Abstract
Peripheral nerve injury impairs motor, sensory, and autonomic function, incurring substantial financial costs and diminished quality of life. For large nerve gaps, proximal lesions, or chronic nerve injury, the prognosis for recovery is particularly poor, even with autografts, the current gold standard for treating small to moderate nerve gaps. In vivo elongation of intact proximal stumps towards the injured distal stumps of severed peripheral nerves may offer a promising new strategy to treat nerve injury. This review describes several nerve lengthening strategies, including a novel internal fixator device that enables rapid and distal reconnection of proximal and distal nerve stumps.
Keywords: peripheral nerve, nerve regeneration, nerve injury, nerve transfer, mechanical loading, biomedical device
Peripheral nerve injuries affect approximately 5% of all trauma patients and up to 1% of the entire population of the United States by age 70 (Asplund et al., 2009), and incur substantial financial costs and lost costs associated with occupational and functional limitations (Evans, 2001). Nerve injuries are especially devastating in the trauma population, who tend to be younger and particularly impacted by decreased independence and diminished quality of life.
Injuries are typically classified based on the integrity of axons and the surrounding connective tissue (Seddon, 1943; Sunderland, 1951), and recovery from injury correlates strongly with severity. Incomplete injuries, especially those where axons retain some continuity, typically recover reliably. However, severed nerves offer a greater challenge; axons must re-extend through the injury site and also the progressively degenerating distal stump before reconnecting with their targeted muscle or end organ. In addition, nerves retract after transection, adding to the size of the gap to be bridged and disrupting alignment of fascicles and endoneurial compartments. Recovery from these injuries is rarely complete, particularly for proximal lesions, which require extensive neuronal outgrowth, or for chronically injured nerves, whose distal stumps provide a poor regenerative environment. As a consequence, individuals experience persistent impairment of motor function, chronic pain, and inappropriate autonomic responses.
While short gaps (typically < 20 mm in human extremities) may be directly repaired end to end, longer gaps require the interposition of a graft or scaffold between the stumps. Autografts have proven to be the gold standard in nerve repair (Figure 1), but present multiple challenges, most notably donor site morbidity, limited graft supply, increased duration under anesthesia, and geometric mismatch between donor and recipient nerve sites (Schmidt and Leach, 2003). While alternatives such as guidance channels and allografts have been utilized, they also present issues such as immunogenicity, and for longer gaps, have yet to match the success of autografts (Evans, 2000; Khuong and Midha, 2013).
Figure 1.
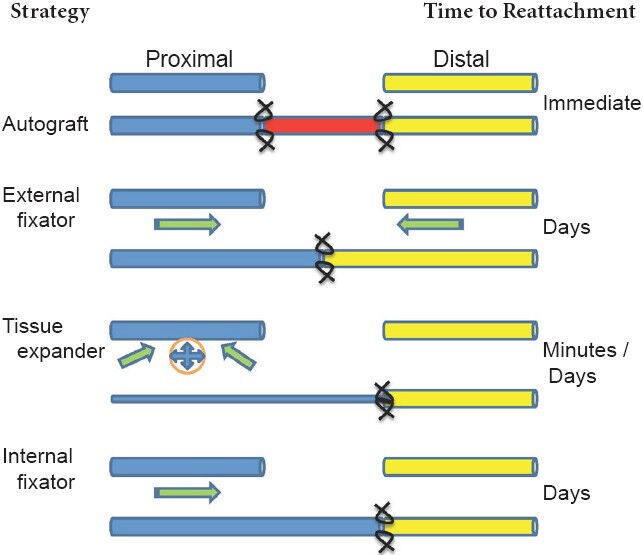
Schematics of nerve lengthening strategies.
Blue: Proximal stump; yellow: distal stump; red: autograft (or other graft). Arrows represent direction of tension placed on respective stumps. Sutures are indicated at stump-graft or stump-stump interfaces.
