Abstract
Mental practice is a new rehabilitation method that refers to the mental rehearsal of motor imagery content with the goal of improving motor performance. However, the relationship between activated regions and motor recovery after mental practice training is not well understood. In this study, 15 patients who suffered a first-ever subcortical stroke with neurological deficits affecting the right hand, but no significant cognitive impairment were recruited. 10 patients underwent mental practice combined with physical practice training, and 5 patients only underwent physical practice training. We observed brain activation regions after 4 weeks of training, and explored the correlation of activation changes with functional recovery of the affected hands. The results showed that, after 4 weeks of mental practice combined with physical training, the Fugl-Meyer assessment score for the affected right hand was significantly increased than that after 4 weeks of practice training alone. Functional MRI showed enhanced activation in the left primary somatosensory cortex, attenuated activation intensity in the right primary motor cortex, and enhanced right cerebellar activation observed during the motor imagery task using the affected right hand after mental practice training. The changes in brain cortical activity were related to functional recovery of the hand. Experimental findings indicate that cortical and cerebellar functional reorganization following mental practice contributed to the improvement of hand function.
Keywords: nerve regeneration, brain activation, cortical activation, somatosensory cortex, cortical reorganization, stroke, mental practice, functional recovery, motor imagery, neural regeneration
Introduction
In the months following stroke, a critical question is how does functional recovery happen and how does rehabilitation training accelerate the process of functional recovery? Evidence from animal (Nakagomi et al., 2009; Ziemka-Nalecz and Zalewska, 2012) and human (Ward, 2011) studies suggests that neurogenesis and brain reorganization may play a role in functional recovery (Fridman et al., 2004). Brain reorganization includes changes in sensory and motor areas (Seitz et al., 2005) that enable new functions or compensate for lost functions following stroke (Li et al., 2014). After stroke, the ipsilesional primary motor cortex (M1) generates less transcallosal inhibition (Shimizu et al., 2002; Stinear et al., 2008). This may contribute to increased excitability and reduced intracortical inhibition in the contralesional M1 (Butefisch et al., 2003), which are changes associated with reduced functional recovery (Hummel and Cohen, 2005). Thus, a key question arises; after rehabilitation training, is cortical reorganization of decreased excitability in the contralesional M1 related to enhanced functional recovery?
Mental practice (MP) is a new and economical mental training intervention in which individuals imagine performing a given task. Motor imagery (MI) has been used to specifically describe this mental task (Schuster et al., 2011). Several investigators have recently proposed that MP combined with MI could serve as a therapeutic tool to improve a patient's motor performance (Jackson et al., 2004). However, cortical changes are rarely reported after performing MP coupled with physical practice (PP), and there is little research on the relationship between cortical reorganization and functional outcomes after MP training.
Functional magnetic resonance imaging (fMRI) can reveal changes in the contralesional and ipsilesional motor cortex, and can provide a relatively objective evaluation for functional recovery after rehabilitation training (Kimberley et al., 2008; Kwon et al., 2013). There is controversy around cortical changes during the movement execution (ME) and MI task performed with the affected hand after stroke. Kim et al. (2004) found the ME task elicited M1 and primary somatosensory cortex (S1) activation bilaterally. Similarly, Butefisch and colleagues (Butefisch et al., 2005) found the ME task could activate the pre-motor cortex and M1 bilaterally. Additionally, the MI task has been shown to follow the same “rules” as the ME task that influences motor behavior (Parsons et al., 1995; Jeannerod and Frak, 1999). A significant increase in fMRI signal intensity was observed in M1 and S1 cortex during the MI task of a finger to thumb opposition (Porro et al., 1996). Moreover, the same task induced activation of M1 and premotor cortex (Roth et al., 1996). Activation of the ipsilesional S1 and M1 cortex (Lang et al., 1996) and contralesional cerebellum (Luft et al., 1998) have been reported during the MI task.
In the current study, it was hypothesized that changes in brain activation of the S1 and M1 cortex during the MI task would be correlated with hand functional recovery after MP training. fMRI was used to investigate brain-activated regions involved in the MI task of thumb to palm opposition, and Fugl-Meyer assessment of hand function was evaluated.
Subjects and Methods
Subjects
All 15 subjects were right-handed as assessed by the Edinburgh scale (Oldfield, 1971) and gave written consent in accordance with the Declaration of Helsinki. Ethical approval was given by the China Rehabilitation Research Center Ethics Committee, China. Stroke was diagnosed according to the criteria of the Fourth National Academic Conference on Cerebrovascular Disease in 1995.
Inclusion criteria
Inclusion criteria included a first-ever subcortical stroke with neurological deficits affecting the right hand (nadir hand function level beyond Brunnstrom stage IV) and no significant cognitive impairment. Additionally, subjects had to pass the kinesthetic and visual imagery questionnaire (KVIQ score ≥ 25 scores) (Malouin et al., 2007) and the Chaotic Motor Imagery Assessment (CMIA) (Simmons et al., 2008).
Exclusion criteria
Exclusion criteria were in accordance with those used by Sharma et al. (2009), which consisted of carotid artery stenosis/occlusion, persistent language deficit, neglect/inattention, significant renal/liver disease, treatment with selective serotonin reuptake inhibitors/benzodiazepines, visual impairment, depression, left-handedness, significant small vessel disease on routine CT, and contraindications to MRI.
