Abstract
Danhong injection (DHI), a Chinese Materia Medica standardized product extracted from Radix Salviae miltiorrhizae and Flos Carthami tinctorii, is widely used in China for treating acute ischemic stroke. In the present study, we explored the neuroprotective efficacy of DHI in a rat model of temporary middle cerebral artery occlusion, and evaluated the potential mechanisms underlying its effects. Pretreatment with DHI (0.9 and 1.8 mL/kg) resulted in a significantly smaller infarct volume and better neurological scores than pretreatment with saline. Furthermore, DHI significantly reduced the permeability of the blood-brain barrier, increased occludin protein expression and decreased neutrophil infiltration, as well as profoundly suppressing the upregulation of matrix metallopeptidase-9 expression seen in rats that had received vehicle. Matrix metallopeptidase-2 expression was not affected by ischemia or DHI. Moreover, DHI (1.8 mL/kg) administered 3 hours after the onset of ischemia also improved neurological scores and reduced infarct size. Our results indicate that the neuroprotective efficacy of DHI in a rat model of cerebral ischemia-reperfusion injury is mediated by a protective effect on the blood-brain barrier and the reversal of neutrophil infiltration.
Keywords: nerve regeneration, Danhong injection, Radix Salviae Miltiorrhiae, Flos Carthami, cerebral ischemia-reperfusion, neutrophil infiltration, matrix metallopeptidase, blood-brain barrier, NSFC grant, neural regeneration
Introduction
Over the past few decades, hundreds of neuroprotective agents have reached clinical trials of ischemic stroke (Ginsberg, 2008; Turner et al., 2013). However, thrombolytic therapy with intravenous tissue plasminogen activator remains the only US Food and Drug Administration-approved treatment for acute ischemic stroke since 1996 (Matsuo et al., 2013). But its narrow therapeutic window and risks of cerebral hemorrhage limit its widespread application (De Keyser et al., 2005). Therefore, the development of a new drug for stroke remains an urgent priority.
In contrast with western developed countries, China has many herbal preparations for the clinical treatment of acute ischemic stroke. Danhong injection (DHI) is a Chinese Materia Medica standardized product extracted from Radix Salviae miltiorrhizae and Flos Carthami tinctorii (Li et al., 2011; Liu et al., 2013). Both of these are traditional Chinese herbs widely used in the treatment of cerebrovascular disorders and coronary artery disease (Zhou et al., 2005; Sun et al., 2009). The main components of this preparation have been identified in previous studies from our research group (Liu et al., 2013). DHI contains danshensu (tanshinol); salvianolic acids A, B, and C; rosmarinic acid; protocatechuic acid; and saccharides. The pharmacologic activities of these components have been extensively documented. For example, salvianolic acid B inhibits platelet aggregation (Ma et al., 2011), ameliorates microcirculatory disturbance (Han et al., 2008), and has anti-inflammatory (Wang et al., 2010) and anti-oxidant properties (Zhao et al., 2008; Wang et al., 2010). Salvianolic acid B also exerts neuroprotective effects against Aβ neurotoxicity (Lee et al., 2013) and traumatic brain injury (Chen et al., 2011) in vivo. Salvianolic acid A has similar activities (Ho and Hong, 2011). Danshensu is an anti-oxidant (Zhao et al., 2008), inhibits nuclear factor-κB expression (Jiang et al., 2013), and protects endothelial cells (Yang et al., 2010). It also suppresses cardiomyocyte apoptosis and provides significant cardioprotection against myocardial ischemia and reperfusion injury by activating the PI3K/Akt and ERK1/2 signaling pathways (Yin et al., 2013). Rosmarinic acid was also proposed as a potential cardiovascular drug in a recent review (Ferreira et al., 2013). Growing evidence suggests that rosmarinic acid alleviates diabetic cerebral ischemia-reperfusion injury and attenuates blood-brain barrier (BBB) breakdown via high mobility group box-1 protein and the nuclear factor-κB signaling pathway (Luan et al., 2013). The combined effects of the individual components are the basis of the neuroprotective effects of DHI.
In the present study, we confirm the neuroprotective efficacy of DHI in a rat model of cerebral ischemia-reperfusion injury, and evaluate the potential mechanisms underlying this process.
