positive correlation (Tables 2 and 3). However, one study adjusted for energy intake and the association became inverse (Lenders et al., 1994). In contrast, Rogers & Emmett (2002) commented that when using absolute nutrient intakes their results were extremely similar to the %E analysis, i.e., an inverse relationship, though the data were not reported in their paper.
The results of other analyses in which %E or energy adjusted intakes for fat or sugar were compared with absolute intakes of the other gave inconsistent results between studies (Table 3).
Associations Between Sugars Subtypes and Total Fat
Extrinsic Sugars: Percentage Energy Analyses
Studies that measured the correlation between extrinsic sugars and fat mostly showed statistically significant inverse associations on the basis of %E (Table 4). The evidence was weaker and less consistent than for total sugars and fat. Nevertheless, an inverse relationship was observed in all population groups investigated, including children, adolescents, and adults. Of three larger studies, two reported an inverse relationship (Bolton Smith and Woodward, 1994; Gibson, 1996) and one found no association (Drewnowski et al., 1997). Studies in children, adolescents, and adults analyzed by sugar or fat categorization also provided evidence for an inverse relationship between extrinsic sugars and fat on a %E basis (Table 5), though again the evidence was less consistent than for total sugars and fat. The exceptions included two studies (Haraldsdottir and Andersen, 1994; Drewnowski et al., 1997) that reported a lack of association in adults, and a larger study that reported an inverse association in men but not in women (Baghurst et al., 1994).
Table 4 . Correlation coefficients for sugars subtype and total fat intakes.
| Reference | Analysis | Correlation coefficient |
|---|---|---|
| Measurement: % energy | ||
| (Baghurst et al., 1988) | Total fat (% total energy) vs. natural sugars (% total energy) Total fat (% total energy) vs. refined sugars (% total energy) | Adults: c. r = −0.97* Adults: r ≤ −0.97* (no statistical analysis) |
| (Bolton Smith and Woodward, 1994) | Extrinsic sugars vs. fat (% total energy) Intrinsic sugars vs. fat (% total energy) Lactose vs. total fat (% total energy) | Men: −0.36 P < 0.001 Women: −0.29 P < 0.001 Men: NS Women: −0.20 P < 0.001 Men: 0.15 P < 0.001 Women: r < −0.10 |
| (Drewnowski et al., 1997) | Sucrose (% energy) vs. total fat (% energy) | Adults: r = −0.01 NS |
| (Garemo et al., 2007) | Sucrose (% energy) vs. total fat (% energy) | Children: r = −0.35 P < 0.0001 |
| (Haraldsdottir and Andersen, 1994) | Refined/industrial mono/disaccharides (% energy) vs. fat (% energy) | Men: −0.30 P < 0.05 Women: 0.04 NS |
| (Gibson, 1996) | Fat (% food energy) vs extrinsic sugars (% food energy) | Men: −0.43 P < 0.001 Women: −0.45 P < 0.001 Men most reliable records: −0.44 P < 0.001 Women most reliable records: −0.53 P < 0.001 |
| Measurement: g/day | ||
| (Drewnowski et al., 1997) | Sucrose (g/day) vs. total fat (g/day) | Adults: r = 0.51 P < 0.001 |
*Conclusions do not rely on these values, which are unrealistically high.
Table 5 . Association between sugars subtype or total fat categorization and intake of total fat or sugar subtype.
| Reference | Fat/sugar type categorization | Sugar type or fat measurement | Method of statistical analysis | Association for population group |
|---|---|---|---|---|
| Measurement: % energy | ||||
| (Alexy et al., 2003) | Added sugars quintiles (% energy) | Total fat (% energy) | Mixed linear model (PROC MIXED) | Children & adolescents: inverse P < 0.0001 |
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Total fat (% total energy) | Chi-squared test | Men & women: inverse P < 0.001 |
| (Baghurst et al., 1994) | Fat quintile (% total energy) | Natural sugars (% total energy) Added sugars (% total energy) | Chi-squared test on one dietary factor for linear trend | Men: inverse P < 0.001 Women: inverse P < 0.001 Men: inverse P < 0.01 Women: NS |
| (Drewnowski et al., 1997) | Added sucrose deciles (g/1000 kcal/day) | Fat (% total energy) | Stepwise regression analyses | Adults: NS |
| (Erkkola et al., 2009) | Added sucrose quartiles (% energy) | Total fat (% energy) | One-way ANOVA | Children: inverse P < 0.001 |
| (Gibney et al., 1987) | Fat: 3 groupings (% food energy) | Sugars (minus lactose) (% food energy) | N/A | Adults: inverse (no statistical analysis provided) |
| (Gibney et al., 1995) | Total sugars minus lactose: 3 groupings (g/1000 kcal) | Fat (g/1000 kcal) | One-way analysis of variance and series of Student–Newman–Keuls range tests | 1 year to 51+years: inverse P < 0.05 |
| (Gibson, 1996) | NMES quintiles (% energy) | Fat (% energy) | Multiple range test | Adults: inverse P < 0.05 |
| (Gibson, 1997) | NMES quintiles (% energy) | Fat (% energy) | One-way ANOVA & Bonferroni test | Children: inverse P < 0.0001 |
| (Haraldsdottir and Andersen, 1994) | Fat tertiles (% total energy) | Refined/industrial sugars (% total energy) | t-test | Adults: NS |
| (Kranz et al., 2005) | Added sugars: 5 groupings (% energy) | Fat (% energy) | Nonparametric test for trend (z score) | Children: inverse P < 0.001 |
| (Lewis et al., 1992) | Added sugars tertiles (% total energy) | Fat (% total energy) | Bonferroni test | Children & adults: Trend for high added sugar consumers to have lower % energy from fat (no statistical analysis provided) |
| (Parnell et al., 2008) | Fat quartiles (% total energy) | Sucrose (% total energy) | Linear regression | Children, men & women: inverse P < 0.001 |
| (Rogers and Emmett, 2002) | Fat quartiles (% energy) | NMES (% energy) | One-way ANOVA | Children: inverse P < 0.001 |
| (Ruottinen et al., 2008) | Sucrose: 3 groupings (% energy) | Fat (% energy) | ANOVA, F-test | Children: NS |
| (Tonstad and Sivertsen, 1997) | Fat quartiles (% energy) | “Sugar” (% energy) assume not total sugars | Unpaired t-test & ANOVA | Children: inverse P < 0.0001 |
| (Ylönen et al., 1996) | Fat: 4 groupings (% energy) | Sucrose (% energy) | One-way ANOVA & Bonferroni test | Children: inverse P < 0.001 |
| Measurement: g/day | ||||
| (Linseisen et al., 1998) | Sucrose intake (not energy adjusted) (g/day) | Fat (g/day) | Multiple regression analysis | Children & adults: positive P< 0.001 |
| (Linseisen et al., 1998) | Sucrose intake (energy adjusted) (g/day) vs. fat (g/day) | Fat (g/day) | Multiple regression analysis | Children & adults: inverse P < 0.001 |
| (Gibney and Lee, 1989, Gibney et al., 1989) | Fat above/below 120 g/day | Sugars excluding lactose (g/day) | N/A | Men: inverse (no statistical analyses provided) |
| Other measurements | ||||
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Total fat (g/day) | Chi squared test | Men: positive P < 0.05 Women: positive P < 0.01 |
| (Cade and Booth, 1990) | Refined sugars above/below 12% total energy | Fat (g/day) | SPSS | Men: inverse P < 0.05 Women: NS |
| (Drewnowski et al., 1997) | Added sucrose deciles (g/1000 kcal/day) | Fat (g/day) | Stepwise regression analyses | Adults: positive P < 0.0001 |
| (Flynn et al., 1996) | Fat quartiles (% total energy) | Fruit sugars (g/day) Vegetable sugars (g/day) Breads and cereals sugars (g/day) Milk sugars (g/day) Total added sugars (g/day) | Mann–Whitney U-test | Women: NS Women: NS Women: NS Women: NS Women: inverse P = 0.009 |
| (Gibney and Lee, 1989a, Gibney et al., 1989b) | Fat 3 groups (% food energy) | Sugars excluding lactose (g/day) | N/A | Men: inverse Women: no change (no statistical analyses provided) |
| (Lewis et al., 1992) | Added sugars tertiles (g/kg body wt) | Fat (% total energy) | Bonferroni test | Children & adults: Trend for high added sugar consumers to have lower % energy from fat (no statistical analysis provided) |
| (Nicklas et al., 1992) | Fat: 4 groups (% energy) | Sucrose (g/day Lactose (g/day) | Pairwise comparisons | Children: NS Children: NS |
| (Räsänen and Ylönen, 1992) | Fat: 4 groupings (% total energy) | Sucrose (g/day Lactose (g/day) | One-way ANOVA & Bonferroni test | Children: NS Children: NS |
| (Ruottinen et al., 2008) | Sucrose: 3 groupings (% energy) | Fat (g/day) | ANOVA, F-test | Children: NS (data not provided) |
Extrinsic Sugars: Absolute Intake and Other Analyses
One study investigated the correlation between sucrose and total fat intake in adults on the basis of absolute intakes and reported a positive association (Table 4). The data were from a large survey (n = 837) using the dietary history method (Drewnowski et al., 1997). Additionally, a positive association was reported between extrinsic sugars and fat on the basis of sucrose percentiles in both children and adults (Linseisen et al., 1998). However, when adjusted for energy intakes the relationship was inverse (Table 5).
