Abstract
Background:
Bispectral index (BIS) monitoring in multiple trauma patients has become a common practice in monitoring the sedation levels. We aimed to assess the utility of BIS in the trauma intensive care unit (ICU).
Methods:
A prospective observational study was conducted in the trauma ICU at Hamad General Hospital in Qatar between 2011 and 2012. Patients were divided in two groups: Group I (without BIS monitoring) and Group II (with BIS monitoring). The depth of sedation was clinically evaluated with Ramsey Sedation Scale, changes in vital signs and Glasgow Coma Scale (GCS) level. Use of sedatives, analgesics, and muscle relaxants were also recorded. Data were compared using Chi-square and Student t-tests.
Results:
A total of 110 mechanically ventilated trauma patients were enrolled with a mean age of 36 ± 14 years. The rate of head injury was greater in Group I when compared with Group II (94% vs. 81%, P = 0.04). In comparison to Group I, patients in Group II had lower GCS and higher mean Injury Severity Score (ISS) (6.3 ± 2.5 vs. 7.4 ± 2.7 and 25.5 ± 8.5 vs. 21.2 ± 4.7, respectively, P = 0.03). The used midazolam dose was less in Group II in comparison to Group I (5.2 ± 2.3 vs. 6.1 ± 2.1, P = 0.03). Also, fentanyl dose was less in Group II (152 ± 58 vs. 187 ± 59, P = 0.004). The rate of agitation, failure of extubation and tracheostomy in Group II were lower than those in Group I, P = 0.001. The length of stay for patients Group I was longer (14.6 ± 7.1 vs. 10.2 ± 5.9 days) in comparison to group II, P = 0.001.
Conclusion:
Management of multiple trauma patients in the trauma ICU with BIS monitoring was found to be associated with better outcomes. BIS monitoring is a guide for adjusting the dosage of sedative agents. It can also minimize agitation, failure of extubation, and length of stay in ICU.
Keywords: Analgesia, bispectral index, head injury, sedation, trauma
INTRODUCTION
Optimal management of multi-traumatized patients in intensive care units (ICUs), especially in those who are connected to mechanical ventilators includes use of sedation and analgesia. Analgesia and sedation are needed in order to tolerate clinical procedures, synchrony with ventilators, optimize oxygenation and to secure patient safety and comfort.[6] A recent study reported that approximately 69% of patients in ICU were inappropriately sedated (54% were over-sedated, and 15% were under-sedated).[15] Inadequate level of sedation may lead to patient's anxiety, patient-ventilator dyssynchrony, agitation, and posttraumatic stress disorder (PSTD). Undersedation may also cause increased stress symptoms, sodium and water retention, increased catabolism and mobilization of substrates to provide energy sources. It also associated with tachycardia, hypertension, increased oxygen consumption, and altered levels of respiratory rates. Moreover, it induces changes in gastro intestinal mobility and variations in coagulability such as clotting time, and platelet aggregation, in addition to delayed wound healing.[24] On the other hand, oversedation may lead to delayed weaning that may result in ventilator associated pneumonia. Furthermore, it may prolong artificial ventilation and length of ICU stay, and consequently increases the cost associated with patient care.[14]
Sedation monitoring can be challenging because of variations in patient's requirement of medications. The synergistic action of drugs in different combinations also makes it difficult to achieve the goal of optimal sedation. Moreover, there is no gold standard for measuring sedation in ICU patients. Most of the time, clinicians monitor vital signs such as heart rate and blood pressure and use subjective sedation scales to monitor sedation level. Commonly used subjective methods include Riker sedation-agitation scale (SAS), motor activity assessment scale (MAAS), Richmond agitation-sedation scale (RASS), Adaptation to the intensive care environment scale (ATICE), and Ramsay sedation scale (RSS).[14] Vital signs do not reflect the depth of sedation always. RSS and SAS rely on patient movement and response and therefore may not precisely measure the deep states of sedation. Weinert et al. added that subjective assessment of sedation is not optimal because of the influence of social, personal, and professional factors on sedation monitoring.[26] Furthermore, differences in the subjective assessment of sedation are more likely to occur.[11] An objective measure such as bispectral Index (BIS) monitoring may be a useful tool in monitoring optimal sedation.[6,7,8] BIS index is derived from patient's electroencephalogram (EEG) data and scaled from 100 to 0 denoting fully awake to unconscious. BIS value decreases linearly with increasing doses of anesthesia.
