Abstract
Endothelial progenitor cells are resident in the bone marrow blood sinusoids and circulate in the peripheral circulation. They mobilize from the bone marrow after vascular injury and home to the site of injury where they differentiate into endothelial cells. Activation and mobilization of endothelial progenitor cells from the bone marrow is induced via the production and release of endothelial progenitor cell-activating factors and includes specific growth factors and cytokines in response to peripheral tissue hypoxia such as after acute ischemic stroke or trauma. Endothelial progenitor cells migrate and home to specific sites following ischemic stroke via growth factor/cytokine gradients. Some growth factors are less stable under acidic conditions of tissue ischemia, and synthetic analogues that are stable at low pH may provide a more effective therapeutic approach for inducing endothelial progenitor cell mobilization and promoting cerebral neovascularization following ischemic stroke.
Keywords: endothelial progenitor cells, mobilization, growth factor, cytokine, neovascularization, ischemic stroke
Introduction
Worldwide, stroke is the second leading cause of death responsible for 4.4 million (9%) of the total 50.5 million deaths each year. In the USA stroke is the third leading cause of death, behind heart disease (with which it is closely linked) and cancer (Stroke Statistics. www.uhnj.org/stroke/stats.htm). Of all strokes, 87% are ischemic and 10% are intracerebral hemorrhagic strokes, while 3% are subarachnoid hemorrhage strokes (Go et al., 2013).
Regulation of cerebral blood flow is critical for the maintenance of neural function (Pratt et al., 2004). On exposure to hypoxic conditions, blood vessel networks of the cerebral microvasculature expand to meet the growing oxygen demands and brain capillary density increases over a 2-week period (Xu and LaManna, 2006). This neovascularization response requires new vessel formation as well as the remodeling of existing vasculature to form new collaterals. Growth factor-driven angiogenesis leads to increased capillary density, restoring tissue oxygen levels. After transient middle cerebral artery occlusion in rats followed by reperfusion, microvessel density increased especially within the inner margin of the cystic infarct, and was accompanied by increased leakiness to immunoglobulin (IgG) and fluorescein-dextran (Yu et al., 2007). Magnetic resonance imaging demonstrated increased angiogenesis 4 weeks after initiation of embolic stroke in rat brain (Ding et al., 2008).
Neovascularization (new blood vessel formation) occurs through vasculogenesis, angiogenesis, and/or arteriogenesis; all three can occur in response to tissue hypoxia and injury. Vasculogenesis and angiogenesis are the fundamental processes during formation of new blood vessels after vascular injury e.g., following ischemic stroke. Angiogenesis is defined as the growth of new vessels from preexisting ones, whereas vasculogenesis is the formation of new blood vessels by ‘de novo’ production of endothelial cells e.g., differentiation of precursor cells into endothelial cells (Risau, 1997; Eguchi et al., 2007). Bone marrow-derived endothelial progenitor cells (EPCs) are considered to play an important role in endogenous vascular repair after vascular injury and in the maintenance of endothelial integrity.
Activation and mobilization of endothelial progenitor cells
EPCs are immature endothelial cells that share common stem/progenitor cells and hematopoietic characteristics and circulate in the peripheral blood. The discovery of EPCs in the peripheral blood was first shown in 1997 (Asahara et al., 1997). These cells are mobilized from the bone marrow after vascular injury and home to the site of injury where they differentiate into endothelial cells (Figure 1). EPCs possess functional and structural characteristics of stem cells as well as of mature endothelial cells. They coexpress the surface markers CD34, CD133 and vascular endothelial growth factor receptor 2 (VEGFR2). The coexpression of specific surface markers like CD34, CD133, and VEGFR2 characterizes a specific progenitor cell subset at a specific maturation stage. The expression of CD34 decreases over time as EPCs differentiate towards endothelial cells (Asahara et al., 1997). Other surface markers that have been described to define subpopulations of EPCs are von Willebrand factor (vWF), CD31 (platelet endothelial cell adhesion molecule-1, PECAM-1), CD144 (vascular endothelial cadherin, VE-cadherin) and CXC chemokine receptor type 4 (CXCR4) (Sabatier et al., 2009).
Figure 1.