Grouping
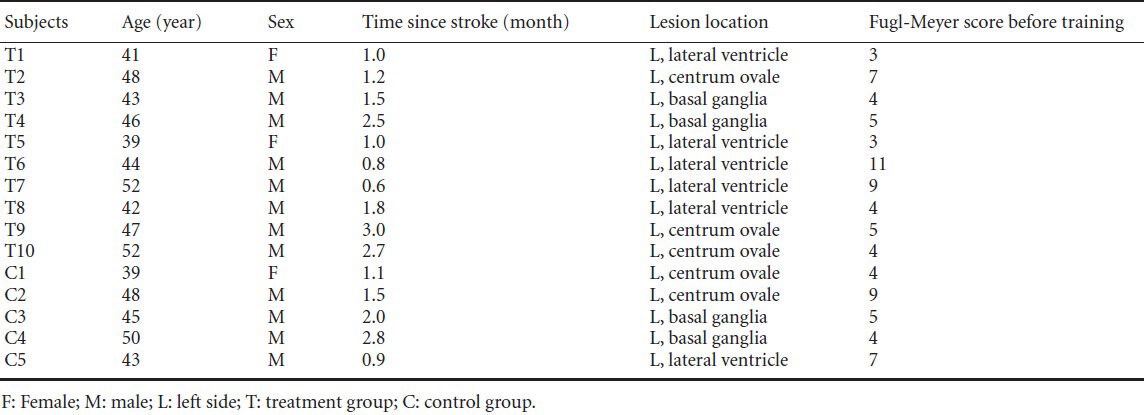
Using the above inclusion and exclusion criteria, 10 subjects (two females; average age, 45.80 ± 4.43 years; average duration from stroke, 1.61 ± 0.85 months; average score for Fugl-Meyer assessment before training, 5.5 ± 2.7) were randomly screened as the treatment group that underwent MP combined with PP training. Five age-matched subjects according to the above inclusion and exclusion criteria (one female; age, 45.0 ± 4.3 years; average duration from stroke, 1.66 ± 0.76 months; average score for Fugl-Meyer assessment before training, 5.8 ± 2.2) were randomly selected as the control group and subsequently underwent PP training. The clinical data for all subjects is presented in Table 1.
Table 1.
Clinical data of all subjects
fMRI and Fugl-Meyer assessment
Subjects who were able to perform MI undertook the remainder of the examination with fMRI and Fugl-Meyer assessment 2 days before training (baseline measurement point). The intervention was conducted once each working day for 4 weeks. Except for the CMIA (outcome measure point), examinations including fMRI and Fugl-Meyer assessment were performed on the last treatment day or the day after fMRI and Fugl-Meyer assessment, and were performed after 4 weeks of training by the same examiners who administered them before training. The examiners were blinded to all participant group assignments.
Intervention
Control group subjects underwent PP training and used the Neurodevelopmental Treatment-Bobath (NDT-Bobath) method (Bobath, 1990). PP training was implemented for 45 minutes each weekday, for 4 weeks. The exercises concentrated on hand movements that were intended to maintain a range of motion, induce isolated hand movement, elicit grasping and gripping, and promote refined activity training.
The treatment group underwent MP combined with MI (MP + MI) training in addition to PP training. MP + MI training specifically refers to MP focused on the mental rehearsal of MI contents with the goal of improving motor performance. The MP + MI training method used was similar to the method of Simmons et al. (2008). Each MP + MI session was performed by adopting the appropriate position, followed by explanation of rules and instructions by the physiotherapist, and then performance of the required tasks by the subjects. For a MP + MI session, subjects sat in a chair at a table with hips, knees, and ankles at 90°, and adopted a hand position appropriate to the task to be imagined during training. The rules of the MI task were explained as imagining in the first person. MP + MI training was also implemented for 45 minutes each weekday, for 4 weeks. Each day, MP + MI training included three sessions with a 5-minute break between the two types. In the MP + MI training sessions, the participant imagined themselves performing an instructed movement without actually performing the movement. The participant received the following instructions before each session: “During this session there are some MI activities including flexion/extension of the thumb, abduction/adduction of all digits, making a fist/spreading the hand, moving extended fingers backwards and forwards, moving the hand between the ulnar and radial deviation that you are going to imagine doing with your paretic hand.” Each MI activity was performed as follows: first, the physiotherapist explained the MI task to be imagined by the subject and then asked the subject to imagine two times. Second, subjects used the non-paretic hand to physically perform the task twice. Third, subjects imagined the task using the non-paretic hand. The instructions given were, “Close your eyes. Concentrate on your hand, but do not move it. Concentrate on how it feels just resting there. Do not move your fingers, hand, or arm. Just imagine it and do not move anything. Open your eyes when you have done this action two times.” Last, subjects imagined the MI task using their paretic hand three times. The same verbal instructions were given for the paretic hand and the non-paretic hand.
Assessment of hand function
Hand function was measured using the hand section of the Fugl-Meyer assessment scale (Fugl-Meyer et al., 1975). Each task in the Fugl-Meyer assessment is scored on a 3-point ordinal scale (0 = cannot perform; 2 = can perform fully), and all items are summed to provide a total score (maximum = 20). The Fugl-Meyer assessment has high test-retest reliability (total = 0.98–0.99 scores; subtests = 0.87–1.00 scores), inter-rater reliability, and construct validity (Duncan et al., 1983). An experienced examiner blinded to each participant's intervention determined the Fugl-Meyer assessment score.
fMRI paradigm of thumb to palm opposition
The task in the fMRI experiment included a block design with auditory-paced (1 Hz) movements in which the thumb touched the palm in an opposition sequence (Baeck et al., 2012). The task duration was 6 minutes 24 seconds. All subjects performed the ME/MI task with the affected right hand inside a magnetic resonance (MR) scanner, and following a brief familiarization period. A control condition, in which subjects did not move and remained at rest, was included in the task. The two blocks were each run twice, with the MI task performed first and the ME task performed second (Sharma et al., 2008). During each run, subjects received auditory prompts every 30 seconds, asking them to either rest or to perform the thumb-to-palm opposition task with the affected hand, and always starting from rest.
For the affected right hand MI paradigm, subjects were instructed to mentally rehearse thumb-to-palm opposition movements by a pre-recorded voice that said “imagery”, and to change to the rest condition when the voice said “rest”. Auditory prompts were presented through sound-insulated earphones connected to the computer's audio output. The imagery condition was then tested against the rest condition. For the rest control condition, subjects were instructed not to imagine anything. The subjects alternated between imagery and rest tasks for 6 cycles beginning with the rest task. Data for the imagery and rest conditions were obtained within 30 seconds of one another (Figure 1).
Figure 1.
