Abstract
Oxidative stress is closely associated with secondary cell death in many disorders of the central nervous system including stroke, Parkinson's disease, Alzheimer's disease. Among many aberrant oxidative stress-associated proteins, DJ-1 has been associated with the oxidative stress cell death cascade primarily in Parkinson's disease. Although principally expressed in the cytoplasm and nucleus, DJ-1 can be secreted into the serum under pathological condition. Recently, a close pathological association between DJ-1 and oxidative stress in stroke has been implicated. To this end, we and others have demonstrated the important role of mitochondria in neuroprotection for stroke by demonstrating that the translocation of DJ-1 in the mitochondria could potentially mitigate mitochondrial injury. Here, we discuss our recent findings testing the hypothesis that DJ-1 not only functions as a form of intracellular protection from oxidative stress, but that it also utilizes paracrine and/or autocrine cues in order to accomplish extracellular signaling between neighboring neuronal cells, resulting in neuroprotection. This article highlights recent evidence supporting the status of DJ-1 as key anti-oxidative stress therapeutic target for stroke.
Keywords: cerebral ischemia, neuroprotection, mitochondria, translocation, extracellular signaling
Targeting oxidative stress pathway for arresting stroke secondary cell death
Despite significant scientific progress in the understanding and treatment of stroke, developing new and effective therapies to combat the disease continues to be a primary clinical concern. Neural tissue damage caused by stroke is attributable to a disruption in blood flow to the brain, resulting in a lack of glucose, oxygen, and other nutrients. This in turn results in an infarcted core, which quickly becomes necrotic and the formation of an ischemic penumbra. This penumbra has been shown to be receptive to therapeutic interventions during the sub-acute period (Heiss, 2000; Warach, 2003; Lo, 2008). As time progresses, the penumbra assimilates to become part of the necrotic core via various mechanisms of secondary cell death. Among the many neurodegenerative pathways, oxidative stress has been proven to aggravate the process of secondary cell death in the central nervous system not only in stroke, but in Parkinson's disease (PD) and Alzheimer's disease as well (Dawson and Dawson, 2003; Nakamura and Lipton, 2010). DJ-1 is a multifunctional redox-sensitive protein that has been associated with the oxidative stress cell death cascade. DJ-1 provides neuroprotection from this form of cell death in multiple pathways, but most importantly by reducing mitochondrial oxidative stress (Canet-Avilés et al., 2004), molecular chaperoning of PD-aggregating protein α-synuclein (Dawson and Dawson, 2003), stimulating anti-apoptotic and antioxidative gene expression (Clements et al., 2006; Fan et al., 2008), promoting the pro-survival Akt pathway while impeding the apoptosis signal-regulating kinase pathway (Junn et al., 2005; Yang et al., 2005; Gorner et al., 2007), and by acting as a positive regulator of androgen receptor-dependent transcription (Takahashi et al., 2001; Dawson and Dawson, 2003; Niki et al., 2003; Tillman et al., 2007). Typically found in the cytoplasm and nucleus, DJ-1 can be secreted into the serum in the presence of pathology, such as melanoma or breast cancer (Tsuboi et al., 2008; Waak et al., 2009), and can also be translocated into the mitochondria of various mammalian cells by mitogen stimulation or, of particular interest, oxidative stress (Canet-Avilés et al., 2004; Tsuboi et al., 2008, Junn et al., 2009). As an additional testament to the close association between DJ-1 and oxidative stress following stroke, as well as the importance of mitochondria in preventing free radical generation, this migration to the mitochondria has been linked to a decrease in aberrant formation of free radicals such as mitochondrial reactive oxygen species (Canet-Avilés et al., 2004; Nakamura and Lipton, 2010). Here, we discuss our recent findings that DJ-1 not only functions as a form of intracellular protection from oxidative stress, but that it also utilizes paracrine and/or autocrine cues in order to accomplish extracellular signaling between neighboring neuronal cells, resulting in neuroprotection (Kaneko et al., 2014a, b). Our commitment to the advancement of cell therapy from the laboratory to the clinical setting led us to the use of human neural progenitor cells (hNPCs) as a medium to determine whether DJ-1 translocated into the mitochondria and secreted under hypoxic-ischemic conditions (Kaneko et al., 2014a). We confirmed the hypothesis that DJ-1 translocated into the mitochondria with subsequent DJ-1 protein secretion into the serum, using cultured primary rat neural cells (PRNCs; ratio of astrocytes/neurons is 6/4) exposed to an experimental stroke condition (Kaneko et al., 2014b).