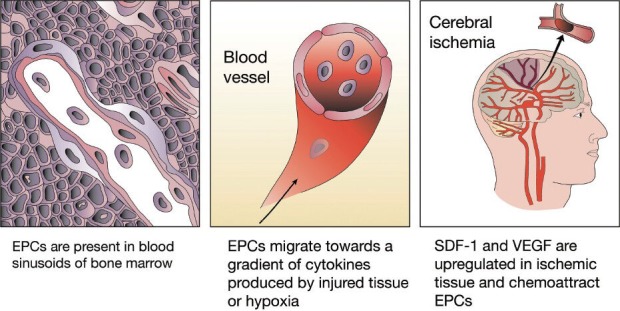
Homing and functional role of endothelial progenitor cells (EPCs) in post-cerebral ischemia.
EPCs migrate from the bone marrow via blood vessels to sites of tissue injury and ischemia. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 are critical mediators for ischemia-specific recruitment of circulating EPCs. Hypoxia-induced vascular endothelial growth factor (VEGF) expression precedes neovascularization after cerebral ischemia. Early EPCs secrete mainly proangiogenic cytokines including VEGF, placental growth factor, transforming growth factor-β, thrombopoietin, hepatocyte growth factor, fibroblast growth factor, macrophage migration inhibitory factor, macrophage colony stimulating factor, interleukin-8, as well as a few antiangiogenic cytokines, and also neurotrophic and neuroregulatory cytokines including brain-derived neurotrophic factor.
EPCs are obtained from isolated peripheral mononuclear cells (MNCs) in culture, and can be divided into two morphologically and functionally different populations, namely the ‘early outgrowth EPCs’ or circulating angiogenic cells (CACs), and the ‘late outgrowth EPCs’ or endothelial colony-forming cells (ECFCs). The first type are largely quiescent cells, while the second type form highly proliferative endothelial colonies derived from single cells and spontaneously display capillary tube-like formation in Matrigel. Early EPCs are derived from a myeloid lineage and only early EPCs express the myeloid markers CD45 and CD14 (Yoder et al., 2007; Zhang et al., 2009). Early EPCs secrete predominantly proangiogenic cytokines including vascular endothelial growth factor (VEGF), placental growth factor (PlGF), transforming growth factor-β (TGF-β), thrombopoietin (TPO), hepatocyte growth factor (HGF), fibroblast growth factors (FGFs), macrophage migration inhibitory factor (MIF), macrophage colony stimulating factor (MCSF), interleukin-8 (IL-8), as well as a few antiangiogenic cytokines, and also neurotrophic and neuroregulatory cytokines including brain-derived neurotrophic factor (BDNF) which may enable EPCs to exert an important trophic influence on neuronal tissue (He et al., 2004).
Activation and mobilization of EPCs from the bone marrow is induced via the production and release of EPC-activating factors e.g. hypoxia-inducible factor-1α (HIF-1α), VEGF or erythropoietin in response to peripheral tissue hypoxia such as after acute ischemic stroke or trauma (Hoenig et al., 2008). In the periphery, stromal cell-derived factor 1 (SDF-1) mediates migration and homing of EPCs to the vascular endothelium through a CXCR4 dependent mechanism (Figure 1) (Zheng et al., 2007; Tilling et al., 2009).
HIF-1α expression in hypoxic tissue is upregulated in a time-related manner around the ischemic boundary zone and induces various signaling pathways, one of which involves upregulation of VEGF (Marti et al., 2000; Althaus et al., 2006). VEGF mRNA expression is evident 1 hour after reperfusion following middle cerebral artery occlusion in rat and peaks at 3 to 24 hours thereafter (Hayashi et al., 1997; Plate et al., 1999). VEGF mediates endothelial nitric oxide synthase (eNOS) expression of nitric oxide (NO) following cerebral ischemia (Chen et al., 2005a). eNOS regulates BDNF expression in the ischemic brain and influences neuroprogenitor cell proliferation, neuronal migration, and neurite outgrowth and affects functional recovery after stroke in mice (Chen et al., 2005a).
Mobilization of EPCs from the bone marrow is also dependent on the production of NO and the local activity of matrix metalloproteinases (Heissig et al., 2002). EPCs are considered to migrate and home to affected sites like the penumbra following ischemic stroke via cytokine gradients, where they act in a paracrine fashion leading to endothelial cell proliferation and stabilization or through differentiation into endothelial cells. Several EPC-activating factors such as VEGF, SDF-1, monocyte chemotactic protein-1 (MCP-1) are also involved in the neovascularization process of the damaged tissue.