The results of other analyses for extrinsic sugars and fat in which %E or energy adjusted intake of one nutrient was compared with absolute intake of the other were inconsistent. Most studies in children reported a nonsignificant association, while positive and inverse relationships were reported for adults (Table 5).
Intrinsic Sugars, Lactose, or “Other Sugars”
Fewer studies investigated correlations between intrinsic sugars and fat intakes (Table 4), one reporting an inverse association in women but not in men, and a positive relationship between total fat and lactose intakes in men and an inverse relationship in women (Bolton Smith and Woodward, 1994). For analyses by sugars or fat categorization (Table 5), one study investigated intakes of natural sugars and fat and reported an inverse association in men and women on a %E basis (Baghurst et al., 1994). A consistent inverse relationship was also reported for all age groups from one year upward (Gibney et al., 1995) based on a large analysis of intakes of total sugars excluding lactose and fat assessed by three-day records. In this study, the potential for a threshold effect was explored, and it was reported that a greater proportion of low-sugar consumers tended to consume above 30% energy from fat and more than 10% energy from saturated (SFA) and monounsaturated fatty acids (MUFA) than was consumed at higher levels of sugar intake.
No analyses addressed absolute intakes of intrinsic sugars, lactose or ‘‘other sugars,’’ and fat intakes. A small study in women, based on a seven-day diet history reported a lack of significant association for absolute intakes of fruit sugars and vegetable sugars with %E from fat (Flynn et al., 1996) and a study in children reported no association between fat intake and lactose (Räsänen and Ylönen, 1992).
Hence overall, no firm conclusions can be drawn about the relationship between intrinsic sugars and fat intakes.
Associations Between Sugar Intakes and Fatty Acids
Analyses for Total Sugars and Fatty Acids
A study in children based on 24-hour dietary recall reported a strong inverse association with SFA on an energy-adjusted basis (Table 6). In three further studies, a consistent inverse association was reported for %E from total sugars and SFA in children, adolescents, and women, though no statistical analysis was reported for the study in women (Flynn et al., 1994). Overall, this provides consistent evidence of an inverse association on the basis of %E between total sugars and SFA intakes. No studies addressed the relationships between total sugars and MUFA or polyunsaturated fatty acids (PUFA). There was insufficient evidence to address the relationship on the basis of absolute intakes. One study published only as an abstract (Flynn et al., 1994) reported an inverse association between SFA and total sugars, but no statistical analysis was provided.
Table 6 . Association between sugars categorization (any type) and fatty acid intake.
| Association with fatty acid (% energy or energy-adjusted basis) for population group |
||||||
|---|---|---|---|---|---|---|
| Method of | ||||||
| Reference | Sugar type categorization | statistical analysis | Saturated/Trans | Monounsaturated | Polyunsaturated | |
| (Farris et al., 1998) | Total sugars quartiles (g/1000 kcal) | Analysis of variance; coefficients of sums of squares for trend analysis | Children (g/1000 kcal): inverse P = 0.0001; negative linear trend P < 0.0001 & negative quadratic trend P < 0.05 | |||
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Chi-squared test | Men & Women: NS | Men & Women: negative linear trend P< 0.001 | Men & Women: negative linear trend P < 0.001 | |
| (Erkkola et al., 2009) | Added sucrose quartiles (% energy) | One-way ANOVA | Children (saturated + trans): NS | Children: inverse P < 0.001 | Children: inverse P < 0.001 | |
| (Gibson, 1996) | NMES quintiles (% food energy) | Pearson Correlation Coefficient | Men & women: inverse P < 0.001 Trans fatty acids men: NS; women: inverse P < 0.01 | Men & women: inverse P < 0.001 | Men & women: inverse P < 0.001 | |
| (Gibson, 1996) | NMES quintiles (% food energy) | Pearson correlation coefficient | Most reliable records Men: inverse P < 0.01; Women: inverse P < 0.001 Trans fatty acids men: NS; women: inverse P < 0.05 | Most reliable records Men & women: inverse P < 0.001 | Most reliable records Men & women: inverse P < 0.001 | |
| (Gibson, 1996) | NMES quintiles (% food energy) | Multiple range test | Men & women: inverse P < 0.05 Trans fatty acids–Men & women: NS | Men & women: inverse P < 0.05 | Men & women: inverse P < 0.05 | |
| (Linseisen et al., 1998) | Sucrose percentiles (g/day) energy adjusted) | Multiple regression analysis model: unpaired t-test | g/day (energy adjusted) Children & adults: inverse P< 0.001 | g/day (energy adjusted) Children & adults: inverse P < 0.001 | g/day (energy adjusted) Children & adults: inverse P < 0.001 | |
| (Ruottinen et al., 2008) | Sucrose low/medium/high (% energy) | ANOVA, F-test | Children: positive P = 0.009 | Children: inverse P = 0.03 | Children: inverse P = 0.0001 | |
| Gibney et al., 1995) | Total sugars minus lactose 3 groupings (g/1000 kcal of total energy) | One-way analysis of variance and series of Student– Newman–Keuls range tests | g/1000 kcal (of total energy) Children & adults: inverse P ≤ 0.05 | |||
| Association with fatty acid (g/day) for population group |
||||||
| Saturated |
Monounsaturated |
Polyunsaturated |
||||
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Chi-squared test | Men & Women: positive linear trend P < 0.001 | Men & Women: positive linear trend P < 0.05 | Men: negative linear trend P < 0.01; Women: NS | |
| Association with sugar/s intake for population group |
||||||
| Saturated fat categorization | Method of statistical | Total sugars | NMES | Total sugars | ||
| (% energy) |
analysis |
(% energy) |
(% energy) |
(g/day) |
NMES (g/day) |
|
| (Flynn et al., 1994) | Quartiles (% total energy) | No statistical analysis | Women: inverse | Women: inverse | Women: inverse | Women: inverse |
| (Matthys et al., 2006) | Tertiles (% energy) | Mann–Whitney U-test | Adolescents: inverse P = 0.000 | |||
| (Ruxton et al., 1996) | Correlation coefficient: total sugars (% energy) vs. saturated fat (% energy) | Pearson's correlation coefficient | Children: inverse r = −0.24 P < 0.01 | |||
Where indicated; NMES: nonmilk extrinsic sugars.
Analyses for Extrinsic Sugars and Fatty Acids
The relationship between intakes of extrinsic sugars and SFA (with or without trans fatty acids) expressed as %E, was inconsistent and there was no clear pattern for different population groups (Table 6). In contrast, associations on a %E basis for extrinsic sugars and both MUFA and PUFA showed consistently strong inverse associations across all population groups studied including children and adults.
No analyses addressed absolute intakes of extrinsic sugars and fatty acids. One large study in adults based on %E from added sugars and absolute intakes of fatty acids found a positive association for both SFA and MUFA and a negative association for PUFA in men and a nonsignificant association in women (Baghurst et al., 1992).
Associations Between Sugars and Other Macronutrients or Alcohol
Overall, on a %E basis (Table 7), the data support an inverse association between total or extrinsic sugars and protein. Based on fewer studies, the data also suggest an inverse association between total or extrinsic sugars and starch, and an inverse association between extrinsic sugars and fiber on a %E basis. There is also consistent evidence for an inverse association between extrinsic sugars and alcohol intakes.
