Abstract
Cervical spinal cord injury (SCI) results in partial to full paralysis of the upper and lower extremities. Traditional primary endpoints for acute SCI clinical trials are too broad to assess functional recovery in cervical subjects, raising the possibility of false positive outcomes in trials for cervical SCI. Endpoints focused on the recovery of hand and arm control (e.g., upper extremity motor score, motor level change) show the most potential for use as primary outcomes in upcoming trials of cervical SCI. As the field moves forward, the most reliable way to ensure meaningful clinical testing in cervical subjects may be the development of a composite primary endpoint that measures both neurological recovery and functional improvement.
Keywords: spinal cord injury, SCI, cervical, clinical trial, endpoint, Cethrin, UEMS
Introduction
Acute spinal cord injury (SCI) is a serious unmet medical need. A traumatic lesion to the spinal cord results in sensory and motor impairment below the level of the injury and spontaneous recovery is very limited in neurologically complete injuries (Fawcett et al., 2007). Cervical SCI, the most devastating and most common type of SCI (Grossman et al., 2012), leaves individuals with impaired or absent function of the upper extremities (arms and hands) in addition to the lower body paralysis seen after thoracic/lumbar/sacral SCI. There are no approved drugs to foster repair after SCI, despite numerous preclinical studies showing the promise of regenerative medicine (Hollis and Tuszynski, 2011; Liu et al., 2011; Filli and Schwab, 2012; Dickendesher et al., 2013; Watzlawick et al., 2014).
Unlike other neurotrauma indications, there have been few clinical trials in SCI, and only very recent trials have focused exclusively on cervical SCI (NCT01828203, NCT01502631, NCT01597518). Clinical trials have traditionally used the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) to broadly track motor/sensory changes in subjects after injury (Ditunno et al., 2005). The most common primary endpoints derived from this neurological assessment are American Spinal Injury Association (ASIA) Impairment Scale conversion and total motor score. These broad endpoints have limited value for the assessment of recovery after cervical injury, as conversion between grades or change in total motor score may occur without significant change in arm and hand control, an area of critical importance to individuals living with quadriplegia after cervical SCI (Anderson, 2004).
As the number of compounds entering late-stage clinical testing for SCI grows, the selection of meaningful primary endpoints for the evaluation of recovery after cervical injury becomes increasingly important. At present, two endpoints used in retrospective analyses of previous studies show the most promise: (1) Upper extremity motor score (UEMS) and (2) motor level change. These endpoints better reflect meaningful recovery in the arms and hands, but can still fail to distinguish functional benefit. For example, very small changes in multiple muscle groups may produce the same overall increase in UEMS score as a more beneficial, full recovery in fewer muscle groups. As the field moves forward, the development of a composite endpoint directly sensitive to both neurological recovery and functional improvement may permit a more meaningful evaluation of drugs for acute cervical SCI.
SCI
Traumatic SCI is a global problem. A SCI can instantly transform an otherwise healthy individual into a person facing a lifetime of disability, and more than 175,000 spinal cord injuries occur globally every year (Lee et al., 2014). In the United States, approximately 12,000 individuals suffer SCIs each year, most commonly from motor vehicle accidents or falls (NSCISC, 2013). Approximately 57–75% of U.S. SCIs are cervical (Grossman et al., 2012; Selvarajah et al., 2014), and complete cervical SCI leads to lifelong quadriplegia (Fawcett et al., 2007). Individuals with SCI also suffer comorbidities, including autonomic dysreflexia, bladder dysfunction, muscle spasticity, and chronic pain (Krassioukov et al., 2003). The combination of these medical complications and corresponding challenges for personal autonomy and community involvement lead individuals with SCI to consistently report a lower quality of life than the nondisabled community (Dijkers, 1997). There are currently no approved treatments to reduce paralysis and improve daily function after SCI.