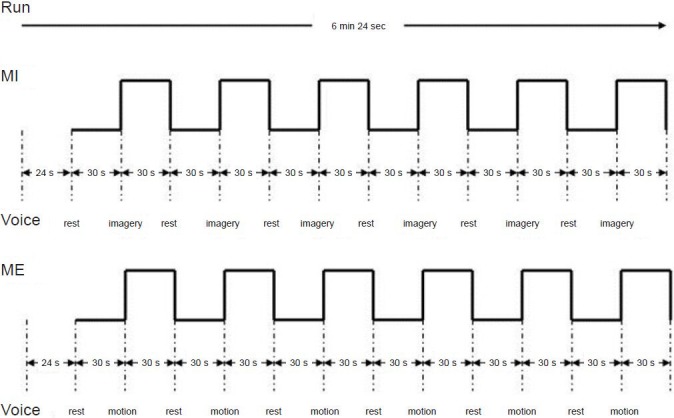
Functional MRI paradigm of motor execution (ME) and motor imagery (MI) tasks.
The total experiment included two runs, one run for the MI task and one run for the ME task. Each run was 6 minutes 24 seconds, and divided into a 24-second preparatory stage and a 6-minute task stage. During each run, subjects received auditory prompts every 30 seconds, asking them to either rest or to perform the MI/ME task of thumb-to-palm opposition with the affected hand, and always starting from rest. Min: Minute; s (sec): second.
fMRI scanning was first performed with the MI task followed by the ME task. For the affected hand (right hand) ME paradigm, subjects were instructed to perform the requested movement by a pre-recorded voice that said “motion”, and to change to the rest condition when the voice said “rest”. Subjects were instructed to alternate between motion and rest conditions for 6 cycles, beginning with the motion condition. Data for the motion and rest conditions were obtained within 30 seconds of one another (Figure 1). The entire functional scanning run lasted approximately 15 minutes. During fMRI scanning, room lights were dimmed and subject's eyes were closed.
fMRI parameters
A 1.5 T General Electric Signa scanner (Signa; General Electric Medical Systems, Milwaukee, WI, USA) equipped with a transmit-receive body coil and a commercial eight-element head coil array was used to obtain blood oxygenation level dependent (BOLD) contrast for each participant. T2*-weighted echo planar imaging was used for fMRI acquisition. The following acquisition parameters were used in the fMRI protocol: echo time (TE) = 40 ms, repetition time (TR) = 3,000 ms, field of view (FOV) = 24 cm, acquisition matrix = 64 × 64. Using a mid-sagittal scout image, 24 contiguous axial slices with a 5-mm thickness were placed along the anterior-posterior commissure plane to cover the entire brain.
Image analysis
The general linear model in statistical parametric mapping 8 (SPM8) implemented in MATLAB was used to perform whole-brain image analysis (Baeck et al., 2012). To adjust for residual head movement, functional images were realigned to the first image. The realigned images were then spatially normalized to fit the Montreal Neurological Institute template based on the standard stereotaxic coordinate system. Additionally, a mask of each participant's stroke lesion was drawn and normalized to the data using a model created from the participant's T1-weighted anatomical scans (Brett et al., 2001). All images were subsequently smoothed with an isotropic Gaussian kernel having a full width of 8 mm at half maximum. SPM8 was used for statistical analysis of preprocessed MRI data on a voxel-by-voxel basis. To identify which cerebral networks were activated under ME and MI, we analyzed the BOLD response under ME/MI conditions. For each subject, a boxcar model convolved with the hemodynamic response function was applied to the fMRI time series at each voxel, and t-maps for contrast ME/MI minus rest were computed. Clusters with < 10 voxels were ignored.
Statistical analysis
Data are expressed as mean ± SD. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used to perform all statistical analyses. Hand function before and after training in each group was compared using paired t-tests. A two-sample t-test was used to compare hand function between treatment and control groups. Before training and after training, a one-sample t-test was used to compare fMRI data between the ME/MI task vs rest in each group. A paired t-test was used to compare fMRI data between post-training with pre-training data in each group and a two-sample t-test was used to compare fMRI data between the treatment and control groups. Statistical significance was accepted at P < 0.05. Correction for multiple comparisons was performed using a false discovery rate (FDR) of 0.05. A Pearson rank correlation analysis was performed between the post- and pre-training activation intensity (T value) of the ipsilesional (left) S1 and contralesional (right) M1 during the ME/MI task, and the post- and pre-training Fugl-Meyer assessment score. A correlation with a P value < 0.05 was considered significant.
Results
Effect of MP combined with PP training on hand function of stroke patients
There was no significant difference in the Fugl-Meyer assessment score between groups before training. However, a paired t-test showed a significant difference between pre- and post-training Fugl-Meyer assessment scores for both groups (P < 0.01; Figure 2), in which the Fugl-Meyer assessment score increased after training. Furthermore, the Fugl-Meyer assessment score was higher in the treatment group that had performed MP combined with PP training compared with the control group that had performed only PP training (P < 0.05; Figure 2).
Figure 2.
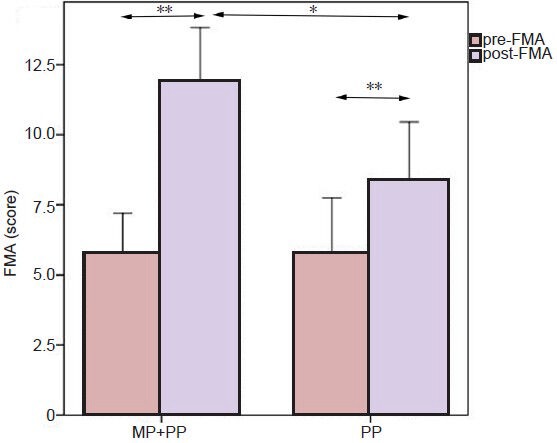
Comparison of pre-training and post-training Fugl-Meyer assessment scores for hand function between the treatment group receiving MP combined with PP training (MP + PP) and the control group receiving PP training alone (PP).