Table 7 . Association between sugars categorization (any type) and intake of macronutrients (other than fat) and alcohol (% energy).
| Reference | Sugars classification | Statistical Test | Energy intake | Protein (% energy) | Carbohydrate (% energy) | Carbohydrate type (% energy) | Alcohol (% energy) |
|---|---|---|---|---|---|---|---|
| Total sugars | |||||||
| (Bolton Smith and Woodward, 1994) | Total sugars (% total energy) | Spearman rank correlation coefficients (two-tailed significance levels) | Men: positive P < 0.001 Women: positive r < 0.10 | Men & Women: inverse P < 0.001 | Starch Men & Women: inverse P < 0.001 | Men & Women: inverse P < 0.001 | |
| (Farris et al., 1998) | Total sugars quartiles (g/1000 kcal) | Analysis of variance; coefficients of sums of squares for trend analysis | Children: NS | Children inverse P = 0.0001 (g/1000 kcal): inverse P = 0.0001 (significant linear trend (< 0.0001) | Children: positive P = 0.0001 (g/1000 kcal): positive P = 0.0001 (significant linear trend P < 0.0001) | Children Dietary Fibre NS Starch(g/1000 kcal): inverse P = 0.001 (significant linear trend P < 0.0001) | |
| Extrinsic sugars | |||||||
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Chi-squared test | Men & Women: positive linear trend P < 0.001 | Men & Women: negative linear trend P < 0.001 | Men & Women: positive linear trend P < 0.001 | Complex Carbohydrate Men & Women: negative linear trend P < 0.001 | Men & Women: negative linear trend P < 0.001 |
| (Bolton Smith and Woodward, 1994) | Extrinsic sugars (% total energy) | Spearman rank correlation coefficients (two-tailed significance levels) | Men & Women: positive P < 0.001 | Men & Women: inverse P < 0.001 | Starch Inverse P < 0.001 | Men: NS Women: inverse P < 0.001 | |
| (Committee on Medical Aspects of Food Policy, 1989) | NMES tertiles (g/1000 kcal) | Method not provided | Men & Women: NS | g/1000 kcal Men & Women: inverse P < 0.01 | g/1000 kcal Men & Women: positive P < 0.001 | g/1000 kcal Men & Women: NS | |
| (Erkkola et al., 2009) | Added sucrose quartiles (% energy) | One-way ANOVA | Children: NS | Children: inverse P < 0.001 | Children: positive P < 0.001 | Fiber Children: inverse P < 0.001 | |
| (Kranz et al., 2005) | Added sugars: five groupings (% energy) | Nonparametric test for trend (z score) | Children 2–3 years: positive P = 0.01; 4–5 years: NS | Children: inverse < 0.001 | Children: positive < 0.001 | Fiber Children: inverse < 0.001 | |
| (Lewis et al., 1992) | Added sugars tertiles (% total energy) | Bonferroni test | Children & adults: positive P < 0.01 for low vs. moderate consumers | Children & adults: positive trend (no statistical analysis) | |||
| (Ruottinen et al., 2008) | Sucrose: three groupings (% energy) | Repeated measures ANOVA F-test | Children: NS | Children: inverse (P < 0.001) | Children: positive (P < 0.001) | Dietary Fibre Children: inverse (P < 0.001) | |
| Intrinsic sugars | |||||||
| (Bolton Smith and Woodward, 1994) | Intrinsic sugars (% total energy) | Spearman rank correlation coefficients (two-tailed significance levels) | Men & Women: inverse P < 0.001 | Men & Women: positive P < 0.001 | Starch Men: positive P < 0.001 Women: NS | Men: inverse P < 0.001 Women: inverse r < 0.10 | |
NMES: Nonmilk extrinsic sugars.
Two studies found a positive association between total sugars and protein measured as absolute intakes (Table 8), which after adjusting for energy became inverse. Only one analysis considered absolute intakes of total sugars and total carbohydrate and reported a positive relationship.
Table 8 . Association between sugars categorization (any type) and intake of energy, macronutrients (other than fat) and alcohol (g/day).
| Reference | Sugars categorization | Method of statistical analysis | Energy | Protein (g/day) | Carbohydrate (g/day) | Carbohydrate type (g/day) | Alcohol (g/day) |
|---|---|---|---|---|---|---|---|
| Total sugars | |||||||
| (Lenders et al., 1994) | Total sugars low/high (g/day) | Students t-test | Adolescents: positive P < 0.001 | Adolescents: positive P < 0.001 | Adolescents: positive P < 0.001 | ||
| (Gibson, 1993) | Total sugars tertiles (g/day) | ANOVA & multiple range test | Children: positive P < 0.001 | Children: positive P < 0.001 | |||
| (Gibson, 1993) | Total sugars tertiles (% food energy) | ANOVA & multiple range test | Children: positive P < 0.001 boys & girls 10–11 years; P < 0.05 boys 14–15 & P < 0.01 girls 14–15 years | Children: inverse P < 0.001 boys 10–11 years; P < 0.05 girls 10–11 years; NS boys & girls 14–15 years | |||
| (Lenders et al., 1994) | Total sugars low/high (g/day) adjusted for dietary energy | Least-squares-means values | Adolescents: inverse P < 0.001 | Adolescents: positive P < 0.001 | |||
| Extrinsic sugars | |||||||
| (Lewis et al., 1992) | Added sugars tertiles (g/kg body wt) | Bonferroni test | Children & adults: positive P < 0.01 | Children & adults: positive trend (no statistical analysis) | |||
| (Linseisen et al., 1998) | Sucrose percentiles (g/day) energy unadjusted | Multiple regression analysis model: unpaired t-test | Children & adults: positive P < 0.001 | Children & adults: positive P < 0.001 | Children & adults: positive P < 0.001 | Polysaccharides Children & adults: positive P < 0.001 | Inverse P < 0.001 |
| (Baghurst et al., 1992) | Added sugars deciles (% total energy) | Chi-squared test | Men & Women: positive linear trend P < 0.001 | Men & Women: NS | Men & Women: positive linear trend P < 0.001 | Complex Carbohydrate Men & Women: NS | Men & Women: negative linear trend P < 0.001 |
| (Committee on Medical Aspects of Food Policy, 1989) | NMES tertiles (g/1000 kcal) | Method not provided | Men & Women: NS | Men: NS; Women: inverse P < 0.05 | Men: NS; Women: positive P < 0.001 | Men: NS; Women: inverse P < 0.05 | |
| (Drewnowski et al., 1997) | Sucrose deciles (g/1000 kcal of total energy) | Stepwise regression analyses | Men & Women: positive P < 0.0001 | Men & Women: inverse P < 0.0001 | |||
| (Linseisen et al., 1998) | Sucrose percentiles (g/day) energy adjusted) | Total sugar low/high (g/day) | Children & adults: inverse P < 0.001 | Children & adults: positive P < 0.001 | Polysaccharides Children & adults: inverse P < 0.001 | Inverse P < 0.001 | |
| (Rasmussen et al., 1998) | Added sugars quintiles (% energy) | No statistical analysis provided | Children: constant across quintiles | Children: inverse | Children: positive | Fiber Children: inverse | |
| Other sugars measurement | |||||||
| (Gibney et al., 1995) | Total sugars minus lactose, three groupings (g/1000 kcal total energy) | One-way analysis of variance and series of Student–Newman– Keuls range tests | Children & adults: inverse P ≤ 0.05 | Children & adults: positive P ≤ 0.05 | Fiber Negative & positive P < 0.05 or NS with age | ||
NMES: Nonmilk extrinsic sugars.
Only one study investigated the relationship between intakes of intrinsic sugars and macronutrients other than fat (Bolton Smith and Woodward, 1994) and reported (%E basis) a positive association with protein, a positive association with starch in men only, and an inverse association with alcohol (Table 7).
Starch–Fat Relationship
The relationship between starch and fat is also of relevance, but there was inconclusive evidence to support a starch−fat seesaw expressed as %E (data not shown), with some studies reporting no association (Gibney and Lee, 1991; Baghurst et al., 1994; Flynn et al., 1996; Cullen et al., 2004), an inverse association (Bolton Smith and Woodward, 1994; Rogers and Emmett, 2002) and one study reporting inconsistent findings in children (Boulton and Magarey, 1995).