Studies among anesthetized patients pointed out the acceptable target ranges; BIS of 40-60 for deep surgical anesthesia and 70-90 for light anesthesia.[14] Beneficial outcomes of use of BIS monitors include improved sedation, decreased sedative requirements, and faster recovery times. BIS monitoring system is useful in minimizing the incidence of awareness with recall in adults. Awareness is a leading cause of patient dissatisfaction with anesthesia. BIS technology enables to assess consciousness and sedation separately from cardiovascular reactivity. It has been demonstrated that BIS monitored patients wake up faster, extubated sooner, and more oriented on arrival at the postanesthesia care unit (PACU). PACU discharge was sooner among BIS monitored patients. Moreover, BIS-guided anesthesia lead to reductions in use of hypnotics.[1,4,11] Recently, its use has been extended to ICU patients.[14,15,24,26] The present study analyzes the utility of BIS monitors in mechanically ventilated patients in trauma ICU.
MATERIAL AND METHODS
The present study was conducted prospectively from December 2011 to December 2012. All adult patients admitted to the trauma ICU at Hamad General Hospital, Doha, Qatar who required mechanical ventilation, sedation, and analgesia were included in the study. Sedation depth was clinically evaluated with RSS, observation of vital signs and Glasgow Coma Scale (GCS). Information on age, gender, mechanism of injury, type of injury, Injury Severity Score (ISS), comorbidity, dose of analgesic and sedative agents used, and outcome measures such as mortality, agitation, failure of extubation, and length of stay in the ICU were recorded. Data were compared between two groups of patients, that is, with BIS monitoring versus without monitoring. Group frequencies were compared with the Chi-square and Student t-test, while the level of significance was set at P < 0.05 for all tests.
In our study, the brain electrical activity was measured using BIS-monitor system (Covidien, Gunbarrel Ave, Boulder, USA). The sensor (BIS Quatro Sensor) used in our study has four electrodes, which were placed at an angle over the forehead of the patient. The first electrode was positioned at the midline, approximately 5 cm above the nose, and the second electrode was placed just lateral and inferior to the first electrode. The third electrode was placed over the temporal region behind the angle of the eye, and the fourth electrode was positioned directly above and adjacent to the eyebrow [Figure 1]. An internal algorithm based on a database of EEGs calculate a number between 0 (absence of brain electrical activity) and 100 (fully awake). This provides a direct measure of level of consciousness in the patients. This study was approved by Hamad Medical Corporation, Medical Research Center, IRB#11222/12.