Materials and Methods
Establishment of cerebral ischemia-reperfusion model
A total of 92 adult male Wistar rats (weighing 250–280 g; specific pathogen-free grade; license No. SCXK (Jing) 2012-0001; Vital River, Beijing, China) were used. Rats were housed five per cage at a stable temperature (22 ± 0.5°C) under a 12 hour light/dark cycle (lights on at 07:00) and had free access to standard rat chow and water. All procedures were approved by the Animal Care and Use Committee at Tianjin University of Traditional Chinese Medicine in China.
The rats were divided into six groups: sham (n = 14), model (n = 23), 0.9 mL/kg DHI (n = 8), 1.8 mL/kg DHI (n = 24), post-treatment model (n = 11) and post-treatment DHI (n = 12). Anesthesia was induced with chloral hydrate (10%, 3 mL/kg). All groups except sham underwent middle cerebral artery occlusion surgery as previously described (Wang et al., 2012). Briefly, the left common carotid artery was exposed and a silicon-coated 4-0 nylon filament was introduced into the left internal carotid artery through the common carotid artery. Sixty minutes after middle cerebral artery occlusion, the intraluminal suture was withdrawn. For the sham group, we cut an incision and closed it without occlusion of the artery. Laser Doppler (Perimed, Jarfalla, Sweden) was used to monitor cerebral blood flow during the procedure to confirm occlusion. Body temperature was monitored by a rectal probe and maintained at 37°C using a feedback-regulated heating system (CMA/Microdialysis AB, Knivsta, Sweden) during surgery.
Drug treatment
DHI was provided by Buchang Pharmaceutical Co., Ltd. (lot No. 130932, 10 mL/vial, Xi’an, Shaanxi Province, China). Quantitative analysis of its components was conducted using high performance liquid chromatography (Waters Corp., Milford, MA, USA) as described previously (He et al., 2012; Liu et al., 2013).
For DHI pretreatment, rats in the DHI groups received a single intraperitoneal injection of DHI (0.9 mL/kg or 1.8 mL/kg) 30 minutes before surgery, and then once daily for 3 additional days. In the sham and model groups, rats received saline (1 mL/200 g) only.
For DHI post-treatment, the first treatment with DHI (1.8 mL/kg) was given 3 hours after the onset of ischemia, and then once daily for 3 subsequent days.
Neurological deficits
The Bederson scale was used to assess general neurological status (Bederson et al., 1986). Tests were performed at 24 and 72 hours after surgery and scored as follows: 0, no observable deficit; 1, forelimb flexion; 2, decreased resistance to lateral push without circling; 3, same behavior as 2 but with circling; 4, ambulation difficulty or inability.
Assessment of cerebral infarct size by TTC staining
After 72 hours of reperfusion, the rats were deeply anesthetized and brains rapidly removed. Six sections (2 mm thick) were cut using a robent brain matrix and were stained with 2% (w/v) 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA). Infarct volume was analyzed with Image J software (Wayne Rasband, National Institutes of Health, USA).
Measurement of BBB permeability
BBB permeability was assessed by measuring Evans blue extravasation as previously described (Ishrat et al., 2010). Briefly, Evans blue dye (2%, 3 mL/kg; Sigma) was injected through the tail vein. 2 hours later, rats were deeply anesthetized and perfused intracardially with normal saline until colorless fluid was obtained from the right atrium. Ipsilateral hemispheres were quickly isolated and weighed. For quantitative measurements, the ipsilateral hemispheres were homogenized in N,N-dimethylformamide and incubated for 72 hours at 37°C and then centrifuged at 21,000 × g for 30 minutes. Evans blue concentrations in the supernatant were determined by a microplate fluorescence reader (Nikon, Tokyo, Japan) at excitation and emission wavelengths of 600 and 650 nm, respectively.
Expression of myeloperoxidase (MPO) in the rat brain detected by immunofluorescent staining
Rats were anesthetized and perfused transcardially with phosphate buffer containing 4% paraformaldehyde. Brains were post-fixed for 24 hours, transferred into 30% sucrose solution for 24 hours, and 30 μm thick coronal brain sections were cut. Sections were incubated with rabbit anti-MPO polyclonal antibody (1:200; Sigma) overnight at 4°C. FITC-conjugated goat anti-rabbit IgG (1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a secondary antibody. Sections were viewed under a confocal microscope (Nikon). The number of MPO+ cells per mm2 in ischemic brain tissue was calculated (one section with four different visual fields per rat).