Under-Reporting and Threshold Effects
Most of the studies did not consider or did not exclude potential under-reporters of dietary intakes. In other studies, the exclusion of under-reporters or subjects on weight-loss diets had no significant effect on the results (Bolton Smith and Woodward, 1994; Haraldsdottir and Andersen, 1994; Macdiarmid et al., 1995; Flynn et al., 1996; Gibson, 1996; Rogers and Emmett, 2002; Alexy et al., 2003; Cullen et al., 2004). Few studies addressed the potential for threshold effects and there was no evidence that a threshold explained inconsistencies in the results.
Influence of Food Group
A variety of measures were used to assess the contribution of food groups to sugar and fat intakes, including daily or weekly food intake, food intake adjusted for energy, grams sugars contributed by a particular food group, contribution of the food group to sugar intake, and servings of food per day, while one study also addressed the issue of using intakes only for consumers of the food as well as for all participants, which made it difficult to compare studies. Flynn et al (1996) identified nonfat confectionery and drinks as the most important food sources in terms of contribution to the reciprocal relationship between fat and sugar. The difference in sugar intakes between the low- and high-fat groups was 53 g per day of which 76% was contributed by added sugars and 23% by natural sugars (Flynn et al., 1996), the latter being derived from foods containing a much lower concentration of sugars compared with jam and table sugar. Overall, in studies supporting the existence of the sugar–fat seesaw, sugar intakes were positively associated and fat intakes were inversely associated with table sugars and preserves and other major sources of sugars.
Intervention Studies
Eligible intervention studies investigated the impact of dietary manipulation or dietary advice to alter sugar and/or fat intakes, though this was not always the primary aim of the study (Online Table 5). Different study designs were used, ranging from studies investigating the addition of sugar to the usual diet to those investigating weight-loss interventions. In all but one study results were on a %E basis and seven studies provided data as absolute intakes.
In studies that investigated reductions in sugars and/or fat, one study compared reduced-fat and reduced-sugar (RFRS) or reduced-fat with-sugar (RFwS) groups against control (Drummond and Kirk, 1998; Drummond and Kirk, 1999). Both intervention groups successfully reduced %E from fat at six weeks and six months (RFRS: 39% energy at baseline, 33.7% at 6 m; RFwS: 40.2% energy at baseline, 32.2% at 6 m). At six weeks %E from NMES was reduced in the RFRS group with no change in total sugar intake, but this reduction was not sustained at six months. In the RFwS group, %E intakes from total sugar and NMES were increased at six weeks but not at six months. Hence, while the RFRS group successfully reduced %E from fat they failed to maintain a reduced-sugar intake for the longer term, a finding consistent with the sugar−fat seesaw hypothesis. On a %E basis carbohydrate intakes were increased in both groups at six months, consistent with the aims of the dietary advice. On the basis of absolute intakes (Drummond and Kirk, 1999), a fall in total sugar and NMES was observed in the RFRS group at six weeks with a fall only in NMES at six months (mean decrease 22.8 g/day). There was no change in sugar or NMES in the RFwS group, and no change in absolute intakes of carbohydrate at six months in either group. Hence, a fall in both fat and extrinsic sugars was sustained when measured as absolute intakes, which is inconsistent with the hypothesis. In a further study, both groups aimed to reduce fat intake, but there was no significant change in either the reduced-fat high-carbohydrate group or the RFRS group on either a %E basis or absolute intakes (Drummond et al., 2003). The RFRS group was successful in reducing %E from NMES and absolute intakes of total sugars and hence advice to reduce both fat and sugar resulted in a decrease in sugar only, which was consistent with the sugar–fat seesaw hypothesis. In a weight-loss study (West and De Looy, 2001), the nutritional aims of the low-fat sugar-containing (LFSC) diet were achieved with no change observed in sucrose intake and a fall in fat intake (by 6% of food energy). However, in the traditional weight-loss diet group that aimed to reduce intake of both sucrose and fat (LFLS) only a reduction in sucrose was achieved (4.1% food energy) with no change in fat intake. Hence consistent with the sugar−fat seesaw hypothesis, this study also demonstrated difficulty in achieving simultaneous reductions in both fat and sucrose on a %E basis.
In a study based on freely selected commercial low-fat or low-sugar foods (Gatenby et al., 1997), fat intake was reduced significantly in the reduced-fat group (37% energy at baseline to 33% at 10 weeks) compared with controls and with the reduced-sugar group, but this was not related to any consistent directional change in sucrose intake. In the reduced-sugar group energy from fat rose by 2% compared with baseline. This suggested that the reduced-sugar group was successful in reducing energy from sugar and sucrose intake at the expense of a small rise in energy from fat, which was consistent with the sugar–fat seesaw hypothesis on a %E basis, though absolute intakes of fat may have been unchanged. In a study rigorously controlling dietary manipulation (Saris et al., 2000), the reduced-fat high-complex carbohydrate group was successful in reducing %E from both fat and sugar. However, the reduced-fat high-sugar intake group achieved a greater reduction in fat on a %E basis compared with the reduced-fat high-complex carbohydrate group, though not on the basis of absolute intakes.
In eligible studies that compared supplementary intake of sugar or sucrose against sweetener or fat, foods were provided for part or for the whole diet on an ad libitum basis. One study compared the incorporation of supplementary sucrose-sweetened or artificially sweetened drinks and foods every day (Raben et al., 2002). There was a significant increase in sucrose intake in the sucrose group (to 28% energy) and a fall in sucrose intake in the sweetener group on both a %E and absolute intake basis. In the sucrose group, the increase in sucrose was accompanied by a fall in fat intake from 33 to 28% energy, but not when assessed by absolute intakes. There were no significant changes in fat intake in the sweetener group (34% energy) by either measurement basis. It was concluded that adding or replacing sugar from the diet was independent of fat intake. The changes were thus consistent with the sugar−fat seesaw hypothesis on a %E basis, but not on the basis of absolute intakes.
A well-designed study that compared supplementary sucrose-sweetened drinks (105 g carbohydrate per day) with diet-drinks in normal weight women eating a low-fat diet reported a greater fall in fat intake in the sucrose group versus baseline than in the sweetener group, on both a %E and absolute intake basis (average fall of 6g per day). There was no change in carbohydrate intake in the sweetener group and an increase in the sucrose group (half the content of the sucrose supplement). Hence this study supported an inverse relationship between sucrose and fat intakes on both a% E basis and absolute intakes (Reid et al., 2007). In a dose-response study using a within-subject repeated-measures design (Mazlan et al., 2006) snacks providing additional sugar resulted in a decrease in %E from fat and no change in absolute intakes when overall intake inclusive of the snacks was considered. When intake was assessed exclusive of the mandatory snacks, absolute intakes of fat were decreased in overweight subjects though not in lean subjects, and there was no change in %E from fat in either group. This demonstrated that, on a %E basis for total consumption, additional sugar resulted in a proportional decrease in energy from fat, but not on the basis of absolute intakes.
Overall on a %E basis the results of all but two studies (Burke et al., 1998; Saris et al., 2000) showed some consistency with the sugar−fat seesaw (Table 9), while on the basis of absolute intakes three studies supported (Naismith and Rhodes, 1995; Drummond et al., 2003; Reid et al., 2007) and four studies did not support the seesaw (Drummond and Kirk, 1999; Saris et al., 2000; Raben et al., 2002; Drummond et al., 2003; Mazlan et al., 2006)
Table 9 . Summary of results of intervention studies.