Figure 1.
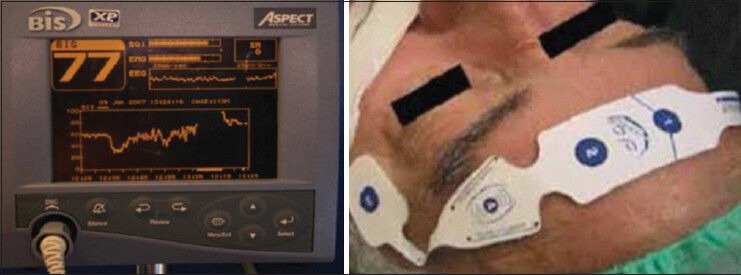
Bispectral index monitoring device
RESULTS
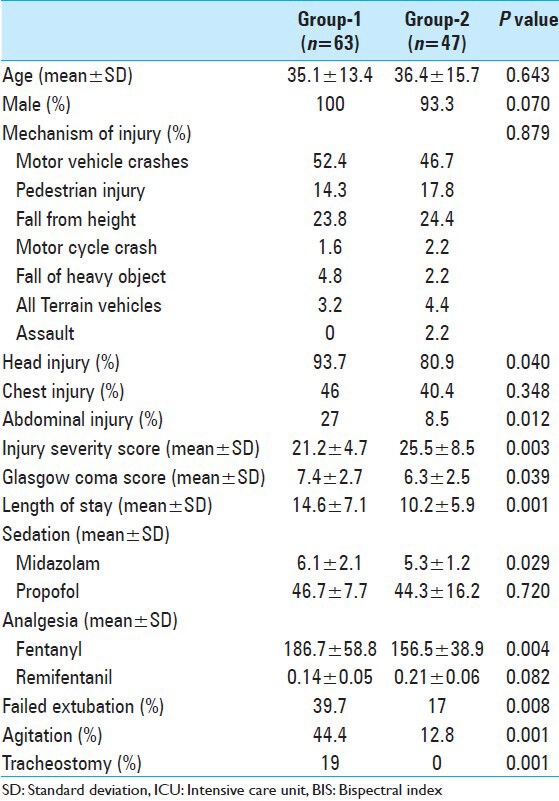
A total of 110 patients admitted to the trauma ICU were enrolled; of them, 97% were males with a mean age of 36 ± 14 years. All these patients were intubated and connected to mechanical ventilators for different indications. Table 1 summarizes demographics, presentation and interventions in all the study population. Sixty-five percent of injuries were traffic-related such as motor vehicle crashes (MVCs) and pedestrian injuries. The main injured body region was the head (88%), followed by chest (44%), and abdomen (19%). The rate of head and abdominal injuries were significantly greater in Group I when compared with Group II (94% vs. 81%, P = 0.04 and 27% vs. 8.5%, P = 0.01, respectively). In comparison to Group I, patients with BIS monitoring (Group II) had significant lower GCS and higher mean ISS (6.3 ± 2.5 vs. 7.4 ± 2.7, P = 0.039 and 25.5 ± 8.5 vs. 21.2 ± 4.7, P = 0.003, respectively) [Table 2]. The used midazolam dose was less in Group II in comparison to Group I (5.2 ± 2.3 vs. 6.1 ± 2.1, P = 0.029). Also, fentanyl dose was less in Group II (152 ± 58 vs. 187 ± 59, P = 0.004). The rate of agitation (44% vs. 13%, P = 0.001) and failure of extubation (40% vs. 17%, P = 0.001), and tracheostomy (19% vs. 0%) were higher in Group I when compared with Group II. The hospital length of stay in Group I was shorter in Group II (10.2 ± 5.9 days), in comparison to group I (14.6 ± 7.1), P = 0.001. The present study observed no correlation between BIS values and clinical sedation scale scores (RSS) (P = 0.49).
Table 1.
Overall demographics, presentation, and interventions (n=110)
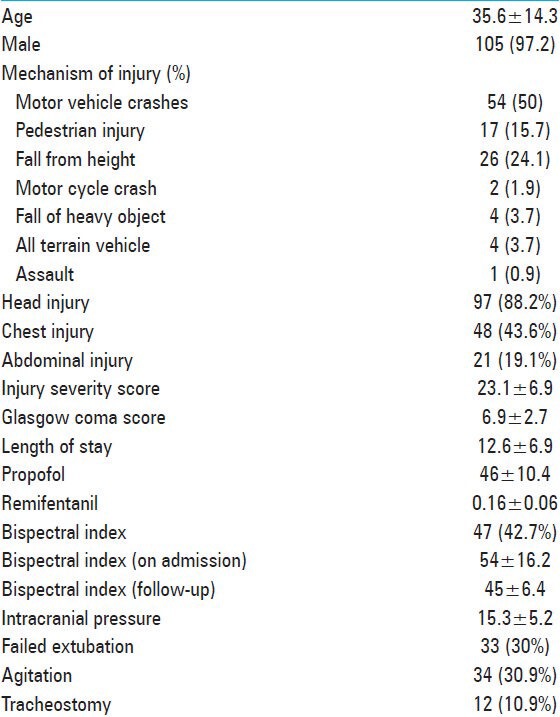
Table 2.
Comparison of ICU patients with and without BIS
DISCUSSION
The current study assesses the utility of BIS monitoring among intubated trauma patients and demonstrates that BIS monitoring has reduced the dose of used analgesics and sedation with a subsequent reduction of the hospital length of stay. It also reduced the rate of agitation, failed extubation, and the need for tracheostomy.