Expression of occludin, MMP-2 and MMP-9 in the rat brain detected by western blot analysis
Brain tissue was homogenized in a commercial lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors. After centrifugation, protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Protein samples were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were subsequently blocked with 5% bovine serum albumin in Tris-buffered saline with Tween-20 solution and incubated with rabbit anti-occludin polyclonal antibody (1:2,000; CST, Beverly, MA, USA), rabbit anti-MMP-2 polyclonal antibody (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-MMP-9 polyclonal antibody (1:1,000; Abcam) and rabbit anti-β-actin polyclonal antibody (1:20,000; Abcam), for 12 hours at 4°C. Peroxidase-linked goat anti-rabbit IgG (1:5,000; Santa Cruz Biotechnology) and ECL reagents (Millipore, Billerica, MA, USA) were used to visualize protein bands. The absorbance of the bands was quantitated using Image J software and protein expression was normalized to the endogenous reference (β-actin) and expressed as fold difference from the sham group.
Statistical analysis
Data are expressed as mean ± SD. Statistical comparison was carried out using an independent-samples t-test with SPSS 11.5 software (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) with least significant difference (LSD) post hoc test was used for measurement of infarct volume. Nonparametric analysis was used for neurological deficit scores. Differences were considered statistically significant at P < 0.05.
Results
Effect of DHI on neurological deficit and infarct volume of rats after middle cerebral artery occlusion
Compared with model rats that received saline, the infarct size of rats pretreated with DHI (1.8 mL/kg) 30 minutes before middle cerebral artery occlusion showed notably smaller infarct volumes (P < 0.01; Figure 1A) and significantly better neurological scores (P < 0.01; Figure 1B).
Figure 1.
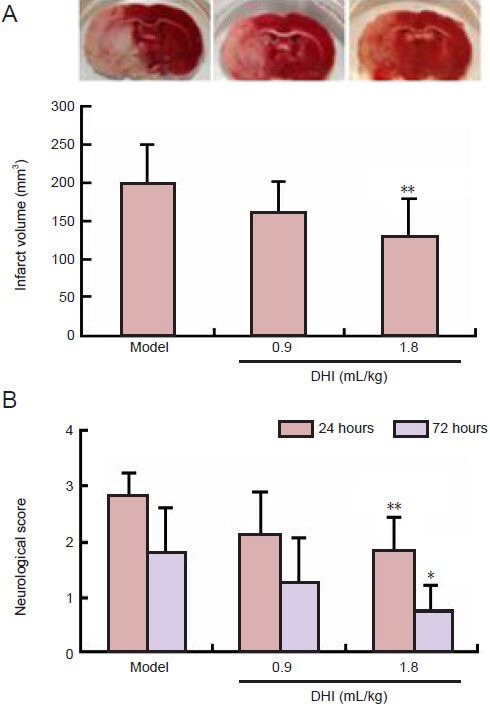
Effect of Danhong injection (DHI) pretreatment on infarct volume and neurological deficit in rats after middle cerebral artery occlusion.
(A) Infarct volume 72 hours after middle cerebral artery occlusion (TTC staining). (B) Neurological deficit score 24 and 72 hours after middle cerebral artery occlusion. Data are expressed as mean ± SD (n = 8–10 rats per group). One-way analysis of variance with least significant difference post hoc test was used for measurement of infarct volume, and nonparametric analysis was used in neurological deficit tests. *P < 0.05, **P < 0.01, vs. model group.
Effect of DHI on BBB permeability and occludin expression in rats after middle cerebral artery occlusion
We speculated that DHI could result in a reduction in ischemic BBB injury. As expected, BBB permeability, assessed by Evans blue extravasation, was significantly greater in the ipsilateral hemisphere of brains from rats in the model group than in the sham group (P < 0.01). Administration of DHI significantly reduced Evans blue leakage and BBB permeability compared with the model group (P < 0.01; Figure 2A). We next examined the effect of DHI on the expression of the tight junction protein occludin in ischemic brain tissue. Occludin expression was significantly lower in the ipsilateral hemisphere of model group rats than in the sham group (Figure 2B; P < 0.01). The downregulation of occludin expression was markedly prevented by DHI treatment (P < 0.01).
Figure 2.