| Reference | Change in intake of fat and/or sugars | Change in energy intake |
|---|---|---|
| (Burke et al., 1998) | % Energy No significant changes at end of intervention or follow-up | |
| (Drummond and Kirk, 1998; Drummond and Kirk, 1999); | % Food Energy 6wk vs. baseline: RFRS: Reduction in fat P < 0.05; NS change in total sugars; reduction in NMES P < 0.05. RFwS: Reduction in fat P < 0.05; Increase in total sugars P < 0.05; NS change in NMES; Control: NS changes in fat, total sugars or NMES | Total energy: 6wk vs. baseline: RFRS: Reduction P < 0.05 RFwS: Reduction P < 0.05 Control: NS change |
| 6m vs. baseline: RFRS: Reduction in fat P < 0.05; NS change in total sugars or NMES; RFwS: Reduction in fat P < 0.05; NS change in total sugars or NMES; Control: NS changes in fat, total sugars or NMES | 6 months vs. baseline: RFRS: Reduction P < 0.05 RFwS: NS change Control: NS change | |
| Absolute Intakes 6wk vs baseline: RFRS: Reduction in fat P < 0.05; Reduction in total sugars and NMES P < 0.05. RFwS: Reduction in fat P < 0.05; NS change in total sugars or NMES. Control: NS changes in fat, total sugars or NMES | ||
| 6m vs baseline: RFRS: Reduction in fat P < 0.05; No significant change in total sugars; Reduction in NMES P < 0.05 RFwS: Reduction in fat P < 0.05; No significant change in total sugars or NMES; Control: NS changes in fat, total sugars or NMES | ||
| (Drummond et al., 2003) | % Energy RF: No significant change in fat or NMES RFRS: No significant change in fat; decrease in NMES P < 0.02 | RF: No significant change RFRS: No significant change |
| Absolute Intakes RF: No significant change in fat or NMES RFRS: No significant change in fat; decrease in sugars P < 0.05 | ||
| (Gatenby et al., 1997) | % Energy 10wk vs. baseline: RF group: Reduction in fat P = 0.017; RF, RS, & control groups: Reduced total sugars intake vs. baseline P = 0.001 with no between group differences RS group: Increase in fat P < 0.05; Reduction in sucrose vs. control and vs. RF group (P < 0.05) Control group: increase in fat (P < 0.05) | Significant time effect for reduction in energy intake in RF, RS and Control groups (P < 0.001); No group effects |
| (Mazlan et al., 2006) | % Energy inclusive of mandatory snacks Sugar increments: decrease in fat (P < 0.001) | Kcal inclusive of mandatory snacks Sugar increments: increase P < 0.016 |
| % Energy exclusive of mandatory snacks Sugar increments: no change in fat | Kcal exclusive of mandatory snacks Sugar increments: no change | |
| Kcal inclusive of mandatory snacks Sugar increments: No change in fat | ||
| Kcal exclusive of mandatory snacks Sugar increments: no change in fat | ||
| (Naismith and Rhodes, 1995) | Displacement of sugar with sweetener: increased fat kcal P < 0.001 Replacement of sweetener with sugar: NS change in fat kcal | Displacement of sugar with sweetener: decrease in energy P < 0.02 Replacement of sweetener with sugar: increase in energy P < 0.05 |
| (Raben et al., 2002) | 10wk vs. baseline: % Energy High sucrose group: Increase in sucrose P < 0.0001 vs. sweetener group, P < 0.05 vs. baseline; Decrease in fat P < 0.01 vs. sweetener group, P < 0.05 vs. baseline Sweetener group: decrease in sucrose P < 0.0001 vs. sucrose group, P < 0.05 vs. baseline; NS change in fat | High sucrose group: increase P < 0.0001 vs. sweetener group, P < 0.05 vs. baseline; Sweetener group: NS change |
| Absolute Intakes High sucrose group: increase in sucrose P < 0.0001 vs. sweetener group, P < 0.05 bs baseline; NS change in fat; Sweetener group: decrease in sucrose P < 0.0001 vs. sucrose grp, P < 0.05 vs. baseline; NS change in fat | ||
| (Reid et al., 2007) | 4wk vs. baseline: % Energy Sucrose drinks: decrease in fat P < 0.001 Drinks with sweetener: decrease in fat P < 0.001 Greater decrease in fat in group receiving sucrose drinks P < 0.001 | 4wk vs. baseline: Sucrose drinks: increase P < 0.001 of approximately half the energy value of the supplementary drinks Drinks with sweetener: decrease P < 0.05 |
| g/day Sucrose drinks: decrease in fat intake Drinks with sweetener: no change | ||
| (Saris et al., 2000) | % Energy LFHS: decrease in fat P < 0.05 and increase in sugars P < 0.0001 vs. LFHC group; P < 0.001 for decrease in fat and increase in sugars vs. control LFHC: decrease in fat P < 0.001 and sugars P < 0.01 vs. control | LFHS: decrease LFHC: decrease Control: no significant change |
| g/day LFHS: Decrease in fat P < 0.001 vs. control; increase in sugars P < 0.001 vs. LFHC group and control LFHC: Decrease in fat P < 0.001 and sugars P < 0.01 vs. control | ||
| (Vandongen et al., 1995) | % Energy 9m vs. baseline: Boys: Change in sugars vs. change in total fat: r = −0.5261 P < 0.0001 Girls: change in sugars vs. change in total fat: r = −0.5437 P < 0.0001 | 9m vs. baseline Boys & Girls: NS change |
| (West and De Looy, 2001) | % Total Energy 8wk vs. baseline: LFSC: nonsignificant change in sucrose; reduction in fat (P < 0.05) LFLS: reduction in sucrose (P < 0.001); nonsignificant change in fat | Total energy Reduced in LFLS and LFSC groups with no significant difference between groups |
NS: nonsignificant; NMES: nonmilk extrinsic sugars; RFRS: reduced-fat and reduced-sugar; RFwS: reduced-fat with-sugar; LFSC: low-fat sugar-containing; LFLS: low-fat low-sugar; LFHS: low-fat high-sugar; LFHC: low-fat high-complex carbohydrate.
DISCUSSION
Following reports from observational data of a reciprocal relationship between sugars and fat intakes it has been suggested that in freely chosen diets, it may not be feasible at the population level to reduce intake of energy from both sugars and fat to comply with dietary guidelines. This reciprocal relationship has been mainly observed when intake of sugars and fat are expressed on a %E basis. The aim of this review was to explore more fully the relationship between the intake of sugars and fat and to determine if a reciprocal relationship also holds on the basis of absolute intakes of sugars and fat and whether the relationship is influenced by any particular type of dietary sugars or fatty acids.
The summary of results (Table 1) demonstrated a consistent inverse association between intakes of total sugars and fat expressed as %E across all population groups. The evidence also supported an inverse relationship for extrinsic sugars though the evidence was less consistent than for total sugars, which may be explained by the challenge of accurately measuring intakes of extrinsic sugars compared with total sugars. Fewer studies analyzed these relationships on the basis of absolute intakes and generally indicated a positive correlation between fat and total or extrinsic sugars. This may be a reflection of higher food consumption in general as the association became inverse when adjusted for energy intake and as the relationship also applied to protein. There was evidence of an inverse relationship between total sugars and SFA measured as %E but the data were inconsistent for extrinsic sugars and SFA measured on this basis. An inverse association was found between extrinsic sugars and MUFA and PUFA expressed as% E. In studies reporting an inverse association between sugars and fat there was also a consistent inverse association for both total and extrinsic sugars with protein intake measured as %E. This association was positive when expressed as absolute intakes but was inverse when adjusted for energy intake. Fewer studies addressed the relationships between intakes of sugars and starch or fiber, but there was evidence for inverse associations between intakes of total or extrinsic sugars and starch and between extrinsic sugars and fiber. Alcohol intake was inversely associated with sugars intake, whatever the basis of intake.
Under-reporting of dietary energy is a potential issue when investigating sugar−fat intake associations because it may reflect selective reporting of sugars or high-fat high-sugar foods. However, there was no clear evidence from the studies reviewed that under-reporting explained or influenced the findings. Studies that analyzed results with and without under-reporters invariably recorded no impact of under-reporters on the results (Haraldsdottir and Andersen, 1994; Gibson, 1996; Tonstad and Sivertsen, 1997; Rogers and Emmett, 2002; Alexy et al., 2003).
Owing to the variety of different measures used to investigate the contribution of food groups to sugar and fat intakes comparison of results was difficult. However, the overall results generally indicated a positive relationship between total and extrinsic sugars with confectionery and sweetened drinks, table sugar and preserves, but did not find any strong evidence that high-sugar high-fat foods explained sugar−fat intake relationships. Hence, the fact that many high-fat foods are low in sugars and vice versa undoubtedly contributes to the seesaw.
Intervention studies are valuable in fully understanding the relationships between intakes of fats and sugars, and different study designs allow for different considerations. Highly controlled studies using a restricted range of foods are able to reduce confounding and investigate true nutritional effects. In contrast, free-living studies are more relevant to real life situations and the influence of usual consumer behavior. Adequate monitoring of dietary compliance is an important consideration and the interpretation of some studies may be limited by poor dietary compliance. Intervention studies reporting data expressed as %E were mostly consistent with the observational data and supported an inverse relationship between fat and sugars. In studies that measured changes in sugar and fat intakes on the basis of absolute intakes three studies were consistent with an inverse sugar−fat relationship and four were not.