Angiogenic potential of endothelial progenitor cells influenced by growth factors and cytokines
The influence of growth factors and cytokines on angiogenic potential of human EPCs in vitro studies has been recently reviewed (Peplow, 2014). Indices of angiogenic potential are chemotactic migration, capillary tube-like formation, proliferation, and apoptosis. Early EPCs are taken to be the cell type obtained by short-term culture of MNCs on fibronectin-coated dishes for up to 7 days, with a spindle-shaped morphology and double positive for DiI-acLDL uptake and Ulex europaeus lectin-1 (UEA-1) binding, and do not spontaneously form capillary tube-like structures in Matrigel. Late EPCs are taken to be the cell type obtained by longer-term culture for 2 to 4 weeks of MNCs on fibronectin or collagen, give a cobblestone-like appearance to the monolayer, are double positive for DiI-acLDL uptake and lectin binding, and spontaneously display capillary-like tube formation when placed on Matrigel coated dishes (Goretti et al., 2013). Members of various families of growth factors and cytokines were tested for effect. These included the proangiogenic factors VEGF and PlGF of VEGF family, FGF-2 of FGF family, monocyte chemoattractant protein-1 (MCP-1) of C-C chemokine family, and SDF-1 which belongs to the intercrine family and is upregulated by factors such as stem cell factor (SCF), IL-6, tumor necrosis factor-α (TNF-α) and downregulated by interferon-β (IFN-β) (Peled et al., 1999; Tran, 2006); nerve growth factor (NGF) and BDNF of the nerve growth factor family; other growth factors such as SCF, TGF-β1, macrophage stimulating protein (MSP), TPO, and the proinflammatory cytokines IL-1β, IL-3, and TNF-α.
In the chemotactic assays, SDF-1, VEGF, IL-1β, MIF, PlGF and TPO when tested alone increased the migration of early EPCs. A much greater migration occurred to MIF at 10 ng/mL (0.8 nmol/L) than to SDF-1 at 200 ng/mL (22 nmol/L), indicating that MIF can influence cell types other than macrophages. Moreover, increased migration was found for SDF-1 and VEGF in combination. Pretreatment of early EPCs with TNF-α at 10–100 μg/mL decreased migration towards VEGF. Increased migration of early CD34+ cells occurred with SDF-1, VEGF and MCP-1 when tested alone. For the assays of capillary tube-like formation, early EPCs were stimulated to form tube-like structures by VEGF, as also were early CD34+ cells by SDF-1 and FGF-2. VEGF increased tube-like formation by late EPCs and late CD34+ cells. TGF-β1 did not modify the capacity for tube-like formation by late EPCs. Pretreatment with TNF-α had the potential to decrease tube-like formation of early and late EPCs. The proliferation of early EPCs was increased by SDF-1 and VEGF, while that of CD34+ cells was increased by NGF, SCF, IL-3, TPO, and granulocyte macrophage colony-stimulating factor (GM-CSF). TNF-α decreased proliferation of early and late EPCs. Apoptosis of early EPCs was reduced by BDNF. Moreover, SDF-1, VEGF2 and TPO, when tested separately, reduced apoptosis of serum-starved early EPCs; SDF-1 and VEGF in combination may exert a synergistic effect on cell survival. All these findings were from studies performed under neutral conditions (pH 7.4).
Extracellular acidosis is a common feature of injured tissues and tumor microenvironment and is an important regulator of cell survival and activation. It is a typical feature of the inflammatory microenviroment and present during the processes of wound healing, tumor growth, hypoxia or ischemia (Trevani et al., 1999; Kumar et al., 2007; Chiche et al., 2010). Transplanted EPCs in animal studies and clinical trials are likely to be exposed to an acidic inflammatory milieu, and it would be pertinent to determine the effect of acidosis on functional features of EPCs including angiogenic potential. The proliferation of CD34+ cells induced by TPO, SCF or IL-3 alone was completely inhibited when the cells were treated for 1 minute prior to acidic exposure (pH 6.5), while that brought about by GM-CSF was markedly reduced when the cells were exposed to acidic conditions for 15 minutes. It was hypothesized that the biological activity of these factors was impaired at low pH values, with the proliferation of CD34+ cells being completely abolished by acidic treatment of VEGF and GM-CSF. A combination of TPO, SCF and IL-3 supported CD34+ proliferation in acidic medium, suggesting that these factors acted synergistically with each other (D’Atri et al., 2011). In addition, VEGF and GM-CSF alone did not reduce apoptosis of CD34+ cells exposed to acidic conditions (pH 6.5). Pretreatment of cells with TPO, SCF or IL-3 resulted in a significant decrease in apoptosis at pH 6.5, with IL-3 having the greatest effect. A combination of TPO, SCF and IL-3 prevented CD34+ cell death. That each growth factor and cytokine alone did not preserve cell functionality indicated that prevention of CD34+ cell death by acidosis required the synergistic action of several overlapping signaling cascades triggered by TPO, SCF and IL-3 (D’Atri et al., 2011).