Limitations of traditional endpoints for evaluation of cervical SCI
The most common primary endpoints in SCI clinical trials are total motor score and ASIA Impairment Scale (AIS) conversion, derivatives of the ISNCSCI assessment (Ditunno et al., 2005; Steeves et al., 2007). Total motor score and AIS conversion were originally designed to assess impairment after SCI (Maynard et al., 1997), but have been subsequently used in clinical trials as measures of drug-based neurological recovery (Steeves et al., 2007). Both endpoints can increase in a manner that may not signify improved function in cervical subjects.
Total motor score (TMS) measures contraction strength in five upper body muscle groups and five lower body muscle groups on either side of the body from 0 (total paralysis) to 5 (normal movement), for a total possible score of 100. The score does not weigh muscle groups by their functional potential or assess the functional value of a score increase. Clinical trial participants could therefore achieve a significant, 20 point improvement in total motor score over placebo in the year following injury due to an isolated recovery from paralysis (0) to normal function (5) in both ankle dorsiflexors and in both knee extensors. A subject could also increase 20 points by improving from full paralysis (0) to active movement with gravity eliminated (2) in all ten leg/foot muscle groups, a change offering similarly limited to nonexistent functional benefit for a quadriplegic individual. The use of a TMS endpoint could thus result in a false positive outcome in a clinical trial for cervical SCI, based on isolated large increases or widely dispersed small increases in lower body muscle groups.
The ASIA Impairment Scale ranks impairment according to body-wide ISNCSCI motor/sensory results, from AIS A (complete paralysis, no motor or sensory function below the level of the injury) to AIS B (complete motor paralysis, sensory function below injury level), AIS C (incomplete motor paralysis, more than half of key muscles below injury level score < 3), AIS D (incomplete motor paralysis, more than half of key muscles below injury level score ≥ 3), or AIS E (normal motor and sensory function). Transitions to higher AIS grades may similarly lack association with functional independence. For example, a two grade transition from AIS A to AIS C, generally regarded as a meaningful clinical outcome, could be due simply to an increase in sensation and the regain in normal control of one large toe (e.g., extensor hallucis longus: 0 to 5).
Both TMS and AIS grade can thus improve based on isolated or widely dispersed increases in lower body control. Though subtle changes in lower body control can hold meaning for individuals with an incomplete SCI or those with paraplegia from a thoracic injury, these changes may have little functional value for an individual with quadriplegia from a cervical SCI. In addition, such changes may simply reflect an incorrect classification of complete paralysis rather than incomplete paralysis during initial baseline measurements. The reliability of baseline measurements is a particular concern in trials of neuroprotective drugs that must be given within 12 hours of injury (Tadie et al., 2003; Casha et al., 2012; Grossman et al., 2014), as baseline assessments for these trials must be conducted while the spinal cord is still in a state of spinal shock (Ditunno et al., 2005). Overall, TMS and AIS conversion are unreliable outcome measures for assessing functional recovery in cervical subjects.
The limitations of these endpoints must be considered with particular care when designing or evaluating trials for drug repositioning, as primary endpoint analysis and scientific reporting in these trials may result in recommendations without the same robust regulatory oversight ensured during the approval process for a new drug. The clinical trial of methylprednisolone as a repositioned treatment for SCI reported total motor score recovery and remains controversial today (Bracken, 2001; Tator, 2006). More recent SCI clinical trials for drug repositioning have also reported primary analyses of total motor score and/or AIS conversion (e.g., the minocycline trial (Casha et al., 2012) and the riluzole trial (Grossman et al., 2014)). The need for standardized reporting of functionally meaningful endpoints in such trials is of critical importance for the evaluation of repositioned therapeutics for cervical SCI.