DJ-1: key anti-oxidative stress therapeutic target for stroke
In our papers (Kaneko et al., 2014a, b), we showed that under oxygen glucose deprivation (OGD), an established experimental model of stroke, DJ-1 had translocated into the healthy mitochondria, and DJ-1 protein was secreted by the injured hNPCs and PRNCs. Capturing of extracellular DJ-1 by using anti-DJ-1 antibody had reduced both cell viability and mitochondrial activity, whereas intracellular glutathione (GSH) levels, a marker of oxidative stress, significantly increased. Interestingly, OGD also reversed the ratio of astrocyte-to-neuron cells, from a ratio of 6:4 to 4:6, indicating that astrocytes rescue neurons by unknown mechanisms. Our findings revealed that DJ-1 plays an active role in the early phase of experimental stroke by integrating the mitochondrial pathway: DJ-1 expression was immediately increased after the injury and had efficiently translocated into the mitochondria in both hNPCs and PRNCs.
Next, we focused on oxidative damage, a major secondary cell death pathway subsequent to the OGD-reperfusion phase (Pompella et al., 2003; Shelly, 2009). We measured GSH production, which may render neuroprotective effects against reactive oxygen species (ROS) and may also modulate cell proliferation. Compared to normoxic conditions, the increase in GSH production in hNPCs after 2-hour reperfusion following OGD was approximately two-fold. This indicates that an endogenous repair mechanism was activated to protect the hNPCs from oxidative damage, by facilitating an increase in intracellular GSH levels in response to cell death injury. This supports previous findings and implicates that secreted type DJ-1 exerts neuroprotective effects in stem/progenitor cells (Mullett et al., 2008; Gao et al., 2012).
After OGD exposure, cell viability and mitochondrial activity were significantly decreased. In contrast, GSH levels were increased, compared with normoxic conditions. Physiologically, the loss of DJ-1 perturbs the endogenous and protective actions of PRNCs against oxidative stress (Martinat et al., 2004; Miyazaki et al., 2008).
Localization of DJ-1 in the PRNCs was viewed under immunefluorescent microscopy. Of interest, the results revealed translocation of DJ-1 into the mitochondrial inner membrane following the hypoxic-ischemic insult (Figure 1). This illustrates that DJ-1 serves as a sensitive biomarker of early protection against neurodegeneration, in response to acute hypoxic-ischemic injury, and may be detected both secreted type DJ-1 and intercellular type DJ-1 (Martinat et al., 2004; Yanagisawa et al., 2008).
Figure 1.
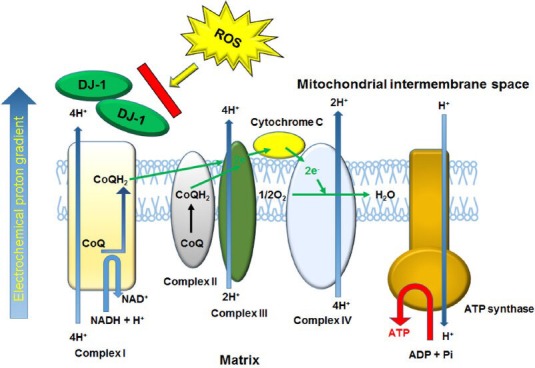
Summarized DJ-1 function for protecting mitochondrial function.
Under ischemic condition, cytoplasmic DJ-1 translocated into the mitochondrial inter membrane where the electron transport chain enzymes (complex I–IV and ATP synthase) generate ATP, and protected mitochondria complex I against ROS such as superoxide. DJ-1 dysfunction not only induces the cytochrome c-mediated apoptosis signaling pathway (Osman et al., 2011) but also damages mitochondrial DNA, leading to neurodegenerative diseases (cf. PD). CoQ: Ubiquinone (oxidized form). CoQH2: ubiquinol (reduced form).
In cultured PRNCs subjected to normoxic and hypoxic-ischemic conditions, we counted the number of MAP2-positive neuronal cells and extrapolated the number of astrocytes. Our analysis revealed that the hypoxia-ischemia had significantly reversed the ratio of astrocytes to neurons (6:4) when compared with normoxic conditions (4:6) (P < 0.01). Additionally, DJ-1 selectively translocated into healthy (polarized) mitochondria comparison with damaged (depolarized) mitochondria, although it is unclear that hypoxic-ischemic condition affects the DJ-1 dynamics. Our experiments demonstrate that DJ-1 is involved with the preservation of functional mitochondria; DJ-1 co-localized with active mitochondria, but not with inactive mitochondria.