Regulation of cerebral blood flow in the ischemic brain
Stroke patients with greater cerebral blood vessel density appear to make better progress and survive longer than patients with lower vascular density (Krupinski et al., 1994). Physical activity improves long-term stroke outcome by eNOS-dependent mechanisms that increase angiogenesis and cerebral blood flow (Gertz et al., 2006). As mentioned earlier, VEGF mediates eNOS expression of nitric oxide (NO) following cerebral ischemia (Chen et al., 2005a). eNOS regulates BDNF expression in the ischemic brain and influences neuroprogenitor cell proliferation, neuronal migration, and neurite outgrowth and affects functional recovery after stroke in mice (Chen et al., 2005a). VEGF and angiopoietin-1 (Ang-1) with its Tie receptor (Tie-2) are important angiogenic factors that control angiogenesis to form large and small vessels in the mature vascular system (Marti and Risau, 1999). However, VEGF also causes blood-brain barrier leakage, inflammation and brain edema. A combination of submaximal doses of VEGF and Ang-1 enhances angiogenesis and is more effective than the maximal dose of either alone (Chae et al., 2000; Valable et al., 2005; Zhu et al., 2006). Coadministration of VEGF with Ang-1 also reduces blood-brain barrier leakage compared to VEGF alone (Valable et al., 2005; Zhu et al., 2006).
Stem cells from multiple sources such as bone marrow cells, embryonic, and peripheral blood stem cells can promote angiogenesis within ischemic tissue (Jackson et al., 2001; Kocher et al., 2001). Post-ischemic angiogenesis requires participation of bone-marrow derived EPCs (Zhang et al., 2002), perivascular microglia, and pericytes (Hill et al., 2004; Kokovay et al., 2006). Stem cell therapeutic approaches have the potential to be the most promising future therapies for poststroke neovascularization and regeneration.
Future perspectives
Despite these recent advances, there remains several challenges facing the translation of cellular therapies for stroke from in vitro and animal studies to clinical trials. Some growth factors may be less stable under the acidic conditions of tissue ischemia and the biological activity of these factors impaired, thereby limiting their usefulness as exogenous mediators. Growth factor analogues that are stable at low pH may provide a better therapeutic strategy. A synthetic SDF polypeptide analog has been engineered that is more effective in inducing EPC migration, including greater stability and function, compared with exogenous administered SDF-1α (Hiesinger et al., 2012). Statins are widely used for preventing stroke and in rodents have been shown to induce the expression of VEGF and BDNF and promote mobilization and proliferation of EPCs (Zhang et al., 2003; Chen et al., 2005b). Circulating EPC levels are increased in patients with ischemic stroke treated with statins during the acute phase (Sobrino et al., 2012). Low circulating EPC level was independently predictive of severe neurological impairment and major adverse clinical outcomes in patients after acute ischemic stroke (Yip et al., 2008). Administration of an inhibitor of TNF-α production up to 6 hours after ischemia reduces brain edema in rats in which the middle cerebral artery has been occluded (Vakili et al., 2011). New possibilities for combinatorial therapies using administered growth factor analogues or inhibitors/antagonists, statins, and EPCs are opened up for the treatment of patients with ischemic stroke.
Footnotes
Conflicts of interest: None declared.