Meaningful endpoints for the evaluation of neurological recovery after cervical SCI
The selection of a meaningful primary endpoint for assessing recovery after cervical SCI requires an understanding of which drug-based neurological improvements would offer subjects the most functional benefit. Since individuals living with quadriplegia overwhelmingly agree that their quality of life would be most improved by a return of arm/hand function (Anderson, 2004), two ISNCSCI neurological assessment derivatives emerge as valuable endpoints for cervical subjects: (1) Upper extremity motor score (UEMS) and (2) Motor level change. Both endpoints have been used extensively in retrospective analyses of cervical subjects in previous SCI clinical trials and databases (Steeves et al., 2011, 2012; Kramer et al., 2012; McKerracher and Anderson, 2013). Though these endpoints are not impervious to the limitations seen in TMS/AIS conversion (e.g., increases in UEMS are also not weighted by comparative functional benefit), both offer a more focused measure of functionally meaningful increases in hand and arm control that could better serve as a primary endpoint to support drug approval for cervical SCI.
Upper extremity motor score (UEMS) measures contraction strength in five key arm and hand muscle groups on either side of the body from 0 (total paralysis) to 5 (active movement), for a maximal possible score of 50. UEMS correlates more closely than total motor score or lower extremity motor score with improvements in self-care and mobility measured by the Functional Independence Measure (FIM) (Marino and Graves, 2004). Increases in UEMS also correspond directly to improvements in functional activities of daily living and self-care measured with the Spinal Cord Independence Measure (SCIM) (Kramer et al., 2012). Furthermore, individuals with a higher UEMS have an increased capacity for self-feeding, as measured by the Quadriplegia Index of Function (QIF) (Marino et al., 1995).
The Motor Level of a cervical SCI corresponds to the lowest spinal segment from which extending neurons permit muscle movement against gravity, and above which motor function is normal. In particular, the key muscle group innervated by the ‘motor level’ must receive an ISNCSCI motor score of at least 3 (contraction against gravity alone), while all key muscle groups innervated by spinal segments above this level receive a 5 (normal function). Since the cervical levels of the spinal cord innervate the arms and hands, the motor level of a cervical subject is a direct measure of arm/hand regions under voluntary control. The recovery of two or more motor levels in the year following a cervical SCI is associated with a significant improvement in functional independence, as measured by the SCIM self-care subcategory (personal grooming, feeding, bathing, and dressing) (Kramer et al., 2012).
As more compounds move into later-stage clinical trials for SCI, the importance of evaluating cervical subjects based on such functionally meaningful primary endpoints must be emphasized. To date, only one trial including acute cervical SCI subjects has selected a primary endpoint with the capacity to measure functional arm and hand recovery (NCT01502631, ≥ 2 motor level recovery). Standardized reporting of a primary UEMS endpoint in future SCI clinical trials will allow assessment of meaningful recovery in cervical subjects and facilitate comparative analyses of drug efficacy between trials. Such reporting will be particularly important for repositioned drug trials, where presented analyses of UEMS recovery have previously been secondary or absent (Bracken et al., 1997; Casha et al., 2012; Grossman et al., 2014).
Meaningful endpoint selection in the phase II/III trial of Cethrin for cervical SCI
The biologic drug Cethrin is a Rho antagonist designed to promote neuroregeneration and neuroprotection when delivered as a topical adjunct to decompression surgery after SCI (McKerracher and Guertin, 2013). An open-label, phase I/IIa trial demonstrated that Cethrin was well-tolerated, and offered a preliminary assessment of efficacy on the traditional SCI endpoints of AIS grade and total motor score (Fehlings et al., 2011). Though improvements by Cethrin-treated cervical subjects on these endpoints were promising (McKerracher and Anderson, 2013), it is clear that an increase in AIS grade conversion or total motor score may not signify improved daily function.