Data are expressed as mean ± SD. A paired t-test showed post-training Fugl-Meyer assessment scores were higher than pre-training Fugl-Meyer assessment scores in the two groups (**P < 0.01). A two-sample t-test showed post-training Fugl-Meyer assessment scores in the MP + PP group were higher than post-training Fugl-Meyer assessment scores in the PP group (*P < 0.05). MP: Mental practice; PP: physical practice; pre-FMA: pre-training Fugl-Meyer assessment score; post-FMA: post-training Fugl-Meyer assessment score.
Effect of MP combined with PP training on brain activation of stroke patients
Comparison of ME/MI task vs. rest before and after training, and comparison of ME and MI task between before and after training in the treatment group
The main effect of the ME and MI tasks before and after training is shown in Table 2 and Figure 3. Before training, the treatment group during the ME task showed increased activity in left S1, the cingulate gyrus, and right anterior cingulate area (Figure 3A). After 4 weeks of combined MP-MI and PP training, left S1 activation intensity (T value) increased, whereas right inferior temporal operculum and left rolandic operculum were found to be inactive (Figure 3B). Before training, the treatment group showed increased activity in the right M1 and inferior frontal operculum during the MI task (Figure 3C). After 4 weeks of combined MP-MI and PP training, the activation intensity (T value) in the right M1 cortex was less compared with before training. The brain regions activated during the MI task after training were the left S1, right supramarginal gyrus and superior temporal gyrus, left sub-gyral and inferior parietal, and the superior temporal gyrus (Figure 3D).
Table 2.
Comparison of activated regions before and after training in the two groups
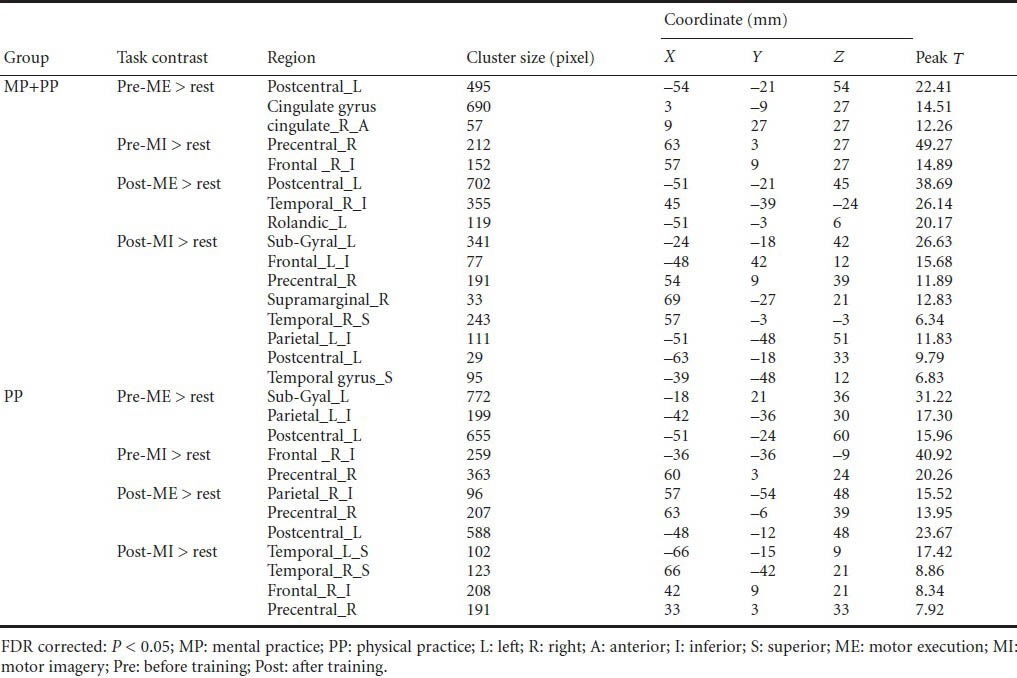
Figure 3.
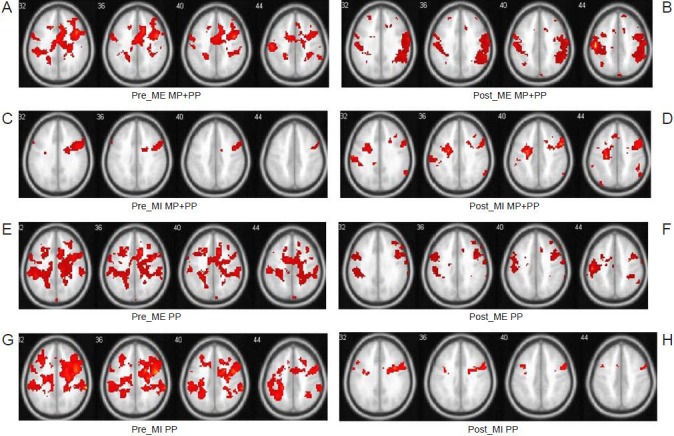
Average brain activation maps contrasted from motor execution (ME) and motor imagery (MI) minus rest in the two groups before and after training.
The eight images (A–H) are cross-sectional images at the MNI coordinate of z from the 32 to 44 mm level. The color in the images represents the activation intensity. Color changes from red to yellow represent increasing activity intensity. (A) Before training, the treatment group (MP + PP) receiving mental practice (MP) combined with physical practice (PP) training during the ME task showed increased activation intensity in the left primary somatosensory cortex (S1), the cingulate gyrus, and right anterior cingulate area. (B) After training, the treatment group during the ME task showed increased left S1 activation intensity. (C) Before training, the treatment group during the MI task showed increased activation intensity in the right primary motor cortex (M1) and inferior frontal operculum. (D) After training, the treatment group showed decreased activation intensity in the right M1, but increased activation intensity in the left S1 during the MI task. (E) Before training, the control group (PP) receiving PP training during the ME task showed increased activation intensity in the left S1 and inferior parietal cortex. (F) After training, the control group showed increased activation intensity in the right inferior parietal cortex, right M1, and left S1 during the ME task. (G) Before training, the control group showed increased activation intensity in the right inferior frontal cortex and right M1 during the MI task. (H) After training, the control group showed increased activation intensity in the left and right superior temporal gyrus, left inferior frontal gyrus, and right M1 during the MI task.