Few studies considered the potential for a threshold effect and where this was considered the results were conflicting. It was reported that a greater proportion of low-sugar consumers tended to consume above 30% energy from fat than did higher sugar consumers (Gibney et al., 1995), but in a French study it was suggested that the lack of association observed for sucrose and fat on a %E basis may have been explained by the lower contribution of energy from added sucrose in the French diet compared with American and British diets (Drewnowski et al., 1997). A further analysis (Mela, 1997) of intervention data (Gatenby et al., 1997) concluded that in subjects with an initially low-fat intake, the use of reduced-sugar foods significantly increased %E from fat compared with baseline, whereas subjects with a higher initial fat intake showed no significant change. Also, high sucrose consumers using reduced-sugar foods increased their %E from fat (Mela, 1997) (data not shown in Mela's paper), whereas for the overall reduced-sugar group the increase in energy from fat was only 2%. Hence, the potential for a threshold effect warrants further investigation, on the basis of both %E and absolute intakes.
The concept of the sugar−fat seesaw has been much debated. The two key outcomes from this review are (1) of an inverse relationship between %E from sugars and fat from observational studies that is generally supported by intervention studies, and (2) indications from observational studies of a positive association on the basis of absolute intakes that may be confounded by energy intake and inconsistent findings from intervention studies, raise the question how these findings can be explained. While absolute intakes may be important at the individual level, intakes on a %E basis form the basis of dietary guidelines and are important at the population level to account for differing energy requirements. Some authors have suggested (Macdiarmid et al., 1995) or concluded (Mazlan et al., 2006) that the seesaw may be a mathematical effect, arising as a function of expressing intakes as %E. However, if the relationship were entirely explained by a mathematical effect, a starch−fat seesaw on the basis of %E may equally be expected, yet this review found inconclusive evidence to support such a relationship. In nutritional terms, the existence of any reciprocal relationships, as suggested by this review not only for sugar−fat, but also for sugar−protein, sugar−alcohol, sugar−starch, and sugar−fiber, is likely to reflect food composition. The fact that key sources of sugar energy are low in energy from fat (e.g., soft drinks, table sugar) and that many sources of fat are low in sugar (e.g., oils, butter, and fat-spreads, cheese, meat products) undoubtedly contributes to the inverse relationship between sugar and fat such that a mathematical effect is unlikely to be the sole explanation. If the sugar−fat seesaw is at least partly explained by food composition this implies that, with appropriate food selection, it is of course possible for individuals to eat less fat and less sugars concurrently. However, the intervention data demonstrate that consumers find this difficult to achieve in practice suggesting that consumer behavior and influences of palatability and satiety are also important considerations in the achievement of multiple dietary guidelines.
A positive relationship between sugar and fat measured as absolute intakes potentially supports an alternative proposal that high-fat high-sugar foods are highly palatable such that sugar acts as a vehicle for dietary fat. However, evidence to support this hypothesis is weak since the positive relationship for absolute intakes reported in this review is based on limited observational data and the finding was subject to confounding since the relationship no longer held when adjusted for energy intake. Also, results of absolute intake data from intervention studies were equivocal. High-sugar intakes may be a marker of higher energy intakes on account of more active lifestyles, and the positive correlation may be partly explained by sugars, fat and protein (on a g/day basis) being positively related to energy intake. There was no evidence from food group analysis to suggest that high-sugar high-fat foods explained the hypothesis (Flynn et al., 1996). No evidence was available to assess the relationship between extrinsic sugars and SFA intakes on an absolute basis, and there was no evidence of a positive relationship for extrinsic sugars and SFA measured as %E. Stubbs et al. have also concluded that there is little evidence that sugar acts as a vehicle for dietary fat and that the area warrants further investigation in intervention trials (Stubbs et al., 2001).
It has further been suggested that increased sugar intake levers fat energy out of the diet (Gibney, 1990). Few observational studies addressed whether a low% E from fat was inversely associated with absolute intake of sugars. One study found an inverse association with extrinsic sugars in women (Flynn et al., 1996), but two other studies found no evidence for an association in children when considering total sugars (Nicklas et al., 1992) or extrinsic sugars (Nicklas et al., 1992; Räsänen and Ylönen, 1992). However, this question can only be confirmed from well-designed and controlled intervention studies. Evidence from one carefully controlled intervention study, where energy density, palatability, taste, texture, and appearance of the intervention foods were equalized across treatments, did not find evidence that sugar displaces absolute intake of fat in the diet (Mazlan et al., 2006). While rigorously controlled interventions can help tease out nutritional from mathematical effects they do not allow for the influence of usual consumer behavior in real-life eating situations.
In summary, results of interventions allowing the free selection of foods demonstrate that it is difficult to reduce concurrently %E of sugar and fat over time. This finding is important because dietary guidelines for fat and sugars are generally expressed as %E. It implies that multiple guidelines may not be easily compatible and that prioritizing guidelines may be constructive.
Strengths and Limitations
Strengths of this review include the large number of studies that were eligible for the analysis, including studies with high numbers of participants and using weighed dietary intake assessment. Data from eligible intervention studies included a range of different study designs, and there was low discrepancy between the reviewers. A wide range of potential relationships have been considered, resulting in a broad examination of the dietary sugar−fat seesaw hypothesis.
Limitations of the review include the challenge of searching for relevant data in studies where investigation of potential sugar−fat relationships may not have been the primary endpoint. Although a number of diverse search terms were used in a serious attempt to capture all the potentially relevant studies it is possible that some data may have been missed. There are also a number of limitations relating to the quality of the published data. Though many large studies were eligible for the review, some of the smaller studies may have been underpowered such that small differences did not reach statistical significance. For studies in which investigation of the sugar−fat relationship was not the primary purpose statistical analysis of the relationship was not always provided and there was insufficient evidence from such studies to support or refute the hypothesis. Validity of the relationship between sugar and fat in the diet relies on the accuracy of dietary intake data and all methods of data collection have inherent limitations that can lead to bias such that they may not accurately reflect true intakes. A further limitation relates to adequate classification of different subtypes of sugars. One analysis that included total, extrinsic, and intrinsic sugars and lactose, provided a full description of the assumptions and estimations on which intake of the sugar subtypes was calculated (Bolton Smith and Woodward, 1994), but such details were not provided in all study reports. Hence, the data from which dietary intakes of sugar subtypes are calculated are not very robust, affecting the validity of comparisons between studies. Finally, there are potentially many existing data sets for which sugar−fat intake relationships and relationships with other macronutrients have not been explored. Such analyses would provide further useful information, on the basis of %E and absolute intakes expressed on a constant energy basis.
CONCLUSIONS
In summary, this review confirms the existence of a sugar−fat seesaw on a %E basis and suggests that conforming to %E guidelines for both sugars and fats is challenging. The implication is that population guidelines to reduce both fat and sugar intakes may be difficult to achieve in practice and more studies investigating aspects of consumer behavior that may influence the relationship are warranted.
Notwithstanding the limitations of dietary intake data, including assessment of different sugar types, the eligible data in this review demonstrate a strong and consistent inverse association between sugars and fat intakes when expressed as %E, in both observational and intervention studies. Overall, the results suggest that this may be partly explained by mathematical and nutritional effects since major sources of sugar are not high in fat, and vice versa. Based on a smaller number of studies, the evidence suggests a positive relationship on the basis of absolute intakes, though this appears to be confounded by energy intake. The finding that the sugar−fat seesaw only holds when nutrients are expressed on a %E or energy-adjusted basis is nevertheless highly relevant because population dietary guidelines are formulated on a %E basis. The finding is also consistent with the conclusions of Gibson and Ashwell (2011) that it may be unrealistic to expect individuals to attain guidelines conceived as population intakes rather than individual targets.
Further work is needed to enable accurate determination of intakes of subtypes of sugars. More ad libitum intervention studies, designed to measure the effect on energy and macronutrient intake of altering fat or sugar intakes, are needed to understand consumer behavior in practice and the factors influencing this. Measuring satiety and palatability scores within such studies, and palatability of low-fat low-sugar diets per se, would help to determine potential drivers of the inverse relationship and to explore reasons for the observed difficulty in sustaining reductions in energy intake from both fat and sugar over time. Studies that quantify the relationship, i.e., determine unit change in fat for a given change in sugar would contribute to further understanding of sugar−fat intake relationships. Further investigation of the relative strength of other potential reciprocal relationships such as sugar−protein, sugar−alcohol, and fat−starch is warranted to understand more about possible mechanisms.