To permit a more meaningful assessment of phase I/IIa Cethrin trial results, the original ISNCSCI assessment data was recently re-analyzed on the endpoint of upper extremity motor score (McKerracher and Anderson, 2013). During the first year after injury, the sixteen Cethrin-treated cervical subjects in all five tested dose groups (0.3, 1, 3, 6, 9 mg) improved an average of 12.2 ± 2.6 points in UEMS from a baseline of complete paralysis (Figure 1). The nine cervical subjects in the three highest dose groups (3, 6, and 9 mg) demonstrated an average UEMS recovery of 14 ± 2.4 points. These improvements would confer functional benefit over the expected spontaneous recovery of 8.8 ± 0.5 points (Model Systems (Marino et al., 2011)) to 9.6 ± 0.4 points (Sygen database (Steeves et al., 2011)) seen in historical cervical individuals with complete paralysis. Even a two point improvement in UEMS recovery can result in increases in hand and arm strength with a tremendous functional impact for subjects with cervical SCI (Steeves et al., 2012).
Figure 1.
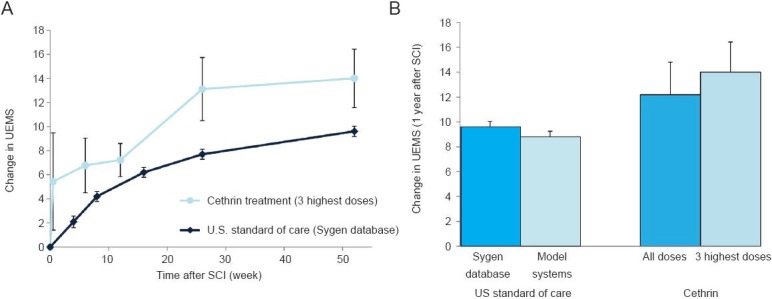
Upper extremity motor score (UEMS): Phase I/IIa Cethrin trial data vs. historical results.
(A) Change in UEMS over time. The average change in UEMS from baseline is displayed at 0, 0.5, 6, 12, 26, and 52 week intervals after initial examination for cethrin-treated cervical subjects in the three highest dose groups (phase I/IIa trial: 3, 6, and 9 mg doses, n = 9), and at 0, 4, 8, 16, 26, and 52 week intervals for historical cervical subjects in the Sygen database (n = 187, C5–7). (B) Change in UEMS at 1 year after SCI. The average change in UEMS from baseline to 1 year post-injury is displayed for cethrin-treated cervical subjects (phase I/IIa trial: n = 16 for all doses [0.3, 1, 3, 6, 9 mg], n = 9 for 3 highest doses 3, 6, 9 mg]) and comparative historical cervical populations analyzed in the literature (Sygen database [n = 187, C5–7], Model systems [n = 315]). Missing data points for dropouts were calculated using the method of Last Observation Carried Forward. Error bars represent standard error.
The upcoming phase II/III Cethrin trial will examine the ability of the drug to reduce paralysis and promote functional recovery after acute cervical SCI (NCT02053883). Upper extremity motor score has been selected as the primary endpoint for this trial. The use of this meaningful primary endpoint for a pivotal trial in cervical SCI will help to shift the field toward the choice of functionally relevant endpoints for future cervical trials.
The phase II/III Cethrin trial will also include secondary endpoints to assess the impact of Cethrin on the serious comorbidities of SCI, such as respiratory impairment, bowel and bladder dysfunction, pain, muscle spasticity, pressure ulcers, and autonomic dysreflexia (Krassioukov et al., 2003; Stein et al., 2010). In addition to the standard International SCI Data Sets, the trial will use a newly developed questionnaire to directly assess personal perceptions of comorbidity severity in Cethrin-treated versus placebo subjects. As the field moves forward, the development and use of other new tools to evaluate SCI comorbidities may permit improved clinical assessments (e.g., an additional test of the ability to generate an effective cough might enhance standard respiratory assessments of forced expiratory volume). By complementing a meaningful primary endpoint (UEMS) with carefully designed secondary tests of SCI comorbidities, the phase II/III Cethrin trial will set the tone for a more patient-centric analysis of cervical SCI treatments.