Comparison of ME/MI task vs. rest before and after training, and comparison of ME and MI task between before and after training in the control group
Before training, the control group showed increased activity in the left S1, left sub-gyral, and inferior parietal cortex during the ME task (Figure 3E). After 4 weeks of PP training, the regions activated during the ME task were the right inferior parietal cortex, right M1, and left S1 cortex (Figure 3F). For the left S1, the activation intensity during the ME task was higher compared with before training. Before training, the control group showed activation in the right inferior frontal cortex and the right M1 during the MI task (Figure 3G). The activated regions during the MI task following 4 weeks of PP training were the left and right superior temporal gyrus, the left inferior frontal gyrus, and the right M1 (Figure 3H).
Comparison of the ME and MI tasks before training between the treatment group and the control group, and comparison of the ME and MI tasks after training in the two groups
Before training, there were no suprathreshold activated clusters for the two groups during the ME and MI task (FDR corrected P < 0.05). After training, activated regions during the ME task were more highly activated in the treatment group compared with the control group (FDR corrected P < 0.05; Table 3). Activated regions included the right cerebellum, right temporal gyrus, left inferior frontal gyrus, and left S1 (Figure 4A). Activated regions during the MI task were more highly activated in the treatment group compared with the control group (FDR corrected P < 0.05; Table 3). Activated regions included the right angular gyrus, right inferior frontal gyrus, right middle frontal gyrus, left sub-gyral, left superior frontal gyrus, and left supplementary motor area (Figure 4B).
Table 3.
Activated regions for ME/MI task before training and after training in the two groups
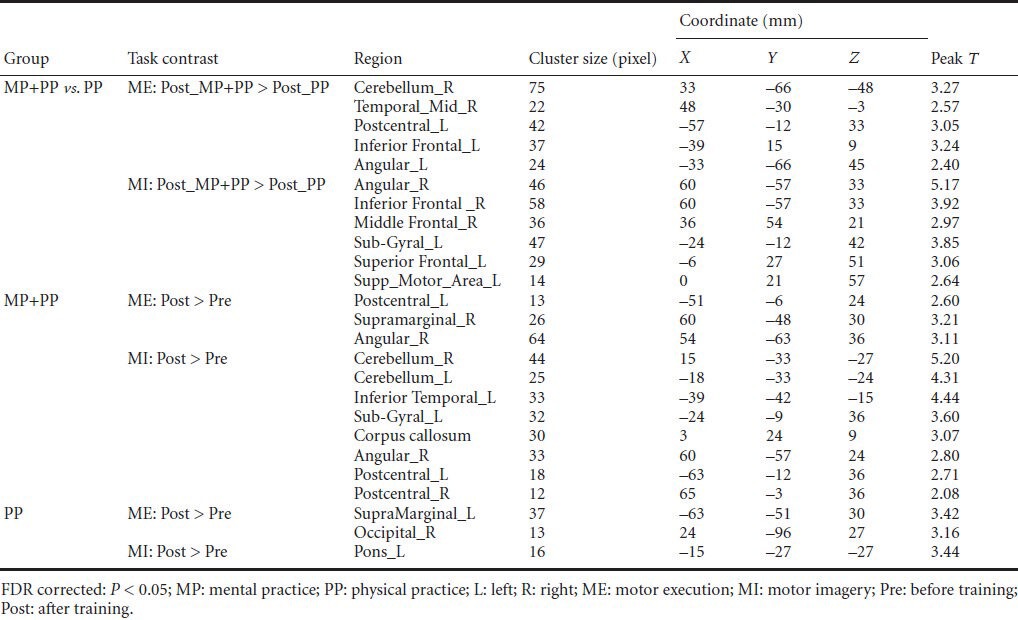
Figure 4.
Average brain activation maps derived from the comparison of motor execution (ME) and motor imagery (MI) between groups (post-training) and within groups (post-training minus pre-training).
Clusters with significant differences were overlapped on render views (posterior, on the left; anterior, on the right (row 1), right, on the left; left, on the right (row 2), inferior, on the left; superior, on the right (row 3). The color in the image represents activated intensity. Red to yellow represents higher activation intensity. (A) After training, activation intensity in the right cerebellum, right temporal gyrus, left inferior frontal gyrus, and left primary somatosensory cortex (S1) was higher in the treatment group (MP + PP) receiving mental practice (MP) combined with physical practice (PP) training, compared with the control group receiving PP training during the ME task. (B) After training, the activation intensity of the right angular gyrus, right inferior frontal gyrus, right middle frontal gyrus, left superior frontal gyrus, and left supplementary motor area in the treatment group was higher compared with the control group during the MI task. (C) In the treatment group, the activation intensity of the left S1, right supramarginal gyrus, and right angular gyrus was higher after training compared with before training during the ME task. (D) In the treatment group, the activation intensity of the cerebellum bilaterally, left inferior temporal gyrus, left sub-gyral gyrus, right angular gyrus, corpus callosum, and S1 bilaterally during the MI task was higher after training compared with before training. (E) In the control group, the activation intensity of the left supramarginal gyrus and right occipital lobe during the ME task was higher after training compared with before training. (F) In the control group, the activation intensity of the left pons during the MI task was higher after training compared with before training.
In the treatment group, the regions activated during the ME task showed higher activity after training compared with before training (FDR corrected P < 0.05; Table 3). These regions were the left S1, right supramarginal gyrus, and right angular gyrus (Figure 4C). The regions activated during the MI task showed higher activity after training compared with before training (FDR corrected P < 0.05; Table 3), which were mainly the cerebellum bilaterally, left inferior temporal gyrus, left sub-gyral gyrus, right angular gyrus, corpus callosum, and S1 bilaterally (Figure 4D).
In the control group, the regions activated during the ME task showed higher activity after training compared with before training (FDR corrected P < 0.05; Table 3), mainly in the left supramarginal gyrus and right occipital lobe (Figure 4E). The regions activated in the MI task showed higher activity after training compared with before training (FDR corrected P < 0.05; Table 3), and mainly in the left pons (Figure 4F).