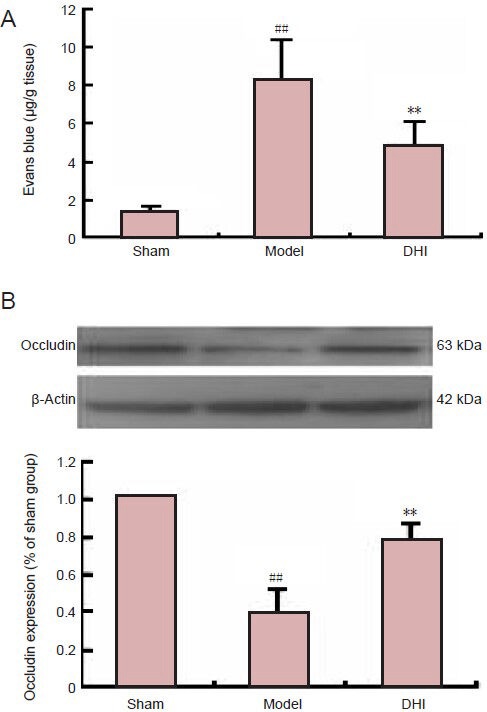
Effect of Danhong injection (DHI) on blood-brain barrier permeability and expression of occludin protein in rats after middle cerebral artery occlusion.
(A) Evans blue leakage at 72 hours after focal ischemia was significantly attenuated by DHI (n = 6 rats per group). (B) Expression of occludin protein, normalized to the endogenous reference (β-actin) and expressed as a percentage of the sham group value (n = 4 rats per group). Data are expressed as mean ± SD. ##P < 0.01, vs. sham group; **P < 0.01, vs. model group (independent-samples t-test).
Effect of DHI on neutrophil infiltration in rats after middle cerebral artery occlusion
MPO is a marker of neutrophil infiltration (Jin et al., 2011). Immunostaining showed that MPO+ cells were not detected either in the contralateral hemisphere of model rats or in sham-operated rats. The number of MPO+ cells in the ischemic hemisphere was notably greater in the model group than in the sham group. Neutrophil infiltration in the DHI-treated group was greater than that in the model group (Figure 3).
Figure 3.
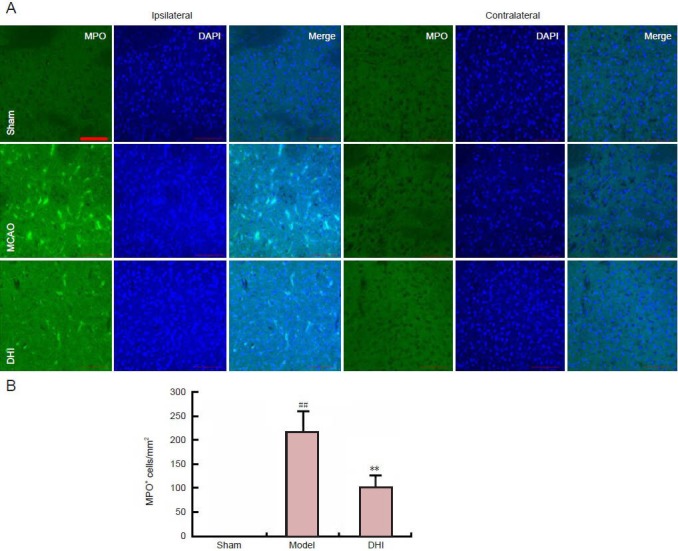
Effect of Danhong injection (DHI) on myeloperoxidase (MPO) expression in rat brain after middle cerebral artery occlusion.
(A) MPO expression in the ipsilateral and contralateral hemispheres (immunofluorescent staining, laser confocal microscopy). The fluorescent indicator is FITC (green). Scale bar: 100 μm. (B) Quantification of MPO-positive cells in the contralateral hemisphere. Data are expressed as mean ± SD (n = 4 per group). ##P < 0.01, vs. sham group; **P < 0.01, vs. model group (independent-samples t-test).
Effect of DHI on MMP expression in rats after middle cerebral artery occlusion
MMPs are responsible for BBB disruption by degrading the basal components of BBB and facilitating immune cell infiltration (del Zoppo et al., 2007). Western blot analysis showed that MMP-9 protein levels were remarkably higher in the ipsilateral hemisphere of model rats than in the sham group (P < 0.01). However, DHI pretreatment profoundly suppressed this upregulation (P < 0.01). Neither ischemia nor DHI affected the expression of MMP-2 (P > 0.05; Figure 4).
Figure 4.