Developing a composite endpoint for cervical SCI
With an overall goal of determining the functional benefit of a trial therapeutic for cervical SCI, the natural complement to selecting an appropriate neurological assessment is the additional use of a direct test to measure functional ability. Tests of functional independence, such as FIM and, more recently, SCIM, have been incorporated as secondary endpoints in SCI clinical trials ((Bracken et al., 1997; Casha et al., 2012), NCT01828203). However, these tests are unsuited for independent use as primary endpoints for cervical SCI, as improvement in a specific category may reflect external factors/rehabilitation, rather than drug-based improvements in body control. For example, a cervical subject could advance in the dressing category of SCIM from a score of (1) (requiring partial assistance with clothing without buttons, zippers, or laces) to a score of (2) (independent with clothing without buttons, zippers, or laces; requires adaptive devices and/or specific settings) by acquiring a relevant adaptive device.
The ideal solution for future clinical testing in acute cervical SCI may be the development of a composite endpoint that incorporates both a relevant neurological assessment and a direct test of functional autonomy. Composite endpoints have become increasingly prevalent in clinical trials for other neurological indications (Cutter et al., 1999; Elm and Investigators, 2012; Kozauer and Katz, 2013). For example, the Multiple Sclerosis Functional Composite evaluates leg function/ambulation (via a timed, 25-foot walk), arm/hand function (via a test involving placing pegs in a pegboard), and cognitive function (via a test of the subject's ability to add numbers heard at 2–3 second intervals) (Cutter et al., 1999). The use of a well-designed composite for cervical SCI could ensure that an approved therapeutic offers neurological improvements conferring direct functional benefit.
The upcoming phase II/III Cethrin trial will take the first step toward the development of such a composite endpoint. The trial has been designed to assess whether the following measures of arm and hand recovery can be combined to form a composite primary endpoint: (1) UEMS, (2) Graded Redefined Assessment of Strength Sensibility and Prehension (GRASSP) (Kalsi-Ryan et al., 2012), (3) Capabilities of Upper Extremity Test (CUE-T) (Marino et al., 2012), and (4) SCIM III Self-Care Subscore (Itzkovich et al., 2007). GRASSP and CUE-T were recently developed to directly measure the recovery of hand and arm capabilities (e.g. reaching forward, pushing down, pinching a key), and the ability to perform basic functional tasks (e.g. pouring water, lifting a container). The Self-Care subsection of SCIM measures a subject's ability to perform complex tasks of daily living, such as feeding, dressing, bathing, and grooming. UEMS offers a functionally relevant assessment of drug-based neurological changes in arm and hand control. UEMS, GRAASP or CUE-T, and the SCIM Self-Care subscore could together form a composite endpoint sensitive to drug-based functional improvement that transforms the trajectory of future cervical SCI clinical trials.
Conclusions
Traditional primary endpoints for acute SCI clinical trials do not adequately assess functional recovery in cervical subjects, raising the possibility of false positive outcomes in trials for cervical SCI. Endpoints focused on the recovery of hand/arm control (UEMS, motor level change) show the most potential for use as primary outcomes for cervical SCI in the immediate future. Selection of the upper extremity motor score as the primary endpoint for the upcoming phase II/III trial of Cethrin will set the tone for the use of this endpoint in clinical development programs. The most reliable way to ensure meaningful clinical testing in acute cervical SCI may be the development of a composite primary endpoint that directly measures both neurological recovery and functional improvement.
Acknowledgments
The authors would like to thank Dr. Kim Anderson-Erisman (Miami Project to Cure Paralysis; University of Miami, USA) for her helpful comments on the manuscript. We are also grateful to Dr. John Ditunno and Dr. Ralph Marino of Thomas Jefferson University for useful discussions on SCI trial endpoints.