Correlation between fMRI activation intensity and Fugl-Meyer assessment score
Among regions activated during the ME and MI task with the affected right hand for both groups, increased activation intensity (T value) in the left S1 between pre- and post-training was positively correlated with Fugl-Meyer assessment score in the treatment group (ME: r = 0.732, P = 0.016; Figure 5A; MI: r = 0.695, P = 0.026; Figure 5B). However, the increase in activation intensity in left S1 with the affected right hand during the ME task between pre- and post-training was not correlated with Fugl-Meyer assessment score in the control group (r = 0.577, P = 0.308), and there was no significant increase in S1 activation in the control group during the MI task when post-training was compared with pre-training. Decreased activation intensity in the right M1 during the MI task with the affected right hand for post-training minus pre-training was negatively correlated with Fugl-Meyer assessment score in the treatment group (r = −0.644, P = 0.044; Figure 5C). Additionally, decreased activation intensity in the right M1 for post-training minus pre-training was not correlated with Fugl-Meyer assessment score in the control group (r = −0.289, P = 0.638; Figure 5D).
Figure 5.
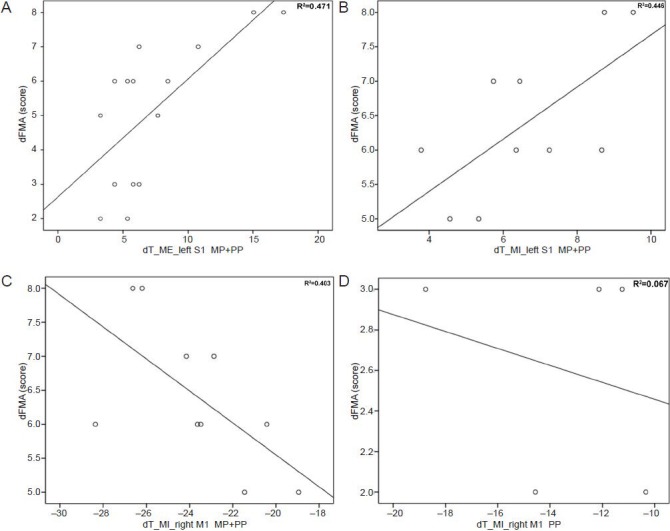
Correlation between increased Fugl-Meyer assessment score and increased ipsilesional S1 and decreased contralesional M1 activation intensity in the two groups before and after training.
(A) Pearson rank correlation analysis showed that increased ipsilesional S1 activation intensity was positively correlated with FMA score for post-and pre-training in the treatment group performing the MI task (r = 0.695, P = 0.026). (B) Pearson rank correlation analysis showed that decreased right M1 activation intensity was negatively correlated with FMA score for post- and pre-training in the treatment group (r = –0.644, P = 0.044). (C) Pearson rank correlation analysis showed that decreased right M1 activation intensity was negatively correlated with FMA score for post- and pre-training in the treatment group (r = –0.644, P = 0.044). (D) Pearson rank correlation analysis showed that decreased right M1 activation intensity was not correlated with FMA score for post- and pre-training in the control group (r = –0.289, P = 0.638). MI: Motor imagery; d: the difference score of post-training minus pre-training; T: activated intensity; S1: primary somatosensory cortex; M1: primary motor cortex; MP: mental practice; PP: physical practice; FMA: Fugl-Meyer assessment; dFMA: the value in the ordinate axis name.
Discussion
The effect of hand function recovery
Reorganization in the nervous system following stroke remains a critical issue for stroke survivors, as some degree of functional recovery is possible (Twitchell, 1965). Specifically, adaptive rehabilitation methods have been shown to assist in brain reorganization and recovery of lost skills (Grefkes et al., 2010), and neural reorganization itself is a critical component of stroke rehabilitation (Mintzopoulos et al., 2009). Clinical imaging findings confirmed that motor functional recovery after stroke correlated with activity changes in M1, and other cortical regions related to motor processing (Weiller et al., 2006). Thus, by comparing fMRI images obtained during MI and ME tasks during different stroke rehabilitation periods, the regions involved and changes in brain activity can be obtained. Unlike PP training, MP in principle is not dependent on residual function but still incorporates voluntary drive. In patients with stroke, MP performed with MI may therefore provide a substitute for ME as a means to activate the motor network (Sharma et al., 2006). MP performed with MI has emerged as a non-invasive strategy that has been shown to improve functioning of the affected arm, even years after stroke (Page et al., 2009). Jackson et al. (2001) considered that MP alone is used as supplementary method and is combined with other traditional rehabilitation trainings in patients with stroke. The combination of MP and PP may be effective in the treatment of Parkinson's disease and the implementation of this treatment regimen extends practice time with negligible risk and low cost (Tamir et al., 2007). The results of a systematic literature review show that those successful MP interventions were added after PP training (Schuster et al., 2011). The current study compared the effects of MP combined with PP training and PP training alone on hand recovery in patients exhibiting stable right hemi-paresis. Furthermore, consistent with our hypotheses, the Fugl-Meyer assessment score after MP combined with PP training was higher compared with after PP training alone, which suggests decreased functional limitation and impairment in the affected right hand.
Changes in ipsilesional (left) S1 activation in the two groups using the affected right hand during the ME task
Our results showed that left S1 activation intensity in the two groups using the affected right hand during the ME task increased more after training compared with before training. Carey et al. (2002) previously studied individuals with chronic stroke, who showed improved tracking accuracy after receiving intensive tracking training. This improvement was accompanied by brain cortical reorganization that was apparent in the treatment group with increased activation in the left S1. Moreover, in the present study, increased activation intensity in the left S1 was correlated with hand function recovery that followed MP+PP training. However after PP training alone, increased activation intensity in S1 was not correlated with hand function recovery. Thus, increased activation in the left S1 cortex played an important role in improving hand function after MP training. The S1 is involved in learning novel motor skills (Luft et al., 2004). Moreover, a wide range of plastic changes has been shown to take place in ipsilesional cortical regions after stroke (Ward, 2005). Thus, the integrity of these areas and their corticospinal output are clearly important for functional recovery (Ward, 2011). Overall, the results show that the left S1 is involved in brain functional reorganization, as represented by enhanced activation intensity associated with sensory information concerning motor processes.