Perspective: advancing DJ-1 for stroke therapeutics
Our recent reports provided insights into the molecular mechanism of DJ-1 for its role in neuroprotection against stroke model. We showed a novel neuroprotective event involving DJ-1 translocation into the mitochondria following hypoxemia in both hNPCs and PRNCs. We found similar outcomes implicating DJ-1 mitochondrial translocation while using either hNPCs or PRNCs; therefore, based on our studies, we can deduce that DJ-1 has a direct action on stem/progenitor cells. Our results indicate that under hypoxic conditions, DJ-1 favors neuronal rescue, as there is an observed reversal of the astrocyte to neuron rescue ratio (6:4 to 4:6). This discovery opens new opportunities for the development of therapeutics aimed at protecting against mitochondrial deficits, including stroke and other neurological disorders. An alternative possibility is that astrocytes are more vulnerable against ROS/lipid peroxides, and this warrants further investigation. Our results also revealed that DJ-1 is secreted from neuronal cells, DJ-1 is sequestered. Decreased levels of DJ-1 correspond to a decline in mitochondrial activity, and ultimately to decreased cell viability under ischemic, or OGD conditions. This observation demonstrates that DJ-1 must undergo mitochondrial translocation following extracellular secretion under ischemic conditions, thus revealing the neuroprotective role DJ-1 has during states of hypoxia, such as in stroke.
A key function of DJ-1 is in maintaining the activity of mitochondrial complex I under oxidative stress (Figure 1) (Hayashi et al., 2009; Kahle et al., 2009; Ircher et al., 2010). Following ischemic neuronal cell death, mitochondrial complex I automatically releases ROS, which subsequently acts upon the neighboring environment, inducing additional mitochondria to generate ROS via permeability transition pore opening, in an attempt to halt progression of secondary cell death. The mitochondrial complex I tightly regulates ATP production in eukaryotic cells; dysfunction of the complexes induces cell death (Taira et al., 2004; Foti et al., 2010). Translocation of DJ-1 into the mitochondria sequesters ROS-induced toxicity endogenously. Analogous findings have been observed in cases of breast cancer and melanoma, where DJ-1 is released under hypoxic conditions into the serum in vitro and in vivo (Le Naour et al., 2001; Miura et al., 2002; Kim et al., 2010). The translocation of DJ-1 into the mitochondria may turn the course of cellular activity toward pro-survival mechanisms, including mitochondrial movement and increased cell-to-cell interaction. In addition, DJ-1 may also act through multi-pronged neuroprotective processes, as evidenced through the varied results received on MTT and cell survival following sequestration of DJ-1.
To date, there have been a number of potential therapeutic molecules that undergo nuclear translocation, and thereafter exerting neuroprotection in stroke (Yang et al., 2008, 2009; Gan et al., 2010; Pallast et al., 2010). Hence, our DJ-1 studies support the postulation that DJ-1 plays a key role in maintaining functioning mitochondria and a dynamic cell structure (Figure 1), thus maintaining a balance between fission and fusion. Our present findings are complemented by recent literature that links DJ-1 to inflammatory pathways and oxidative stress (Aleyasin et al., 2007; Yanagisawa et al., 2008; Mullett et al., 2009). Although at the time of ischemic injury we found increased levels of the antioxidant GSH that correspond to mitochondrial translocation of DJ-1, sequestration of extracellular DJ-1 with antibody resulted in increased GSH levels, but decreased mitochondrial activity and therefore cell viability. Use of stem/progenitor cells, in this case specifically PRNCs, allowed us to represent both cell types. These findings implicate that although GSH plays an important role in neuroprotection during stroke, its effects are inconsequential in the presence of DJ-1 deficit.
The mitochondria stands as a viable therapeutic target for the treatment of stroke as evidenced through various pathways. Indeed, altered expression of the primary inhibitor of tissue-type plasminogen activators, plasminogen activator inhibitor-1, promotes apoptotic sequence involving cytochrome c release from the mitochondria (Soeda et al., 2008). Moreover, the small amino acid N-acetylaspartate, synthesized by neuronal mitochondria, is released into serum following reperfusion in animal models of brain ischemia and in serum of patients following acute ischemic stroke (Elting et al., 2004). Additionally, DJ-1 promotes angiogenesis and osteogenesis by way of activating fibroblast growth factor receptor-1 signaling (Kim et al., 2012).