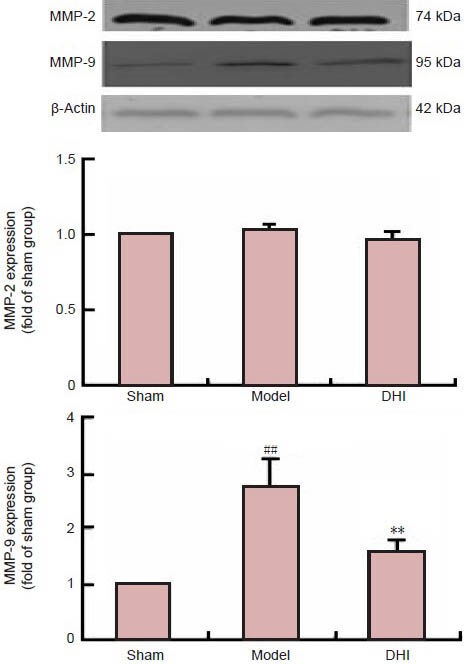
Effect of Danhong injection (DHI) on the expression of matrix metallopeptidase (MMP)-2 and MMP-9 protein in rats after middle cerebral artery occlusion (western blot assay).
Data are expressed as mean ± SD (n = 4 rats per group). MMP-2 and MMP-9 expression was normalized to the endogenous reference (β-actin) and expressed as fold difference from the sham group. ##P < 0.01, vs. sham group; **P < 0.01, vs. model group (independent-samples t-test).
Effect of post-treatment with DHI on neurological deficit and infarct volume in rats after middle cerebral artery occlusion
Because pretreatment of DHI protected the brain against ischemic injury, we also investigated the effect of post-treatment with DHI on ischemia. We administered DHI (1.8 mL/kg) to rats 3 hours after the onset of ischemia and found that DHI could also improve neurological deficit scores and reduce infarct volume in rats after middle cerebral artery occlusion (Figure 5).
Figure 5.
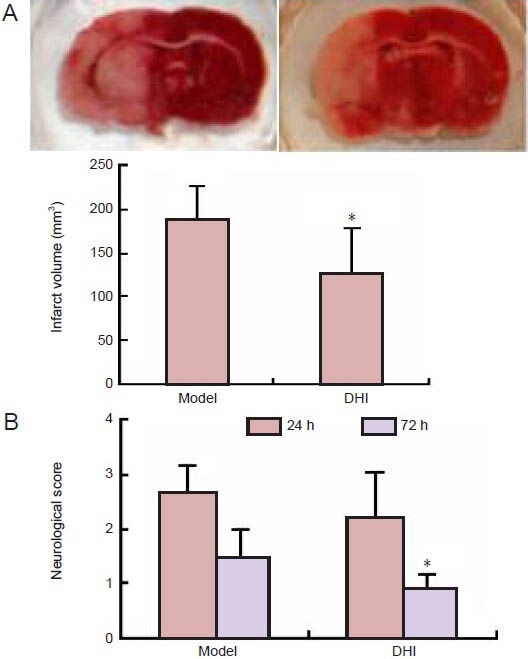
Effect of Danhong injection (DHI) post-treatment (1.8 mL/kg, 3 hours after the onset of ischemia) on brain damage in rats after middle cerebral artery occlusion.
(A) Infarct volume. (B) Neurological score. Higher scores indicate more severe neurological deficits. Data are expressed as mean ± SD (n = 11–12 rats per group). *P < 0.05, vs. model group (one-way analysis of variance with least significant difference post hoc test for infarct volume; nonparametric analysis for neurological deficit score). h: Hours.
Discussion
In the present study, we explored the neuroprotective efficacy of DHI in a rat model of transient middle cerebral artery occlusion. Our results showed that pretreatment with DHI (0.9 and 1.8 mL/kg) resulted in a significantly smaller infarct volume and better neurological function than vehicle pretreatment of model rats. Administration of DHI also resulted in significantly less Evans blue leakage and neutrophil infiltration, as well as preventing the downregulation of occludin expression and upregulation of MMP-9 expression seen in model rats. Neither ischemia nor DHI affected the expression of MMP-2. Importantly, treatment with DHI (1.8 mL/kg) 3 hours after the onset of ischemia also prevented neurological deficit and reduced infarct size.