Changes in ipsilesional (left) S1 activation and contralesional (right) M1 activation in the two groups using the affected right hand during the MI task
After training, the left S1 was active when the affected right hand was used during the MI task. Right M1 activation intensity decreased with the affected right hand after MP + PP training. Puh et al. (2007) showed that contralesional M1 activation decreased from 3 weeks to 3 months after injury. The current study showed a decline in right M1 activation after 4 weeks. This suggests that MP+PP training might accelerate the process of contralesional M1 functional reorganization. Moreover, increased activation intensity in the left S1 correlated with hand function recovery. Additionally, decreased activation intensity in the right M1 correlated with improved hand function after MP+PP training. However, there was a small decrease in activation in the right M1 after PP training alone. There was no correlation between decreased M1 activation and Fugl-Meyer assessment score in the control group. Thus, as left S1 activation increased and right M1 activation decreased, hand functional recovery improved after MP training. Increased left S1 activation played a greater role in functional reorganization, which allowed improvement in hand function after MP training. This is in agreement with Jang's hypothesis that hand motor function associated with infarcted M1 can reorganize into S1 (Jang et al., 2005).
Changes in contralesional (right) cerebellum activation in the treatment group using the affected right hand during the MI task
MI was able to activate the left S1 to the same degree as the ME task with the affected right hand, although activation intensity of the left S1 during the MI task was weaker compared with that during the ME task. Similar to ME, increased left S1 activation correlated with hand function recovery in stroke patients. Moreover, the results also showed that after 4 weeks of training using the affected right hand during MI, the right cerebellum was activated. Fujii and Nakada (2003) showed that functional reorganization in the left hemisphere involved the left M1 and S1 activation and right cerebellum activation, which was associated with better functional recovery. Small et al. (2002) also showed that patients with good recovery had clear changes in cerebellar hemisphere activation contralateral to the injured corticospinal tract, which were related to hemodynamic changes such as diaschisis, or to the definite role of the cerebellum in motor skill learning. Based on these results, we suggest that MP+PP training promoted decreased right M1 activation, increased left S1 activation, and increased right cerebellum activation. Reorganization in these regions correlated with hand function recovery in our stroke patients.
Changes in corpus callosum activation in the treatment group using the affected right hand during the MI task
The corpus callosum, which is the largest white matter structure in the human brain connecting the cerebral hemispheres, was activated during the MI task in the affected right hand after training. The corpus callosum plays a crucial role in maintaining independent processing in the hemispheres and in integrating information between hemispheres (Takeuchi et al., 2012). The timing and accuracy of bimanual motor tasks are thought to be predominantly programmed by one of the hemispheres. To monitor the activity of motor regions of the opposite hemisphere, an efference copy of the planned motor program is sent to the opposite hemisphere through the corpus callosum allowing for optimal timing of movements in both hands (Liuzzi et al., 2011). Thus, the corpus callosum might be involved in functional recovery of the hand.
MP combined with PP activated the left S1 cortex, right cerebellum, and corpus callosum, following decreased activation in the right M1, and this activation was related to motor skill learning and interhemispheric interaction. Functional reorganization may thus be correlated with hand function recovery in stroke patients.
There are some limitations of the current study. Future studies should focus on the dynamic relationship among active brain regions. Moreover, new methods could be used to analyze fMRI data. Functional improvement in daily life is the ultimate goal of rehabilitation, and more research focusing on this outcome is needed.
Acknowledgments:
We thank all physicians and nurses from Neurorehabilitation Department of China Rehabilitation Research Center for their assistance in performing this study.
Footnotes
Conflicts of interest: None declared.
Copyedited by Barrett R, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol A Biol Sci Med Sci [Epub ahead of print] 2013 doi: 10.1093/gerona/glt207. [DOI] [PubMed] [Google Scholar]
- 2.Baeck JS, Kim YT, Seo JH, Ryeom HK, Lee J, Choi SM, Woo M, Kim W, Kim JG, Chang Y. Brain activation patterns of motor imagery reflect plastic changes associated with intensive shooting training. Behav Brain Res. 2012;234:26–32. doi: 10.1016/j.bbr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Bobath B. 3rd ed. London: Heinemann Medical Books; 1990. Adult Hemiplegia: Evaluation and Treatment. [Google Scholar]
- 4.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- 5.Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, Seitz RJ. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology. 2005;64:1067–1069. doi: 10.1212/01.WNL.0000154603.48446.36. [DOI] [PubMed] [Google Scholar]
- 6.Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- 7.Butler T, Imperato-McGinley J, Pan H, Voyer D, Cordero J, Zhu YS, Stern E, Silbersweig D. Sex differences in mental rotation: top-down versus bottom-up processing. Neuroimage. 2006;32:445–456. doi: 10.1016/j.neuroimage.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Callow N, Hardy L. The relationship between the use of kinaesthetic imagery and different visual imagery perspectives. J Sports Sci. 2004;22:167–177. doi: 10.1080/02640410310001641449. [DOI] [PubMed] [Google Scholar]
- 9.Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 10.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 11.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 12.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13, 31. [PubMed] [Google Scholar]
- 13.Fujii Y, Nakada T. Cortical reorganization in patients with subcortical hemiparesis: neural mechanisms of functional recovery and prognostic implication. J Neurosurg. 2003;98:64–73. doi: 10.3171/jns.2003.98.1.0064. [DOI] [PubMed] [Google Scholar]