Excessive mechanical loading has long been implicated in nerve injury, and thus tension is actively avoided during nerve repair (Terzis et al., 1975; Millesi, 1986). However, recent evidence suggests that moderate levels of tension may, in fact, promote neuronal growth. While nerves are not a prototypical load-bearing tissue, they do exist in a dynamic biomechanical state. They are exposed to loading during joint movement and development (Topp and Boyd, 2006), in some cases experiencing regional strains approaching 30% (Wright et al., 1996, 2001). Such deformations far exceed levels typically believed to induce injury (Wall et al., 1992; Brown et al., 1993), and likely reflect regional differences in nerve structure and biomechanical properties, to accommodate increased strain (Phillips et al., 2004; Mason and Phillips, 2011).
This regional heterogeneity may also reflect a nerve's ability to meet its functional demands by adapting to its mechanical environment. Consistent with this notion, recent work at the cell and tissue scales has revealed the importance of tension as a growth modulating signal, and perhaps even a survival signal (Anava et al., 2009). Under physiologic or mildly supraphysiologic loads, tension induces growth. Clinically, empirical evidence from the use of limb lengthening procedures in orthopedic surgery and tissue expanders in plastic surgery has demonstrated that nerve strain is often a rate-limiting factor, but at proper strain rates, large amounts of limb growth or tissue expansion can be produced without impaired nerve function (Stanitski, 1999).
Individual neurons in cell culture are similarly responsive to tension. Early reports revealed that towing axonal growth cones promotes axonal elongation (Bray, 1984; Zheng et al., 1991). This observation was dramatically exploited to elongate parallel tracts of cultured sensory neurons within a bioreactor by means of a microstepper (Pfister et al., 2004). Neurons reached lengths exceeding 10 cm, at a maximal rate of 8 mm per day of regeneration, or 8 × the typical rate of axonal outgrowth. Neurons also maintained their diameters and executed cellular processes such as axonal transport at pre-strain levels (Loverde et al., 2011), demonstrating an active response to deformation.
We and others have hypothesized that in vivo elongation of intact proximal stumps towards the injured distal stumps of severed peripheral nerves can faithfully reproduce the mechanical environment created during the above-mentioned in vivo and in vitro procedures. Such lengthening across a nerve gap may accelerate functional recovery and ensures an anatomic and functional match to the distal stump. A few promising strategies have been developed using this rationale. Ochiai and colleagues describe the use of a modified external fixator to close nerve gaps in rats, rabbits, and monkeys (Saijilafu et al., 2008; Sharula et al., 2010; Hara et al., 2012). This device exerted tension on both the proximal and distal stumps of the nerve. After the gap was closed, direct end-to-end repair was performed following device extraction. Based on electrophysiological and histological measures, nerve elongation groups appeared to match, or in some cases, outperform autografts, offering an exciting preview of the potential for elongation strategies. On the other hand, the device itself appears bulky, is housed extracorporeally, and requires extensive bone anchoring. Also, although extension of the distal stump would serve to reduce a nerve gap, the rationale for doing so is not wholly clear. While it is possible that extracellular matrix and compacted lamina in the distal stump are actively remodeling in response to strain, it is more likely that expansion of the degenerating distal stump is completely passive. As a consequence, imposed strains are well beyond structural tolerances for nerve loading (Georgeu et al., 2005), and regenerating axons would have to extend a longer distance through a mechanically compromised environment to reach their targets.
Borrowing strategies from the plastic surgery literature, some of these drawbacks have been addressed by using modified tissue expanders (Figure 1). Intact rat sciatic nerves were rapidly elongated (< 1 hour) up to 24% through the use of an underlying tissue expander (Arnaoutoglou et al., 2006), but resulted in partially reversible nerve conduction deficits and histological changes, including myelin thinning. Over a slower time frame (days), a tissue expander was used to seal a 20 mm gap in a canine sciatic nerve injury model, with similar outcomes (Wood et al., 1991). On one hand, conductive changes following the use of a tissue expander may be attributed to widened nodes of Ranvier (van der Wey et al., 1996), with blood flow preserved for expansion up to 40%. On the other hand, changes in conduction and nerve morphology are indicative of a compression neuropathy, and possibly, a related tension neuropraxia due to the relatively short time over which these devices act.