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine. 2001;26:S47–54. doi: 10.1097/00007632-200112151-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 4.Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 5.Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker BG, Antel JP, Confavreux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122:871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 6.Dickendesher TL, Duan Y, Giger RJ. Vol. 2. Academic Press; Chapter 8: Axon Regeneration. Cellullar Migration and Formation of Neuronal Connections; pp. 151–176. [Google Scholar]
- 7.Dijkers M. Quality of life after spinal cord injury: a meta analysis of the effects of disablement components. Spinal Cord. 1997;35:829–840. doi: 10.1038/sj.sc.3100571. [DOI] [PubMed] [Google Scholar]
- 8.Ditunno JF, Jr, Burns AS, Marino RJ. Neurological and functional capacity outcome measures: essential to spinal cord injury clinical trials. J Rehabil Res Dev. 2005;42:35–41. doi: 10.1682/jrrd.2004.08.0098. [DOI] [PubMed] [Google Scholar]
- 9.Elm JJ, Investigators NN-P. Design innovations and baseline findings in a long-term Parkinson's trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson's Disease Long-Term Study-1. Mov Disord. 2012;27:1513–1521. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawcett J, Curt A, Steeves J, Coleman W, Tuszynski M, Lammertse D, Bartlett P, Blight A, Dietz V, Ditunno J. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 11.Fehlings M, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman JR, Yee A, Tighe A, McKerracher L. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011;28:787–796. doi: 10.1089/neu.2011.1765. [DOI] [PubMed] [Google Scholar]
- 12.Filli L, Schwab ME. The rocky road to translation in spinal cord repair. Ann Neurol. 2012;72:491–501. doi: 10.1002/ana.23630. [DOI] [PubMed] [Google Scholar]
- 13.Grossman RG, Frankowski RF, Burau KD, Toups EG, Crommett JW, Johnson MM, Fehlings MG, Tator CH, Shaffrey CI, Harkema SJ. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine. 2012;17:119–128. doi: 10.3171/2012.5.AOSPINE12127. [DOI] [PubMed] [Google Scholar]
- 14.Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B, Shaffrey CI, Johnson MM, Harkema SJ, Boakye M, Guest JD, Wilson JR. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollis ER, 2nd, Tuszynski MH. Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics. 2011;8:694–703. doi: 10.1007/s13311-011-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, Tonack M, Hitzig SL, Glaser E, Zeilig G, Aito S, Scivoletto G, Mecci M, Chadwick RJ, El Masry WS, Osman A, Glass CA, Silva P, Soni BM, Gardner BP, Savic G, Bergstrom EM, Bluvshtein V, Ronen J, Catz A. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007;29:1926–1933. doi: 10.1080/09638280601046302. [DOI] [PubMed] [Google Scholar]
- 17.Kalsi-Ryan S, Curt A, Verrier MC, Fehlings MG. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17:65–76. doi: 10.3171/2012.6.AOSPINE1258. [DOI] [PubMed] [Google Scholar]
- 18.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 19.Kramer JL, Lammertse DP, Schubert M, Curt A, Steeves JD. Relationship between motor recovery and independence after sensorimotor-complete cervical spinal cord injury. Neurorehabil Neural Repair. 2012;26:1064–1071. doi: 10.1177/1545968312447306. [DOI] [PubMed] [Google Scholar]
- 20.Krassioukov AV, Furlan JC, Fehlings MG. Medical co-morbidities, secondary complications, and mortality in elderly with acute spinal cord injury. J Neurotrauma. 2003;20:391–399. doi: 10.1089/089771503765172345. [DOI] [PubMed] [Google Scholar]