In general, BIS was used to titrate sedative agents in ICU patients where it showed decreased use of sedatives and less oversedation when compared with use of subjective methods.[14] However, a previous study reported no difference in ventilator days or hospital mortality by the use of BIS method compared with standard care.[17] deWit et al.[7] reported that BIS scores were unable to predict extubation success or failure in medical ICU patients. In trauma patients, BIS was found reliable and advantageous over subjective scales such as RASS during a propofol spontaneous awakening trial (SAT).[18] However, Ogilvie et al.[21] reported that subjective interpretations of BIS are more likely in patients with traumatic brain injury (TBI) or patients receiving paralytic agents. Agitation, irritability, and aggression are the major behavioral complications related to TBI in the ICU. However, the frequency of TBI was significantly higher among non-BIS group in our study. This indicates that the higher incidence of agitation, extubation failure, and tracheostomy observed in our cases might be associated with TBI irrespective of the BIS monitoring. Therefore, patient characteristics differences alone could be associated with higher rate of complication and increased length of hospital stay. Also, it is evident from our analysis that more severely injured patients underwent BIS monitoring, which helps in lowering the rate of sedation and analgesia and reduces the overall hospital length of stay. Consistently, it has been demonstrated that BIS-monitored patients wake up faster, extubated sooner, and more oriented on arrival at the PACU.[11]
Brocas et al.[3] studied the effect of an alfentanil bolus in mechanically ventilated critically ill patients and showed that BIS was lower with alfentanil bolus. Riker et al.[23] compared BIS and Suppression ratio (SR) with burst suppression of the EEG during Pentobarbital infusions in adult intensive care patients and found BIS was correlated well with SR (r2 = 0.79).
The usefulness of the BIS in monitoring the degree of sedation in ICU patients can be demonstrated by its degree of correlation to commonly used sedation scales. Nasraway et al.[20] conducted a prospective study to validate the BIS to SAS. Patients with SAS of 3 or less were included, that is, deeply sedated patients. The correlation was low, but statistically significant between the BIS and SAS. The correlation was increased when the BIS associated with high Electromyographic (EMG) activity was removed from analysis. The study demonstrated that BIS may be useful in patients who are deeply sedated (SAS score ≤3), that is, in patients with lower EMG activity.[20] Ma et al.[16] compared the reliability of BIS with SAS in assessing the depth of sedation in mechanically ventilated patients in ICU and found that SAS was well correlated with BIS; and BIS monitoring was more reliable in measuring the depth of sedation especially when SAS reaches 2-4. Furthermore, a study that compared subjective RSS with BIS concluded that BIS monitoring enables more effective titration of sedatives and offers an objective, safe, and reliable measure of sedation.[9]
A significant correlation between BIS scores and the level of consciousness in brain-injured ICU patients was reported by Jung et al.[13] The investigators pointed out the fact that BIS scores could not indicate a patient's mental status without vagueness except in coma patients. However, the study added that BIS in conjunction with GCS or other neurological evaluation scales will be useful for assessing the level of consciousness in brain-injured patients.[13]
Paul et al.[22] also reported the significant correlation between BIS and GCS scores in patients with mild-to-moderate head injuries. GCS and BIS were measured before surgery, after surgery and once a day for the first 10 days in 29 patients with mild (GCS 13-15) and moderate (GCS 9-12) head injuries who underwent craniotomy. Hsia et al.[12] reported on the positive correlation that existed between GCS and BIS among critically ill pediatric patients. Gill et al.[10] showed the weak correlation between GCS and BIS in emergency department patients. Notably, the level of consciousness and BIS are two scores obtained from different brain regions that may not correlate with each other as BIS score is the measure of frontal lobe electrical activity.[13]
BIS monitors are in fact cerebral function monitors since it records the electrical activity of the cerebral cortex. In severe brain injury patients, the electrical activity of the brain may vary according to cerebral perfusion, cerebral metabolism, hypoxia, sedative pharmacologic agents, and seizure activity.[8,27] Dunham et al.[8] showed that BIS ≥60 suggest that patients with severe brain injury are likely to have an acceptable ICP and cerebral perfusion pressure (CPP). Xifeng et al.[27] and Myles et al.[19] reported that BIS provides useful information that may lead to the identification of patients with good chance of recovery after ischemic hypoxic brain injury.[19,27]
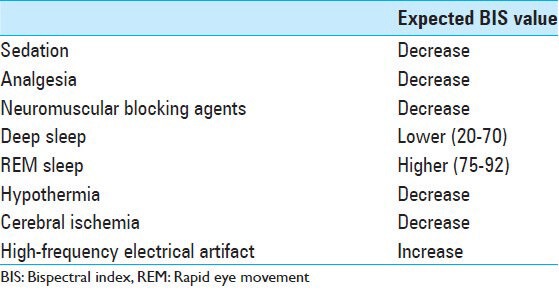
Limitations: BIS values are affected by the choice of anesthetic agents and the depth of sedation among the patients may differ for the same BIS score but with different combinations of drugs.[14,15] BIS scores have not been proved as a useful tool in certain populations such as critically ill patients with unstable body temperatures and patients with dementia.[2] In many studies, different investigators used different versions of BIS monitor that makes it difficult to compare the results obtained because of the changes in the algorithm used. BIS-XP version attempts to filter electro-oculographic and other EMG artifacts. Deogaonkar et al.[5] reported that XP version of BIS showed much higher correlation with sedation scales when comparing with version 2.11. However, Vivien et al.[25] showed overestimation of the BIS due to high EMG activity with both versions and this resulted in undersedation. The data regarding the use of neuromuscular blocking agents, cerebral ischemia, and information for emergence from coma are lacking in our study. Table 3 shows the factors that should be considered during the evaluation of BIS results.