Our group has built upon previous efforts by developing a compact internal fixator device that elongates the proximal stump linearly towards the distal stump, to enable accelerated distal reconnection (Figure 1, (Chuang et al., 2013)). A prototype version of the device was implanted into a rat nerve gap. Observations at early 3–6 week time points revealed successful nerve lengthening, axonal outgrowth into the gap, and no detrimental effects of nerve lengthening for strains up to ~20%, based on axon number, axon density, and inflammatory response (Chuang et al., 2012). However, some fibrosis occurring within the 1-week actuation period limited axonal extension into the distal stump. Thus, we designed the second version of our device for a two stage procedure, in which the proximal transected nerve is placed into the device, elongated linearly beyond the distal stump, and reattached to the distal stump with a primary repair concurrent with device removal (Figure 2). We rationalize that the extra procedure would occur regardless, to explant the device. Delayed reconnection of freshened nerve tips would also allow excision of any fibrotic tissue or neuroma, which could impede axonal progress. Further, regenerating axons would only have to cross a single interface into the distal stump, and would reconnect farther distally than previous approaches (Figure 1). These advantages raise the possibility of excising large swaths of degenerating distal stumps, such as those existing during chronic nerve injuries, prior to repair. Future possibilities include design of a device that is completely self-contained, and independent of external actuation, but must still avoid potential complications, including jammed actuation, excessive fibrotic encapsulation, or slippage of the device from the nerve.
Figure 2.
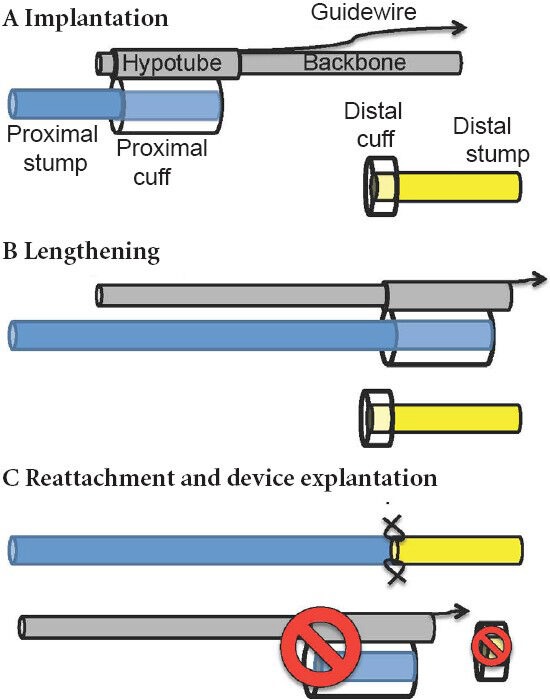
Schematic of nerve internal fixator device modified from (Chuang et al., 2013).
(A, B) Implanted device uses hypotube with nerve cuff to pull intact proximal stump past distal stump. (C) Cuffed nerve regions and device are explanted, allowing clean reattachment of proximal stump to distal stump.
As clinical success of repairing injuries differing in severity and time of intervention, is highly variable, it will be necessary to test lengthening devices in models differing in scale and time, including both acute and chronic models of nerve injury, and models where nerves must be lengthened across moving joints or within an environment of unusual geometry. Complicating interpretation of such studies are gaps in our understanding of mechanisms underlying thresholds of strain (or tension) that maximize nerve growth without inducing nerve dysfunction -- a very delicate balance that may vary by nerve, species, or individual. As a consequence, additional insight into the mechanobiology of nerve lengthening is also of great importance; a better grasp of fundamental accelerators of and barriers to regeneration may enable a more rational dissection of factors dictating successful recovery.