It is well documented that BBB breakdown is an important contributing factor to injury in stroke. Under ischemic stroke conditions, BBB disruption leads to increased paracellular permeability across cerebral vessels and cerebral edema (Petty and Wettstein, 2001). Blood-borne leukocytes can migrate into the brain and subsequently produce inflammatory factors, increase the BBB permeability and cause secondary insults (Chamorro and Hallenbeck, 2006). There is a positive correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in rats (Matsuo et al., 1994; Jin et al., 2011). Our results suggest that DHI protects the BBB from the hyperpermeability induced by middle cerebral artery occlusion and reverses the induction of neutrophil infiltration in ischemic tissue.
MMPs are involved in BBB opening following reperfusion injury (del Zoppo et al., 2007). Our results demonstrate that MMP-9 expression is induced in ischemic brain tissue, while MMP-2 expression is not affected by cerebral ischemia and 24 hour reperfusion. This discrepancy is explained by the difference in timescales between MMP-9 and MMP-2 expression. MMP-9 is detected in ischemic tissue within 24 hours of occlusion and is still observed at the 5 day point, while MMP-2 expression is detected predominantly after 5 days following middle cerebral artery occlusion (Romanic et al., 1998).
DHI shows good free radical scavenging capacity in the 2,2-diphenyl-1-picrylhydrazyl spectrophotometric assay in vitro (Liu et al., 2013). The beneficial effects of DHI in clinical and experimental tests have also been documented. A clinical observation suggests that DHI is conducive to the recovery of patients with traumatic intracranial hematoma (Sun et al., 2009). DHI also inhibits the maturation of dendritic cells induced by oxidatively modified low-density lipoprotein (partly by activating a PPARγ-mediated signaling pathway) (Liu et al., 2012), protects against myocardial reperfusion injury in minipigs, reduces infiltration of inflammatory cells, maintains mitochondrial integrity, and increases superoxide dismutase activity (Ma et al., 2010). In a recent study, DHI was shown to have a strong ameliorative effect on cerebral ischemia-reperfusion damage in rats because of its anticoagulant, antithrombotic, antifibrinolytic and antioxidant activities, and its regulation of Bcl-2 and Bax protein expression (He et al., 2012). Interestingly, in the present study, we report that DHI protects the BBB and reverses neutrophil infiltration. Some active components in DHI, such as salvianolic acid A and B, have a molecular weight of 700 and therefore cannot cross the blood-brain barrier. Our findings elucidat the mechanism action of DHI and provide biological and molecular evidence for its clinical effects of DHI.
In summary, treatment with DHI provides protection against cerebral ischemia, mediated by a reduction in BBB permeability and reversal of neutrophil infiltration. Our results provide new insights into the mechanisms underlying the effects of DHI in the treatment of stroke.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81173592; National Science and Technology Major Project of the Ministry of Science and Technology of China, No. 2011ZX09201-201, 2012ZX09101201-004, 2012ZX09101202, NCET-13-0935, 2013ZX09201020; Tianjin Municipal Applied Basic Research and Cutting-Edge Technology Research Scheme of China, No. 14JCYBJC28900; and Program for Innovation Team Training in Universities in Tianjin, No. TD12-5035.
Conflicts of interest: None declared.
Copyedited by Murphy JS, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 2.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Liu W, Chao X, Zhang L, Qu Y, Huo J, Fei Z. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull. 2011;84:163–168. doi: 10.1016/j.brainresbull.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 4.De Keyser J, Uyttenboogaart M, Koch MW, Elting JW, Sulter G, Vroomen PC, Luijckx GJ. Neuroprotection in acute ischemic stroke. Acta Neurol Belg. 2005;105:144–148. [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira LG, Celotto AC, Capellini VK, Albuquerque AA, Nadai TR, Carvalho MT, Evora PR. Is rosmarinic acid underestimated as an experimental cardiovascular drug? Acta Cir Bras. 2013;28(Suppl 1):83–87. doi: 10.1590/s0102-86502013001300016. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, Hibi T, Tsuneki H, Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117:280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Wan H, Du Y, Bie X, Zhao T, Fu W, Xing P. Protective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2012;144:387–394. doi: 10.1016/j.jep.