In consideration of the our recent findings, specifically that DJ-1 extracellular secretion and mitochondrial translocation occur in cultured hNPCs and PRNCs, it is plausible that an in vivo model may mimic the effects of DJ-1 in the stem progenitor cell-populated neurogenic sites of lateral ventricle and hippocampal dentate gyrus. In concert with other studies (Görner et al., 2007; Vasseur et al., 2009; Aron et al., 2010; Yan and Pu, 2010), we advance the concept that DJ-1 is a protein essential for the maintenance of stem/progenitor cell survival in response to ischemia, which presents a unique strategy for treating stroke.
Footnotes
Funding: NT is funded by USF School of Physical Therapy and Rehabilitation Sciences; CVB, NT, and YK are funded by USF Department of Neurosurgery and Brain Repair, NIH 1R01NS071956-01A1, Department of Defense W81XWH-11-1-0634 and the James and Esther King Biomedical Research Foundation 1KG01-33966.
Conflicts of interest: None declared.
References
- 1.Aleyasin H, Rousseaux MW, Phillips M, Kim RH, Bland RJ, Callaghan S, Slack RS, During MJ, Mak TW, Park DS. The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron L, Klein P, Pham TT, Kramer ER, Klein R. Pro-survival role for Parkinson's associated gene DJ-1 revealed in trophically impaired dopaminergic neurons. PLoS Biol. 2010;6:e1000349. doi: 10.1371/journal.pbio.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1 a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 6.Elting J, Sulter G, Langedij M, Luijckx G, Teelken A, De Keyser J. N-acetylaspartate: serum marker of reperfusion in ischemic stroke. J Stroke Cerebrovasc Dis. 2004;13:254–258. doi: 10.1016/j.jstrokecerebrovasdis.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- 8.Foti R, Zucchelli S, Biagioli M, Roncaglia P, Vilotti S, Calligaris R, Krmac H, Girardini JE, Del Sal G, Gustincich S. Parkinson disease-associated DJ-1 is required for the expression of the glial cell line-derived neurotrophic factor receptor RET in human neuroblastoma cells. J Biol Chem. 2010;285:18565–18574. doi: 10.1074/jbc.M109.088294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neuroscu. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao HH, Yang WW, Qi ZZ, Lu LL, Duan CC, Zhao CC, Yang HH. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J Mol Biology. 2012;423:232–248. doi: 10.1016/j.jmb.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Görner K, Holtorf E, Waak J, Pham TT, Vogt-Weisenhorn DM, Wurst W, Haass C, Kahle PJ. Structural determinants of the c-terminal helix-kink-helix Gorner motif essential for protein stability and survival promoting activity of DJ-1. J Biol Chem. 2007;282:13680–13691. doi: 10.1074/jbc.M609821200. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Ishimori C, Takahashi-Niki K, Taira T, Kim YC, Maita H, Maita C, Ariga H, Iguchi-Ariga SM. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commu. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Heiss WD. Ischemic penumbra: Evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Irrcher I, Aleyasin H, Seifert E, Hewitt S, Chhabra S, Phillips M, Lutz AK, Rousseaux MWC, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park D. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 15.Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci U S A. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junn E, Jang W, Zhao X, Jeong B, Mouradian M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko Y, Shojo H, Burns J, Staples M, Tajiri N, Borlongan CV. DJ-1 ameliorates ischemic cell death in vitro possibly via mitochondrial pathway. Neurobiol Dis. 2014a;62:56–61. doi: 10.1016/j.nbd.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneko Y, Tajiri N, Shojo H, Borlongan CV. Oxygen glucose-deprived rat primary neural cells exhibit DJ-1 translocation into healthy mitochondria: A potent stroke therapeutic target. CNS Neurosci Ther. 2014b;20:275–281. doi: 10.1111/cns.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YC, Kitaura H, Iguchi-Ariga SM, Ariga H. DJ-1 an oncogene and causative gene for familial Parkinson's disease, is essential for sv40 transformation in mouse fibroblasts through up-regulation of c-myc. FEBS Lett. 2010;584:3891–3895. doi: 10.1016/j.febslet.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Shin HI, Cha SS, Lee CS, Hong BS, Lim S, Jang HJ, Kim J, Yang YR, Kim YH, Yun S, Rijal G, Lee-Kwon W, Seo JK, Gho YS, Ryu SH, Hur EM, Suh PG. DJ-1 promotes angiogenesis and osteogenesis by activating FGF receptor-1 signaling. Nat Commun. 2012;3:1296. doi: 10.1038/ncomms2313. [DOI] [PubMed] [Google Scholar]