Finally, though our focus has thus far been on repairing nerve gaps resulting from primary injury, the advent of nerve transfer surgeries for treating cervical spinal cord injuries and for repairing severed nerve trunks (e.g., brachial plexus injuries) suggest another application for nerve lengthening (Brown and Mackinnon, 2008; Dahlin et al., 2009). Nerve transfers require rerouting of redundant branches or fascicles from functional nerves to functionally paralyzed recipient nerves. The transfer restores neuromuscular function in the recipient nerve, enabling individuals to execute critical tasks such as elbow extension or key pinch. However, the choice of donor nerves is often limited by anatomical proximity to the recipient, resulting in the use of an intervening graft or reconnection more proximally than desired. It is possible that sufficiently lengthening a donor nerve, even while intact, could create additional candidate donors for nerve transfers than those currently available. The years to come should offer an exciting expansion of our understanding and application of principles of nerve lengthening towards nerve repair.
Footnotes
Funding: This work was supported by a grant from Department of Defense (W81XWH-10-1-0773), National Science Foundation (CBET1042522), and a grant from the National Skeletal Muscle Research Center at UCSD.
References
- 1.Anava S, Greenbaum A, Ben Jacob E, Hanein Y, Ayali A. The regulative role of neurite mechanical tension in network development. Biophys J. 2009;96:1661–1670. doi: 10.1016/j.bpj.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaoutoglou CM, Sakellariou A, Vekris M, Mitsionis GI, Korompilias A, Ioakim E, Harhantis A, Beris A. Maximum intraoperative elongation of the rat sciatic nerve with tissue expander: functional, neurophysiological, and histological assessment. Microsurgery. 2006;26:253–261. doi: 10.1002/micr.20236. [DOI] [PubMed] [Google Scholar]
- 3.Asplund M, Nilsson M, Jacobsson A, von Holst H. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology. 2009;32:217–228. doi: 10.1159/000197900. [DOI] [PubMed] [Google Scholar]
- 4.Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin. 2008;24:319–340. doi: 10.1016/j.hcl.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J, Kwan M, Garfin SR. Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Relat Res. 1993:288–294. [PubMed] [Google Scholar]
- 7.Chuang TH, Wilson RE, Love JM, Fisher JP, Shah SB. San Diego, CA, USA: Curran Associates; 2012. A novel internal fixator device for peripheral nerve regeneration. 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. [Google Scholar]
- 8.Chuang TH, Wilson RE, Love JM, Fisher JP, Shah SB. A novel internal fixator device for peripheral nerve regeneration. Tissue Eng Part C Methods. 2013;19:427–437. doi: 10.1089/ten.tec.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlin L, Johansson F, Lindwall C, Kanje M. Chapter 28: Future perspective in peripheral nerve reconstruction. Int Rev Neurobiol. 2009;87:507–530. doi: 10.1016/S0074-7742(09)87028-1. [DOI] [PubMed] [Google Scholar]
- 10.Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312–318. doi: 10.1002/1098-2388(200010/11)19:3<312::aid-ssu13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 12.Georgeu GA, Walbeehm ET, Tillett R, Afoke A, Brown RA, Phillips JB. Investigating the mechanical shear-plane between core and sheath elements of peripheral nerves. Cell Tissue Res. 2005;320:229–234. doi: 10.1007/s00441-004-1031-2. [DOI] [PubMed] [Google Scholar]
- 13.Hara Y, Nishiura Y, Ochiai N, Sharula, Nakajima Y, Kubota S, Saijilafu, Mishima H. New treatment for peripheral nerve defects: reconstructiond of a 2cm, monkey median nerve gap by direct lengthening of both nerve stumps. J Orthop Res. 2012;30:153–161. doi: 10.1002/jor.21476. [DOI] [PubMed] [Google Scholar]