References
- 1.Althaus J, Bernaudin M, Petit E, Toutain J, Touzani O, Rami A. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int. 2006;48:687–695. doi: 10.1016/j.neuint.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005a;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005b;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiche J, Brahimi-Horn MC, Pouyssegur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Atri LP, Etulain J, Romaniuk MA, Torres O, Negrotto S, Schattner M. The low viability of human CD34+ cells under acidic conditions is improved by exposure to thrombopoietin, stem cell factor, interleukin-3, or increased cyclic adenosine monophosphate levels. Transfusion. 2011;51:1784–1795. doi: 10.1111/j.1537-2995.2010.03051.x. [DOI] [PubMed] [Google Scholar]
- 8.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Gollapalli L, Panda S, Li Q, Ewing JR, Chopp M. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008;39:1563–1568. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- 10.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, et al. Heart disease and stoke statistics - 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goretti E, Rolland-Turner M, Leonard F, Zhang L, Wagner DR, Devaux Y. MicroRNA-16 affects key functions of human endothelial progenitor cells. J Leukoc Biol. 2013;93:645–655. doi: 10.1189/jlb.1012511. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 14.He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 15.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafil S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of Kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, McCormick RC, Muenzer JR, Yang EC, Levit RD, Yuan LJ, Macarthur JW, Saven JG, Woo YJ. Computational protein design to reengineer stromal cell-derived factor-1α generates an effective and translatable angiogenic polypeptide analog. Circulation. 2012;124(Suppl 11):S18–26. doi: 10.1161/CIRCULATIONAHA.110.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropath Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1á endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Curr Drug Targets. 2008;9:422–435. doi: 10.2174/138945008784221215. [DOI] [PubMed] [Google Scholar]
- 19.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 21.Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 22.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Kasseckert S, Kostin S, Abdallah Y, Schafer C, Kaminski A, Reusch HP, Piper HM, Steinhoff G, Ladilov Y. Ischemic acidosis causes apoptosis in coronary endothelial cells through activation of caspase-12. Cardiovasc Res. 2007;73:172–180. doi: 10.1016/j.cardiores.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Marti HH, Risau W. Angiogenesis in ischemic disease. Thromb Haemost. 1999;82:44–52. [PubMed] [Google Scholar]
- 25.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 27.Peplow PV. Influence of growth factors and cytokines on angiogenic function of endothelial progenitor cells: a review of human in vitro studies. Growth Factors In press. 2014 doi: 10.3109/08977194.2014.904300. [DOI] [PubMed] [Google Scholar]
- 28.Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–666. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Pratt PF, Medhora M, Harder DR. Mechanisms regulating cerebral blood flow as therapeutic targets. Curr Opin Investig Drugs. 2004;5:952–956. [PubMed] [Google Scholar]
- 30.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 31.Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13:454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobrino T, Blanco M, Perez-Mato M, Rodriguez-Yanez M, Castillo J. Increased levels of circulating endothelial progenitor cells in patients with ischemic stroke treated with statins during acute phase. Eur J Neurol. 2012;19:1539–1546. doi: 10.1111/j.1468-1331.2012.03770.x. [DOI] [PubMed] [Google Scholar]
- 33.Stroke Statistics. www.uhnj.org/stroke/stats.htm .
- 34.Tran CA. Ann-Arbor: ProQuest Publishing; 2006. Inhibition of preadipocyte differentiation by TNF-alpha and interferon (alpha, beta, and gamma): global gene expression analysis. [Google Scholar]
- 35.Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68:484–492. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, Geffner JR. Extracellular acidification induces human neutrophil activation. J Immunol. 1999;162:4849–4857. [PubMed] [Google Scholar]
- 37.Vakili A, Mojarrad S, Athavan MM, Rashidy-Pour A. Pentoxifylline attenuates TNF-α protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res. 2011;1377:119–125. doi: 10.1016/j.brainres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Valable S, Montaner J, Bellail A, Berezowski V, Brillaut J, Cecchelli R, Divoux D, MacKenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 39.Xu K, LaManna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol. 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- 40.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 41.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2007;27:755–763. doi: 10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelium growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ingram DA, Murphy MP, Reza Saadatzadeh M, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–1682. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone-marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Shwe Y, Du R, Chen Y, Shen FX, Young WL, Yang GY. Effects of angiopoietin-1 on vascular endothelial growth factor-induced angiogenesis in the mouse brain. Acta Neurochir Suppl. 2006;96:438–443. doi: 10.1007/3-211-30714-1_90. [DOI] [PubMed] [Google Scholar]