ACKNOWLEDGMENT
Authors 1 and 3 reviewed and interpreted the evidence, and 1 and 2 wrote the paper.
FUNDING
This review was made possible by a grant from the Sugar 735 Bureau (UK), under the condition that Sugar Bureau personnel or associates had no part in the selection or assessment of relevant data, in the interpretation of the results or writing of the paper.
APPENDIX A: Search Terms.
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Seesaw / See saw |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Reciprocal$2 |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Relation$4 / Interrelation$4 |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Ratio$1 |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Vehicle |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Negative$2 / Inverse$2 |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Positive$2 |
| Sugar$1/ sucrose / sugar sweetened beverage$1 / soft drink$1 | And | Fat | And | Intake$1 / Consumption |
$x = Wildcard + no. of following lettersThe searches were applied to ‘‘whole document.’’
REFERENCES
- Alexy U. Sichert H.W. Kersting M. Associations between intake of added sugars and intakes of nutrients and food groups in the diets of German children and adolescents. Br. J. Nutr. 2003;90(2):441–447. doi: 10.1079/bjn2003904. [DOI] [PubMed] [Google Scholar]
- Baghurst K.I. Baghurst P.A. Crawford D. The interrelationship between fat and carbohydrate intake in the free-living population. Proc. Nutr. Soc. Aust. 1988;13:80. [Google Scholar]
- Baghurst K.I. Baghurst P.A. Record S.J. Demographic and nutritional profiles of people consuming varying levels of added sugars. Nutr. Res. 1992;12(12):1455–1465. [Google Scholar]
- Baghurst K.I. Baghurst P.A. Record S.J. Demographic and dietary profiles of high and low fat consumers in Australia. J. Epidemiol. Community Health. 1994;48(1):26–32. doi: 10.1136/jech.48.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Smith C Woodward M. Dietary composition and fat to sugar ratios in relation to obesity. Int. J. Obes. 1994;18(12):820–828. [PubMed] [Google Scholar]
- Boulton T.J. Magarey A.M. Effects of differences in dietary fat on growth, energy and nutrient intake from infancy to eight years of age. Acta paediatr. (Oslo Norway: 1992) 1995;84(2):146–50. doi: 10.1111/j.1651-2227.1995.tb13597.x. [DOI] [PubMed] [Google Scholar]
- Burke S.J. McCarthy S.N. O'Neill J.L. Hannon E.M. Kiely M. Flynn A. Gibney M.J. An examination of the influence of eating location on the diets of Irish children. Public Health Nutr. 2007;10(6):599–607. doi: 10.1017/S1368980007258379. [DOI] [PubMed] [Google Scholar]
- Burke V. Milligan R.A. Thompson C. Taggart A.C. Dunbar D.L. Spencer M.J. Medland A. Gracey M.P. Vandongen R. Beilin L.J. A controlled trial of health promotion programs in 11-year-olds using physical activity “enrichment” for higher risk children. J. Pediatr. 1998;132(5):840–848. doi: 10.1016/s0022-3476(98)70315-4. [DOI] [PubMed] [Google Scholar]
- Cade J. Booth S. What can people eat to meet the dietary goals: And how much does it cost? J. Hum. Nutr. Diet. 1990;3(3):199–207. [Google Scholar]
- Committee on Medical Aspects of Food Policy Dietary Sugars and Human Disease (Report on Health and Social Subjects). London, UK: Stationery Office Books 1989 [Google Scholar]
- Cullen M. Nolan J. Moloney M. Kearney J. Lambe J. Gibney M.J. Effect of high levels of intense sweetener intake in insulin dependent diabetics on the ratio of dietary sugar to fat: A case–control study. Eur. J. Clin. Nutr. 2004;58(10):1336–1341. doi: 10.1038/sj.ejcn.1601969. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Henderson S.A. Shore A.B. Fischler C. Preziosi P. Hercberg S. The fat–sucrose seesaw in relation to age and dietary variety of French adults. Obes. Res. 1997;5(6):511–518. doi: 10.1002/j.1550-8528.1997.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Drummond S. Kirk T. The effect of different types of dietary advice on body composition in a group of Scottish men. J. Hum. Nutr. Diet. 1998;11(6):473–485. [Google Scholar]
- Drummond S. Kirk T. Assessment of advice to reduce dietary fat and non-milk extrinsic sugar in a free-living male population. Public Health Nutr. 1999;2(2):187–197. doi: 10.1017/s1368980099000245. [DOI] [PubMed] [Google Scholar]
- Drummond S. Kirk T. Jackson J. Hendry J. Panton S. Gray F. Effectiveness of dietary advice given by community dietitians to men with elevated blood cholesterol in a clinical setting: A pilot study. J. Hum. Nutr. Diet. 2003;16(2):81–83. doi: 10.1046/j.1365-277x.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Erkkola M. Kronberg K.C. Kyttälä P. Lehtisalo J. Reinivuo H. Tapanainen H. Veijola R. Knip M. Ovaskainen M.L. Virtanen S.M. Sucrose in the diet of 3-year-old Finnish children: Sources, determinants and impact on food and nutrient intake. Br. J. Nutr. 2009;101(8):1209–1217. doi: 10.1017/S0007114508057619. [DOI] [PubMed] [Google Scholar]
- Farris R.P. Nicklas T.A. Myers L. Berenson G.S. Nutrient intake and food group consumption of 10-year-olds by sugar intake level: The Bogalusa heart study. J. Am. Coll. Nutr. 1998;17(6):579–585. doi: 10.1080/07315724.1998.10718806. [DOI] [PubMed] [Google Scholar]
- Flynn M.A. T. Codd M.B. Sugrue D.D. Gibney M.J. An approach to the formulation of healthy eating advice. Proc. Nutr. Soc. 1994;53(2) [Google Scholar]
- Flynn M.A. T. Sugrue D.D. Codd M.B. Gibney M.J. Women's dietary fat and sugar intakes: Implications for food based guidelines. Eur. J. Clin. Nutr. 1996;50(11):713–719. [PubMed] [Google Scholar]
- Garemo M. Lenner R.A. Strandvik B. Swedish pre-school children eat too much junk food and sucrose. Acta Paediatr. 2007;96(2):266–272. doi: 10.1111/j.1651-2227.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- Gatenby S.J. Aaron J.I. Jack V.A. Mela D.J. Extended use of foods modified in fat and sugar content: Nutritional implications in a free-living female population. Am. J. Clin. Nutr. 1997;65(6):1867–1873. doi: 10.1093/ajcn/65.6.1867. [DOI] [PubMed] [Google Scholar]
- Gibney M.J. Dietary guidelines: A critical appraisal. J. Hum. Nutr. Diet. 1990;3:245–54. [Google Scholar]
- Gibney M.J. Lee P. Patterns of food and nutrient intake in the chronically unemployed consuming high and low levels of table sugar. Proc. Nutr. Soc. 1989;48(2) [Google Scholar]
- Gibney M.J. Lee P. Formulation of practical advice for reducing dietary fat intakes in unemployed in Dublin. J. Hum. Nutr. Diet. 1991;4(3):179–183. [Google Scholar]
- Gibney M. Maloney M. Shelly E. The Kilkenny health project: Patterns of food intake in individuals consuming low, moderate and high fat diets. Proc. Nutr. Soc. 1987;46 [Google Scholar]
- Gibney M.J. Moloney M. Shelley E. The Kilkenny Health Project: Food and nutrient intakes in randomly selected healthy adults. Br. J. Nutr. 1989;61(2):129–137. doi: 10.1079/bjn19890103. [DOI] [PubMed] [Google Scholar]
- Gibney M. Sigman G.M. Stanton J.L. Jr. Keast D.R. Consumption of sugars. Am. J. Clin. Nutr. 1995;62(1):178S–193S. doi: 10.1093/ajcn/62.1.178S. discussion 194S. [DOI] [PubMed] [Google Scholar]
- Gibson S.A. Consumption and sources of sugars in the diets of British schoolchildren: Are high-sugar diets nutritionally inferior? J. Hum. Nutr. Diet. 1993;6(4):355–371. [Google Scholar]