- 14.Khuong HT, Midha R. Advances in nerve repair. Curr Neurol Neurosci Rep. 2013;13:322. doi: 10.1007/s11910-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 15.Loverde JR, Ozoka VC, Aquino R, Lin L, Pfister BJ. Live imaging of axon stretch growth in embryonic and adult neurons. J Neurotrauma. 2011;28:2389–2403. doi: 10.1089/neu.2010.1598. [DOI] [PubMed] [Google Scholar]
- 16.Mason S, Phillips JB. An ultrastructural and biochemical analysis of collagen in rat peripheral nerves: the relationship between fibril diameter and mechanical properties. J Peripher Nerv Syst. 2011;16:261–269. doi: 10.1111/j.1529-8027.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Millesi H. The nerve gap. Theory and clinical practice. Hand Clin. 1986;2:651–663. [PubMed] [Google Scholar]
- 18.Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips JB, Smit X, De Zoysa N, Afoke A, Brown RA. Peripheral nerves in the rat exhibit localized heterogeneity of tensile properties during limb movement. J Physiol. 2004;557:879–887. doi: 10.1113/jphysiol.2004.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saijilafu, Nishiura Y, Hara Y, Yoshii Y, Ochiai N. Simultaneous gradual lengthening of both proximal and distal nerve stumps for repair of peripheral nerve defect in rats. Muscle Nerve. 2008;38:1474–1480. doi: 10.1002/mus.21147. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 22.Seddon HJ. Three types of nerve injury. Brain. 1943;66:247–288. [Google Scholar]
- 23.Sharula, Hara Y, Nishiura Y, Saijilafu, Kubota S, Ochiai N. Repair of the sciatic nerve defect with a direct gradual lengthening of proximal and distal nerve stumps in rabbits. Plast Reconstr Surg. 2010;125:846–854. doi: 10.1097/PRS.0b013e3181ccdbd4. [DOI] [PubMed] [Google Scholar]
- 24.Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7:143–153. doi: 10.5435/00124635-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Sunderland S. A classification of peripheral nerve injury produced by loss of function. Brain. 1951;74:491–495. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 26.Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975;56:166–170. [PubMed] [Google Scholar]
- 27.Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86:92–109. doi: 10.1093/ptj/86.1.92. [DOI] [PubMed] [Google Scholar]
- 28.van der Wey LP, Polder TW, Stegeman DF, Gabreels-Festen AA, Spauwen PH, Gabreels FJ. Peripheral nerve elongation by laser Doppler flowmetry-monitored expansion: an experimental basis for future application in the management of peripheral nerve defects. Plast Reconstr Surg. 1996;97:568–576. doi: 10.1097/00006534-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg Br. 1992;74:126–129. doi: 10.1302/0301-620X.74B1.1732240. [DOI] [PubMed] [Google Scholar]
- 30.Wood RJ, Adson MH, VanBeek AL, Peltier GL, Zubkoff MM, Bubrick MP. Controlled expansion of peripheral nerves: comparison of nerve grafting and nerve expansion/repair for canine sciatic nerve defects. J Trauma. 1991;31:686–690. [PubMed] [Google Scholar]
- 31.Wright TW, Glowczewskie F, Wheeler D, Miller G, Cowin D. Excursion and strain of the median nerve. J Bone Joint Surg Am. 1996;78:1897–1903. doi: 10.2106/00004623-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Wright TW, Glowczewskie F, Jr, Cowin D, Wheeler DL. Ulnar nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg Am. 2001;26:655–662. doi: 10.1053/jhsu.2001.26140. [DOI] [PubMed] [Google Scholar]
- 33.Zheng J, Lamoureux P, Santiago V, Dennerll T, Buxbaum RE, Heidemann SR. Tensile regulation of axonal elongation and initiation. J Neurosci. 1991;11:1117–1125. doi: 10.1523/JNEUROSCI.11-04-01117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