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Ho JH, Hong CY. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci. 2011;18:30. doi: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Zhou M, Han Y, Xing L, Zhao H, Dong L, Bai G, Luo G. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol. 2013;147:426–433. doi: 10.1016/j.jep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Jin R, Song Z, Piazza SY A, Nanda A, Penninger JM, Granger DN, Li G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YW, Kim DH, Jeon SJ, Park SJ, Kim JM, Jung JM, Lee HE, Bae SG, Oh HK, Ho Son KH, Ryu JH. Neuroprotective effects of salvianolic acid B on an Aβ25–35 peptide-induced mouse model of Alzheimer's disease. Eur J Pharmacol. 2013;704:70–77. doi: 10.1016/j.ejphar.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Tang J, Meng F, Li C, Xie Y. Study on 10 409 cases of post-marketing safety Danhong injection centralized monitoring of hospital. Zhongguo Zhong Yao Za Zhi. 2011;36:2783–2785. [PubMed] [Google Scholar]
- 16.Liu H, Wang S, Sun A, Huang D, Wang W, Zhang C, Shi D, Chen K, Zou Y, Ge J. Danhong inhibits oxidized low-density lipoprotein-induced immune maturation of dentritic cells via a peroxisome proliferator activated receptor ã-mediated pathway. J Pharmacol Sci. 2012;119:1–9. doi: 10.1254/jphs.11226fp. [DOI] [PubMed] [Google Scholar]
- 17.Liu HT, Wang YF, Olaleye O, Zhu Y, Gao XM, Kang LY, Zhao T. Characterization of in vivo antioxidant constituents and dual-standard quality assessment of Danhong injection. Biomed Chromatogr. 2013;27:655–663. doi: 10.1002/bmc.2842. [DOI] [PubMed] [Google Scholar]
- 18.Luan H, Kan Z, Xu Y, Lv C, Jiang W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation response. J Neuroinflammation. 2013;10:28. doi: 10.1186/1742-2094-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C, Yao Y, Yue QX, Zhou XW, Yang PY, Wu WY, Guan SH, Jiang BH, Yang M, Liu X, Guo DA. Differential proteomic analysis of platelets suggested possible signal cascades network in platelets treated with salvianolic acid B. PLoS One. 2011;6:e14692. doi: 10.1371/journal.pone.0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma XJ, Yin SJ, Jin JC, Wu CF, Huang Y, Shi DZ, Yin HJ. Synergistic protection of Danhong injection and ischemic postconditioning on myocardial reperfusion injury in minipigs. Chin J Integr Med. 2010;16:531–536. doi: 10.1007/s11655-010-0567-0. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo R, Kamouchi M, Ago T, Hata J, Shono Y, Kuroda J, Wakisaka Y, Sugimori H, Kitazono T. Thrombolytic therapy with intravenous recombinant tissue plasminogen activator in Japanese older patients with acute ischemic stroke: Fukuoka Stroke Registry. Geriatr Gerontol Int [Epub ahead of print] 2013 doi: 10.1111/ggi.12205. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, Kogure K. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke. 1994;25:1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- 23.Petty MA, Wettstein JG. Elements of cerebral microvascular ischaemia. Brain Res Rev. 2001;36:23–34. doi: 10.1016/s0165-0173(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 24.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Zhang JJ, Shan JZ, Zhang H, Jin CY, Xu S, Wang YL. Clinical observation of Danhong Injection (herbal TCM product from Radix Salviae miltiorrhizae and Flos Carthami tinctorii) in the treatment of traumatic intracranial hematoma. Phytomedicine. 2009;16:683–689. doi: 10.1016/j.phymed.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Turner R, Lucke-Wold B, Lucke-Wold N, Elliott A, Logsdon A, Rosen C, Huber J. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sci. 2013;14:1890–1917. doi: 10.3390/ijms14011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SX, Hu LM, Gao XM, Guo H, Fan GW. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res. 2010;35:1029–1037. doi: 10.1007/s11064-010-0151-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang SX, Guo H, Hu LM, Liu YN, Wang YF, Kang LY, Gao XM. Caffeic acid ester fraction from Erigeron breviscapus inhibits microglial activation and provides neuroprotection. Chin J Integr Med. 2012;18:437–444. doi: 10.1007/s11655-012-1114-y. [DOI] [PubMed] [Google Scholar]
- 29.Yang RX, Huang SY, Yan FF, Lu XT, Xing YF, Liu Y, Liu YF, Zhao YX. Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol Sin. 2010;31:1395–1400. doi: 10.1038/aps.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan W, Guo C, Zhou D, Wang Y, Xi M, Wen A. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol. 2013;699:219–226. doi: 10.1016/j.ejphar.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan YJ, Guo ZX, Zhao LB. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]