- 21.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 23.Marino RJ, Graves DE. Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Arch Phys Med Rehabil. 2004;85:1804–1810. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Marino RJ, Rider-Foster D, Maissel G, Ditunno JF. Superiority of motor level over single neurological level in categorizing tetraplegia. Paraplegia. 1995;33:510–513. doi: 10.1038/sc.1995.111. [DOI] [PubMed] [Google Scholar]
- 25.Marino RJ, Burns S, Graves DE, Leiby BE, Kirshblum S, Lammertse DP. Upper- and lower-extremity motor recovery after traumatic cervical spinal cord injury: an update from the national spinal cord injury database. Arch Phys Med Rehabil. 2011;92:369–375. doi: 10.1016/j.apmr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Marino RJ, Patrick M, Albright W, Leiby BE, Mulcahey M, Schmidt-Read M, Kern SB. Development of an objective test of upper-limb function in tetraplegia: the capabilities of upper extremity test. Am J Phys Med Rehabil. 2012;91:478–486. doi: 10.1097/PHM.0b013e31824fa6cc. [DOI] [PubMed] [Google Scholar]
- 27.Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 28.McKerracher L, Anderson KD. Analysis of recruitment and outcomes in the phase I/IIa Cethrin clinical trial for acute spinal cord injury. J Neurotrauma. 2013;30:1795–1804. doi: 10.1089/neu.2013.2909. [DOI] [PubMed] [Google Scholar]
- 29.McKerracher L, Guertin P. Rho as a target to promote repair: translation to clinical studies with cethrin. Curr Pharm Des. 2013;19:4400–4410. doi: 10.2174/1381612811319240007. [DOI] [PubMed] [Google Scholar]
- 30.NSCISC. Alabama, United States: 2013. Spinal Cord Injury. Facts and Figures at a Glance. National Spinal Cord Injury Statistics Center Birmingham; pp. 1–2. [Google Scholar]
- 31.Selvarajah S, Hammond ER, Haider AH, Abularrage CJ, Becker D, Dhiman N, Hyder O, Gupta D, Black JH, 3rd, Schneider EB. The burden of acute traumatic spinal cord injury among adults in the United States: an update. J Neurotrauma. 2014;31:228–238. doi: 10.1089/neu.2013.3098. [DOI] [PubMed] [Google Scholar]
- 32.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A International Campaign for Cures of Spinal Cord Injury P. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 33.Steeves J, Kramer J, Fawcett J, Cragg J, Lammertse D, Blight A, Marino R, Ditunno J, Coleman W, Geisler F. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord. 2011;49:257–265. doi: 10.1038/sc.2010.99. [DOI] [PubMed] [Google Scholar]
- 34.Steeves JD, Lammertse DP, Kramer JLK, Kleitman N, Kalsi-Ryan S, Jones L, Curt A, Blight AR, Anderson KD. Outcome measures for acute/subacute cervical sensorimotor complete (AIS-A) spinal cord injury during a phase 2 clinical trial. Top Spinal Cord Inj Rehabil. 2012;18:1–14. doi: 10.1310/sci1801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein DM, Menaker J, McQuillan K, Handley C, Aarabi B, Scalea TM. Risk factors for organ dysfunction and failure in patients with acute traumatic cervical spinal cord injury. Neurocrit Care. 2010;13:29–39. doi: 10.1007/s12028-010-9359-9. [DOI] [PubMed] [Google Scholar]
- 36.Tadie M, Gaviria M, Mathe J-F, Menthonnex P, Loubert G, Lagarrigue J, Saint-Marc C, Argenson C, Kempf C, D’Arbigny P, Kamenka J-M, Privat A, Carli P. Early care and treatment with a neuroprotective drug, gacyclidine, in patients with acute spinal cord injury. Rachis. 2003;15:363–376. [Google Scholar]
- 37.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-957. [DOI] [PubMed] [Google Scholar]
- 38.Watzlawick R, Sena ES, Dirnagl U, Brommer B, Kopp MA, Macleod MR, Howells DW, Schwab JM. Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury: a systematic review and meta-analysis. JAMA Neurol. 2014;71:91–99. doi: 10.1001/jamaneurol.2013.4684. [DOI] [PubMed] [Google Scholar]