Table 3.
Factors that affect bispectral index value
CONCLUSION
Clinically, use of a BIS monitor may help standardize clinical practice and improve patients care. BIS is a guide for adjusting the dosage of sedative agents. It can also minimize the clinical consequences such as sedation overdose, prolonged intubation, failed extubation, and hospital stay. It can also provide financial benefits by limiting excessive use of costly sedatives and decreasing time to extubation. Although the BIS monitor is relatively new to the intensive care setting, it can be a beneficial tool in monitoring neurological patients.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/141/141890
Contributor Information
Saeed Mahmood, Email: saeedagm@hotmail.com.
Ashok Parchani, Email: aparchani@yahoo.com.
Ayman El-Menyar, Email: aymanco65@yahoo.com.
Ahmad Zarour, Email: ahmad.zarour@yahoo.com.
Hassan Al-Thani, Email: althanih@hotmail.com.
Rifat Latifi, Email: latifi@surgery.arizona.edu.
REFERENCES
- 1.Bae JY, Choi do Y, Woo CH, Kwak IS, Mun SH, Kim KM. The BIS and hemodynamic changes in major burn patients according to a slow infusion of propofol for induction. Korean J Anesthesiol. 2011;60:161–6. doi: 10.4097/kjae.2011.60.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigham C, Bigham S, Jones C. Does the bispectral index monitor have a role in intensive care? J Intensive Care Soc. 2012;13:314–9. [Google Scholar]
- 3.Brocas E, Dupont H, Paugam-Burtz C, Servin F, Mantz J, Desmonts JM. Bispectral index variations during tracheal suction in mechanically ventilated critically ill patients: Effect of an alfentanil bolus. Intensive Care Med. 2002;28:211–3. doi: 10.1007/s00134-001-1189-y. [DOI] [PubMed] [Google Scholar]
- 4.Choi SS, Kim JS, Park IK, Lee G, Hahm KD. Changes in the bispectral index and cerebral oxygen saturation during neuroendovascular intervention under general anesthesia. Korean J Anesthesiol. 2012;62:98–100. doi: 10.4097/kjae.2012.62.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deogaonkar A, Gupta R, DeGeorgia M, Sabharwal V, Gopakumaran B, Schubert A, et al. Bispectral index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32:2403–6. doi: 10.1097/01.ccm.0000147442.14921.a5. [DOI] [PubMed] [Google Scholar]
- 6.Devlin JW. The pharmacology of oversedation in mechanically ventilated adults. Curr Opin Crit Care. 2008;14:403–7. doi: 10.1097/MCC.0b013e32830280b3. [DOI] [PubMed] [Google Scholar]
- 7.De Wit M, Epstein SK. Administration of sedatives and level of sedation: Comparative evaluation via the Sedation-Agitation Scale and the Bispectral Index. Am J Crit Care. 2003;12:343–8. [PubMed] [Google Scholar]
- 8.Dunham CM, Ransom KJ, McAuley CE, Gruber BS, Mangalat D, Flowers LL. Severe brain injury ICU outcomes are associated with Cranial-Arterial Pressure Index and noninvasive Bispectral Index and transcranial oxygen saturation: A prospective, preliminary study. Crit Care. 2006;10:R159. doi: 10.1186/cc5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gélinas C, Tousignant-Laflamme Y, Tanguay A, Bourgault P. Exploring the validity of the bispectral index, the critical-care pain observation tool and vital signs for the detection of pain in sedated and mechanically ventilated critically ill adults: A pilot study. Intensive Crit Care Nurs. 2011;27:46–52. doi: 10.1016/j.iccn.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Gill M, Green SM, Krauss B. Can the bispectral index monitor quantify altered level of consciousness in emergency department patients? Acad Emerg Med. 2003;10:175–9. doi: 10.1111/j.1553-2712.2003.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 11.Gurudatta C. Sedation in Intensive Care Unit patients: Assessment and awareness. Indian J Anaesth. 