- Gibson S.A. Are diets high in non-milk extrinsic sugars conducive to obesity? An analysis from the Dietary and Nutritional Survey of British Adults. J. Hum. Nutr. Diet. 1996;9(4):283–292. doi: 10.1111/j.1365-277X.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- Gibson S.A. Non-milk extrinsic sugars in the diets of pre-school children: Association with intakes of micronutrients, energy, fat and NSP. Br. J. Nutr. 1997;78(3):367–378. doi: 10.1079/bjn19970157. [DOI] [PubMed] [Google Scholar]
- Gibson S. Ashwell M. Dietary patterns among British adults: Compatibility with dietary guidelines for salt/sodium, fat, saturated fat and sugars. Public Health Nutr. 2011;14(8):1323–36. doi: 10.1017/S1368980011000875. [DOI] [PubMed] [Google Scholar]
- Haraldsdottir J. Andersen L.B. Clustering of dietary variables. A study of young men and women. Scand. J. Nutr. /Naringsforskning. 1994;38(3):112–116. [Google Scholar]
- Kranz S. Smiciklas-Wright H. Siega-Riz A.M. Mitchell D. Adverse effect of high added sugar consumption on dietary intake in American preschoolers. J. Pediatr. 2005;146(1):105–111. doi: 10.1016/j.jpeds.2004.08.077. [DOI] [PubMed] [Google Scholar]
- Lenders C.M. Hediger M.L. Scholl T.O. Khoo C.S. Slap G.B. Stallings V.A. Effect of high-sugar intake by low-income pregnant adolescents on infant birth weight. J. Adolesc. Health. 1994;15(7):596–602. doi: 10.1016/1054-139x(94)90145-s. [DOI] [PubMed] [Google Scholar]
- Lewis C.J. Park Y.K. Dexter P.B. Yetley E.A. Nutrient intakes and body weights of persons consuming high and moderate levels of added sugars. J. Am. Diet. Assoc. 1992;92(6):708–713. [PubMed] [Google Scholar]
- Linseisen J. Gedrich K. Karg G. Wolfram G. Sucrose intake in Germany. Z. Ernahrungswiss. 1998;37(4):303–314. doi: 10.1007/s003940050030. [DOI] [PubMed] [Google Scholar]
- Macdiarmid J.I. Cade J.E. Blundell J.E. Extrinsic sugar as vehicle for dietary fat. Lancet. 1995;346(8976):696–697. [PubMed] [Google Scholar]
- Matthys C. De H.S. Bellemans M. De M.M. De B.G. Sources of saturated fatty acids in Belgian adolescents’ diet: Implications for the development of food-based dietary guidelines. Br. J. Nutr. 2006;95(3):546–554. doi: 10.1079/bjn20051600. [DOI] [PubMed] [Google Scholar]
- Mazlan N. Horgan G. Whybrow S. Stubbs J. Effects of increasing increments of fat- and sugar-rich snacks in the diet on energy and macronutrient intake in lean and overweight men. Br. J. Nutr. 2006;96(3):596–606. [PubMed] [Google Scholar]
- Mela D.J. Impact of macronutrient-substituted foods on food choice and dietary intake. Ann. N. Y. Acad. Sci. 1997;819:96–107. doi: 10.1111/j.1749-6632.1997.tb51800.x. [DOI] [PubMed] [Google Scholar]
- Miller T.M. Abdel-Maksoud M.F. Crane L.A. Marcus A.C. Byers T.E. Effects of social approval bias on self-reported fruit and vegetable consumption: A randomized controlled trial. Nutr. J. 2008;7:18. doi: 10.1186/1475-2891-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith D.J. Rhodes C. Adjustment in energy intake following the covert removal of sugar from the diet. J. Hum. Nutr. Diet. 1995;8(3):167–175. [Google Scholar]
- Nicklas T.A. Webber L.S. Koschak M. Berenson G.S. Nutrient adequacy of low fat intakes for children: The Bogalusa heart study. Pediatrics. 1992;89(2):221–228. [PubMed] [Google Scholar]
- Parnell W. Wilson N. Alexander D. Wohlers M. Williden M. Mann J. Gray A. Exploring the relationship between sugars and obesity. Public Health Nutr. 2008;11(8):860–866. doi: 10.1017/S1368980007000948. [DOI] [PubMed] [Google Scholar]
- Payne J.A. Belton N.R. Nutrient intake and growth in preschool children. I. Comparison of energy intake and sources of energy with growth. J. Hum. Nutr. Diet. 1992;5(5):287–298. [Google Scholar]
- Raben A. Vasilaras T.H. Møller A.C. Astrup A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am. J. Clin. Nutr. 2002;76(4):721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Räsänen L. Ylönen K. Food consumption and nutrient intake of one- to two-year-old Finnish children. Acta Paediatr. (Oslo Norway: 1992) 1992;81(1):7–11. doi: 10.1111/j.1651-2227.1992.tb12069.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen L.B. Lyhne N. Ovesen L. Sugar: A harmless indulgence? Int. J. Food Sci. Nutr. 1998;49(4):253–264. [Google Scholar]
- Reid M. HammersleyHill R.J.A.J. Skidmore P. Long-term dietary compensation for added sugar: Effects of supplementary sucrose drinks over a 4-week period. Br. J. Nutr. 2007;97(1):193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- Rogers I. Emmett P. Fat content of the diet among pre-school children in Britain, relationship with food and nutrient intakes. Eur. J. Clin. Nutr. 2002;56(3):252–263. doi: 10.1038/sj.ejcn.1601335. [DOI] [PubMed] [Google Scholar]
- Ruottinen S. Niinikoski H. Lagström H. Rönnemaa T. Hakanen M. Viikari J. Jokinen E. Simell O. High sucrose intake is associated with poor quality of diet and growth between 13 months and 9 years of age: The special turku coronary risk factor intervention project. Pediatrics. 2008;121(6):e1676–e1685. doi: 10.1542/peds.2007-1642. [DOI] [PubMed] [Google Scholar]
- Ruxton C.H. S. Kirk T.R. Belton N.R. Energy and nutrient intakes in a sample of 136 Edinburgh 7-8 year olds: A comparison with United Kingdom dietary reference values. Br. J. Nutr. 1996;75(2):151–160. doi: 10.1079/bjn19960121. [DOI] [PubMed] [Google Scholar]
- Ryan R Hill S Broclain D Horey D Oliver S Prictor M Cochrane Consumers and Communication Review Group. Study Quality Guide. March 2007. www.latrobe.edu.au/cochrane/resources.html. [Google Scholar]
- Saris W.H. Astrup A. Prentice A.M. Zunft H.J. Formiguera X. Verboeket-van de Venne W.P. Raben A. Poppitt S.D. Seppelt B. Johnston S. Vasilaras T.H. Keogh G.F. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The CARMEN study. The Carbohydrate Ratio Management in European National diets. Int. J. Obes. Relat. Metab. Disord. 2000;24(10):1310–8. doi: 10.1038/sj.ijo.0801451. [DOI] [PubMed] [Google Scholar]
- Stubbs R.J. Mazlan N. Whybrow S. Carbohydrates, appetite and feeding behavior in humans. J. Nutr. 2001;131(10):2775. doi: 10.1093/jn/131.10.2775S. [DOI] [PubMed] [Google Scholar]
- Tonstad S. Sivertsen M. Relation between dietary fat and energy and micronutrient intakes. Arch. Dis. Child. 1997;76(5):416–420. doi: 10.1136/adc.76.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandongen R. Jenner D.A. Thompson C. Taggart A.C. Spickett E.E. Burke V. Beilin L.J. Milligan R.A. Dunbar D.L. A controlled evaluation of a fitness and nutrition intervention program on cardiovascular health in 10- to 12-year-old children. Prev. Med. 1995;24(1):9–22. doi: 10.1006/pmed.1995.1003. [DOI] [PubMed] [Google Scholar]
- West J.A. De Looy A.E. Weight loss in overweight subjects following low-sucrose or sucrose-containing diets. Int. J. Obes. 2001;25(8):1122–1128. doi: 10.1038/sj.ijo.0801652. [DOI] [PubMed] [Google Scholar]
- Ylönen K. Virtanen S.M. Ala V.E. Räsänen L. Composition of diet in relation to fat intake of children aged 1–7 years. J. Hum. Nutr. Diet. 1996;9(3):207–218. [Google Scholar]
- Ziegler P. Nelson J.A. Barratt F.A. Fiveash L. Drewnowski A. Energy and macronutrient intakes of elite figure skaters. J. Am. Diet. Assoc. 2001;101(3):319–325. doi: 10.1016/S0002-8223(01)00083-9. [DOI] [PubMed] [Google Scholar]