- 22.Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, Hanash SM. Proteomics-based identification of rs/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–3335. [PubMed] [Google Scholar]
- 23.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nature medicine. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 24.Martinat C, Shendeiman S, Alan I, Thomas L, Flint Beal MM, Yang L, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: An ES-derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:1754–1763. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura K, Bowman ED, Simon R, Peng AC, Robles AI, Jones RT, Katagiri T, He P, Mizukami H. Laser capture microdissection and microarray expression analysis of lung adenocarcinoma reveals tobacco smoking- and prognosis-related molecular profiles. Cancer Res. 2002;62:3244–3250. [PubMed] [Google Scholar]
- 26.Miyazaki S, Yanagida T, Nunome K, Ishikawa S, Inden M, Kitamura Y, Nakagawa S, Taira T, Hirota K, Ariga H. DJ-1-binding compounds prevent oxidative stress-induced cell death and movement defect in Parkinson's disease model rats. J Neurochem. 2008;105:2418–2434. doi: 10.1111/j.1471-4159.2008.05327.x. [DOI] [PubMed] [Google Scholar]
- 27.Mullett SJ, Hamilton RL, Hinkle DA. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology. 2009;29:125–131. doi: 10.1111/j.1440-1789.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Lipton SA. Redox regulation of mitochondrial fission protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis. 2010;15:1354–1363. doi: 10.1007/s10495-010-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SMM, Ariga H. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res. 2003;1:247–261. [PubMed] [Google Scholar]
- 30.Osman C, Voelker DR, Thomas Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:14499–14503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 33.Shelly CL. Regulation of glutathione synthesis. Mol Asp Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemos. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- 35.Taira T, Saito Y, Niki T, Iguchi-Ariga S, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. Embo Reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi T, Taira T, Niki T, Seino C, Iguchi-Ariga SMM, Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 37.Tillman JE, Yuan J G, Gu G, Fazli L, Ghosh R, Flynt AS, Gleave M, Rennie PS, Kasper S. DJ-1 binds androgen receptor directly and mediates its activity in hormonally treated prostate cancer cells. Cancer Res. 2007;67:4630–4637. doi: 10.1158/0008-5472.CAN-06-4556. [DOI] [PubMed] [Google Scholar]
- 38.Tsuboi Y, Munemoto H, Ishikawa S, Matsumoto K, Iguchi-Ariga SM, Ariga H. DJ-1, a causative gene product of a familial form of Parkinson's disease, is secreted through microdomains. FEBS Lett. 2008;582:2643–2649. doi: 10.1016/j.febslet.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 39.Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci U S A. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waak J, Weber SS, Waldenmaier A, Görner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham TT, Reumers V, Baekelandt V, Wurst W, Kahle PJ. Regulation of astrocyte inflammatory responses by the Parkinson's disease-associated gene dj-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 41.Warach S. Measurement of the ischemic penumbra with MRI: it's about time. Stroke. 2003;34:2533–2534. doi: 10.1161/01.STR.0000092395.19554.9A. [DOI] [PubMed] [Google Scholar]
- 42.Yan H, Pu XP. Expression of the Parkinson's disease-related protein DJ-1 during neural stem cell proliferation. Biol Pharm Bull. 2010;33:18–21. doi: 10.1248/bpb.33.18. [DOI] [PubMed] [Google Scholar]
- 43.Yanagisawa D, Kitamura Y, Inden M, Takata K, Taniguchi T, Morikawa S, Morita M, Inubushi T, Tooyama I, Taira T, Iguchi-Ariga SM, Akaike A, Ariga H. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J Cereb Blood Flow Metab. 2008;28:563–78. doi: 10.1038/sj.jcbfm.9600553. [DOI] [PubMed] [Google Scholar]
- 44.Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemiainduces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008;28:892–896. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Ma Q, Mackensen GB, Paschen W. Deep hypothermia markedly activates thesmall ubiquitin-like modifier conjugation pathway; implications for the fate ofcells exposed to transient deep hypothermic cardiopulmonary bypass. J Cereb Blood Flow Metab. 2009;29:886–980. doi: 10.1038/jcbfm.2009.16. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]