2011;55:553–5. doi: 10.4103/0019-5049.90607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsia SH, Wu CT, Wang HS, Yan DC, Chen SC. The use of bispectral index to monitor unconscious children. Pediatr Neurol. 2004;31:20–3. doi: 10.1016/j.pediatrneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Jung JY, Cho CB, Min BM. Bispectral index monitoring correlates with the level of consciousness in brain injured patients. Korean J Anesthesiol. 2013;64:246–50. doi: 10.4097/kjae.2013.64.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBlanc JM, Dasta JF, Kane-Gill SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006;40:490–500. doi: 10.1345/aph.1E491. [DOI] [PubMed] [Google Scholar]
- 15.Luebbehusen ML. Bispectral Index Monitoring: No longer used only in the OR, Bispectral Index Monitoring can now be used to help ensure that a patient is sedated safely and effectively. Technol Today. 2005;68:50–6. [Google Scholar]
- 16.Ma PL, Zhao JZ, Su JW, Li Q, Wang Y. Comparison of reliability of bispectral index and sedation-agitation scale in assessing the depth of sedation in patients treated with mechanical ventilation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:323–6. [PubMed] [Google Scholar]
- 17.Memis D, Turan A, Karamanlioglu B, Oguzhan N, Pamukcu Z. Comparison of sufentanil with sufentanil plus magnesium sulphate for sedation in the intensive care unit using bispectral index. Crit Care. 2003;7:R123–8. doi: 10.1186/cc2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondello E, Siliotti R, Noto G, Cuzzocrea E, Scollo G, Trimarchi G, et al. Bispectral Index in ICU: Correlation with Ramsay Score on assessment of sedation level. J Clin Monit Comput. 2002;17:271–7. doi: 10.1023/a:1021250320103. [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Daly D, Silvers A, Cairo S. Prediction of neurological outcome using bispectral index monitoring in patients with severe ischemic-hypoxic brain injury undergoing emergency surgery. Anesthesiology. 2009;110:1106–15. doi: 10.1097/ALN.0b013e31819daef6. [DOI] [PubMed] [Google Scholar]
- 20.Nasraway SA SA, Jr, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM. How reliable is the Bispectral Index in critically ill patients. A prospective, comparative, single-blinded observer study? Crit Care Med. 2002;30:1483–7. doi: 10.1097/00003246-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie MP, Pereira BM, Ryan ML, Gomez-Rodriguez JC, Pierre EJ, Livingstone AS, et al. Bispectral index to monitor propofol sedation in trauma patients. J Trauma. 2011;71:1415–21. doi: 10.1097/TA.0b013e3182178b8b. [DOI] [PubMed] [Google Scholar]
- 22.Paul DB, Umamaheswara Rao GS. Correlation of Bispectral Index with Glasgow Coma Score in mild and moderate head injuries. J Clin Monit Comput. 2006;20:399–404. doi: 10.1007/s10877-006-9045-9. [DOI] [PubMed] [Google Scholar]
- 23.Riker RR, Fraser GL, Wilkins ML. Comparing the bispectral index and suppression ratio with burst suppression of the electroencephalogram during pentobarbital infusions in adult intensive care patients. Pharmacotherapy. 2003;23:1087–93. doi: 10.1592/phco.23.10.1087.32766. [DOI] [PubMed] [Google Scholar]
- 24.Tonner PH, Paris A, Scholz J. Monitoring consciousness in intensive care medicine. Best Pract Res Clin Anaesthesiol. 2006;20:191–200. doi: 10.1016/j.bpa.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Vivien BT, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Weinert CR, Chlan L, Gross C. Sedating critically ill patients: Factors affecting nurses’ delivery of sedative therapy. Am J Crit Care. 2001;10:156–67. [PubMed] [Google Scholar]
- 27.Xifeng W, Lianshuang Z, Dawei W. Prediction of neurological outcome using bispectral index in patients with severe acute brain injury. Turk J Med Sci. 2013;43:718–25. [Google Scholar]