- 14.Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- 16.Jackson PL, Doyon J, Richards CL, Malouin F. The efficacy of combined physical and mental practice in the learning of a foot-sequence task after stroke: a case report. Neurorehabil Neural Repair. 2004;18:106–111. doi: 10.1177/0888439004265249. [DOI] [PubMed] [Google Scholar]
- 17.Jackson PL, Lafleur MF, Malouin F, Richards C, Doyon J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch Phys Med Rehabil. 2001;82:1133–1141. doi: 10.1053/apmr.2001.24286. [DOI] [PubMed] [Google Scholar]
- 18.Jang SH, Ahn SH, Yang DS, Lee DK, Kim DK, Son SM. Cortical reorganization of hand motor function to primary sensory cortex in hemiparetic patients with a primary motor cortex infarct. Arch Phys Med Rehabil. 2005;86:1706–1708. doi: 10.1016/j.apmr.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol. 1999;9:735–739. doi: 10.1016/s0959-4388(99)00038-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Jang SH, Byun WM, Han BS, Lee KH, Ahn SH. Ipsilateral motor pathway confirmed by combined brain mapping of a patient with hemiparetic stroke: a case report. Arch Phys Med Rehabil. 2004;85:1351–1353. doi: 10.1016/j.apmr.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 21.Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Exp Brain Res. 2008;186:183–190. doi: 10.1007/s00221-007-1221-8. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HG, Choi BY, Chang CH, Kim SH, Jung YJ, Jang SH. Recovery of an injured corticospinal tract during a critical period in a patient with intracerebral hemorrhage. NeuroRehabilitation. 2013;32:27–32. doi: 10.3233/NRE-130820. [DOI] [PubMed] [Google Scholar]
- 23.Lang W, Cheyne D, Hollinger P, Gerschlager W, Lindinger G. Electric and magnetic fields of the brain accompanying internal simulation of movement. Brain Res Cogn Brain Res. 1996;3:125–129. doi: 10.1016/0926-6410(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Li Y, Zhu W, Chen X. Changes in brain functional network connectivity after stroke. Neural Regen Res. 2014;9:51–60. doi: 10.4103/1673-5374.125330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liuzzi G, Horniss V, Zimerman M, Gerloff C, Hummel FC. Coordination of uncoupled bimanual movements by strictly timed interhemispheric connectivity. J Neurosci. 2011;31:9111–9117. doi: 10.1523/JNEUROSCI.0046-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luft AR, Skalej M, Stefanou A, Klose U, Voigt K. Comparing motion- and imagery-related activation in the human cerebellum: a functional MRI study. Hum Brain Mapp. 1998;6:105–113. doi: 10.1002/(SICI)1097-0193(1998)6:2<105::AID-HBM3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther. 2007;31:20–29. doi: 10.1097/01.npt.0000260567.24122.64. [DOI] [PubMed] [Google Scholar]
- 29.Mintzopoulos D, Astrakas LG, Khanicheh A, Konstas AA, Singhal A, Moskowitz MA, Rosen BR, Tzika AA. Connectivity alterations assessed by combining fMRI and MR-compatible hand robots in chronic stroke. Neuroimage. 2009;47(Suppl 2):T90–97. doi: 10.1016/j.neuroimage.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T. Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci. 2009;29:1842–1852. doi: 10.1111/j.1460-9568.2009.06732.x. [DOI] [PubMed] [Google Scholar]
- 31.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.Page SJ. Imagery improves upper extremity motor function in chronic stroke patients: a pilot study. Occupational Therapy Journal of Research. 2000;20:200–215. [Google Scholar]
- 33.Page SJ, Szaflarski JP, Eliassen JC, Pan H, Cramer SC. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 35.Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puh U, Vovk A, Sevsek F, Suput D. Increased cognitive load during simple and complex motor tasks in acute stage after stroke. Int J Psychophysiol. 2007;63:173–180. doi: 10.1016/j.ijpsycho.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Decorps M, Jeannerod M. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–1284. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- 38.Schuster C, Hilfiker R, Amft O, Scheidhauer A, Andrews B, Butler J, Kischka U, Ettlin T. Best practice for motor imagery: a systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011:9. doi: 10.1186/1741-7015-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seitz RJ, Kleiser R, Butefisch CM. Reorganization of cerebral circuits in human brain lesion. Acta Neurochir Suppl. 2005;93:65–70. doi: 10.1007/3-211-27577-0_9. [DOI] [PubMed] [Google Scholar]
- 40.Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- 41.Sharma N, Jones PS, Carpenter TA, Baron JC. Mapping the involvement of BA 4a and 4p during Motor Imagery. Neuroimage. 2008;41:92–99. doi: 10.1016/j.neuroimage.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Sharma N, Simmons LH, Jones PS, Day DJ, Carpenter TA, Pomeroy VM, Warburton EA, Baron JC. Motor imagery after subcortical stroke: a functional magnetic resonance imaging study. Stroke. 2009;40:1315–1324. doi: 10.1161/STROKEAHA.108.525766. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 44.Simmons L, Sharma N, Baron JC, Pomeroy VM. Motor imagery to enhance recovery after subcortical stroke: who might benefit, daily dose, and potential effects. Neurorehabil Neural Repair. 2008;22:458–467. doi: 10.1177/1545968308315597. [DOI] [PubMed] [Google Scholar]
- 45.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- 46.Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi N, Oouchida Y, Izumi S. Motor control and neural plasticity through interhemispheric interactions. Neural Plast 2012. 2012:823285. doi: 10.1155/2012/823285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamir R, Dickstein R, Huberman M. Integration of motor imagery and physical practice in group treatment applied to subjects with Parkinson's disease. Neurorehabil Neural Repair. 2007;21:68–75. doi: 10.1177/1545968306292608. [DOI] [PubMed] [Google Scholar]
- 49.Twitchell TE. Variations and abnormalities of motor development. Phys Ther. 1965;45:424–430. doi: 10.1093/ptj/45.5.424. [DOI] [PubMed] [Google Scholar]
- 50.Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol. 2011;589:5625–5632. doi: 10.1113/jphysiol.2011.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward NS. Plasticity and the functional reorganization of the human brain. Int J Psychophysiol. 2005;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Weiller C, May A, Sach M, Buhmann C, Rijntjes M. Role of functional imaging in neurological disorders. J Magn Reson Imaging. 2006;23:840–850. doi: 10.1002/jmri.20591. [DOI] [PubMed] [Google Scholar]
- 53.Ziemka-Nalecz M, Zalewska T. Endogenous neurogenesis induced by ischemic brain injury or neurodegenerative diseases in adults. Acta Neurobiol Exp (Wars) 2012;72:309–324. doi: 10.55782/ane-2012-1904. [DOI] [PubMed] [Google Scholar]


