Abstract
Recently, we have demonstrated the ability of naringin, a well-known flavanone glycoside of grapefruits and citrus fruits, to prevent neurodegeneration in a neurotoxin model of Parkinson's disease. Intraperitoneal injection of naringin protected the nigrostriatal dopaminergic projection by increasing glial cell line-derived neurotrophic factor expression and decreasing the level of tumor necrosis factor-alpha in dopaminergic neurons and microglia, respectively. These results suggest that naringin can impart to the adult dopaminergic neurons the ability to produce glial cell line-derived neurotrophic factor against Parkinson's disease with anti-inflammatory effects. Based on these results, we would like to describe an important perspective on its possibility as a therapeutic agent for Parkinson's disease.
Keywords: naringin, parkinson's disease, GDNF, inflammation, mTORC1, neurodegeneration
Naringin is a well-known flavanone glycoside of grapefruits and citrus fruits (Jagetia and Reddy, 2002). It exerts a variety of biological and pharmacological effects such as anti-inflammatory, antioxidant and lipid-lowering activities (Chanet et al., 2012). Recently, Gopinath and Sudhandiran (2012) have reported that naringin possess neuroprotective effect against neurodegenerative disorders through modulating the oxidative stress and inflammatory responses. In addition, several studies have shown that naringin has neuroprotective effects by the induction of neurotrophic factors such as brain-derived neurotrophic factor and vascular endothelial growth factor, and by the activation of anti-apoptotic pathways (Kim et al., 2009; Choi et al., 2010; Rong et al., 2012). Although these evidences suggest that naringin may be a potential natural compound involved in the prevention and treatment against neurodegenerative diseases (Jagetia and Reddy, 2005; Kim et al., 2009; Choi et al., 2010; Golechha et al., 2011; Chanet et al., 2012; Rong et al., 2012), it has not been clarified so far whether naringin has beneficial effects against degeneration of the nigrostriatal dopaminergic (DA) projection in the adult brain, which is associated with Parkinson's disease. Therefore, we have recently investigated whether daily intraperitoneal injection of naringin can have neuroprotective roles in a rat model of 1-methyl-4-phenylpyridinium (MPP+)-induced Parkinson's disease (Leem et al., 2014). Our results have showed that naringin treatment significantly increases glial cell line-derived neurotrophic factor (GDNF) expression and mammalian target of rapamycin complex 1 (mTORC1) activity in the nigral DA neurons, and naringin-induced production of GDNF contributes to the protection of the nigrostriatal DA projection in a neurotoxin model of Parkinson's disease (Leem et al., 2014). Moreover, naringin decreases the level of tumor necrosis factor-alpha (TNF-α) in microglia increased by MPP+ -induced neurotoxicity. Taken together, our findings suggest that naringin may be a potential natural product to prevent degeneration of the nigrostriatal DA projection in the adult brain.
Over the last few years, there has been a growing interest in a number of pharmacological approaches to prevent and improve the neuronal dysfunction and death associated with neurodegenerative diseases. Parkinson's disease is a chronic and progressive movement disorder by the degeneration of DA neurons and biochemical reduction in striatal dopamine levels of the central nervous system, and is associated with major clinical symptoms including tremor at rest, rigidity of the limbs, slowness and paucity of voluntary movement (bradykinesia) and postural instability (a tendency to fall even in the absence of weakness or cerebellar balance disturbance) which are worsen over time (Savitt et al., 2006; Burke and O’Malley, 2013). It affects nearly 1% of the global population aged 65 and older (Lee and Trojanowski 2006; Recchia et al., 2008). Age-related Parkinson's disease is a complex multifactor disease, and no drugs are available to stop its progression. For several years, trophic factors have been suggested as potential therapeutic agents since they can regulate the survival of specific neuronal populations in the central nervous system (Bäckman et al., 2006). One such trophic factor is GDNF, a member of the transforming growth factor-β family of trophic factors.
GDNF is a protein that promotes the survival of many types of neurons including DA neurons (Pochon et al., 1997). The neurotrophic properties of GDNF were first described by Lin et al. (1993) who have demonstrated that GDNF promotes cell survival and increases dopamine uptake in the DA neuron cultures derived from embryonic rodent midbrain. Subsequent experiments have demonstrated that the direct injection of GDNF into the substantia nigra or striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice increases the density of DA fibers and improves motor function (Tomac et al., 1995). The neurotrophic and protective effects of GDNF were also observed in many different animal models of Parkinson's disease (Siegel and Chauhan, 2000; Manfredsson et al., 2009; Allen et al., 2013). In addition, a conditional GDNF-null mouse showed a delayed and progressive loss of the DA neurons (Pascual et al., 2008). Although these evidences suggest that GDNF is a potent neurotrophic factor for the survival and protection of DA neurons, there is a limitation of using GDNF for Parkinson's disease patients. Since GDNF does not cross the blood-brain barrier, which is the membrane protecting the brain, direct application of GDNF to the brain is needed. Moreover, in clinical trials, intracerebroventricular injection and intraputaminal infusion of GDNF did not improve parkinsonism and caused several side effects such as nausea, anorexia and vomiting (Nutt et al., 2003; Peterson and Nutt, 2008). These effects may be caused by the limited diffusion into the appropriate target sites of brain. Thus, despite the potential clinical importance of GDNF, the utilization of GDNF in clinical pharmacology and other therapeutics for Parkinson's disease will be dependent upon sustained delivery of the appropriate amount of GDNF to the target areas in a safe and efficacious manner without producing adverse effects.
Physiological responses to GDNF are mediated by a two-component receptor complex: the Ret tyrosine kinase (encoded by the c-ret proto-oncogene) and a glycosylphosphatidyl inositol-linked protein called GDNF family receptor α-1 (GFRα1) (Treanor et al., 1996; Trupp et al., 1996). The binding of GDNF to Ret and GFRα1 leads to Ret phosphorylation (Kjær and Ibáñez, 2003; Sariola and Saarma, 2003), and thereby leads to the activation of several intracellular signaling pathways, including RAS/mitogen-activated protein kinase/extracellular signal-regulated kinase pathway and phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (mTOR) pathway, which in turn promotes neuronal survival and differentiation (Creedon et al., 1997; Uesaka et al., 2013).
MPP+ treatment decreases Akt phosphorylation in mice, which results in loss of mTORC1 activation, and a loss of Akt phosphorylation is also observed in the substantia nigra of Parkinson's disease patients (Selvaraj et al., 2012). Moreover, the activation of mTORC1 by adeno-associated virus 1 transduction with a gene encoding the constitutively active form of Akt or ras homolog enriched in the brain (Rheb) in substantia nigra DA neurons induces neurotrophic effects, including abilities to both protect and restore the nigrostriatal DA projections, in a neurotoxin mouse model of Parkinson's disease (Cheng et al., 2011; Kim et al., 2011, 2012). These results suggest that the activation of mTORC1 is required for the survival of DA neurons and functional maintenance of the DA system in the adult brain. In accordance with previous reports, we have recently reported that naringin-mediated neuroprotection in the MPP+ rat model of Parkinson's disease may be associated with the activation of mTORC1 (Leem et al., 2014). However, it is still unclear whether the activation of mTORC1 can reproduce neurotrophic factors through intracellular signaling pathways in adult neurons of the brain. We have recently found that increased expression of Rheb (S16H) by a viral vector induces a robust ability to induce GDNF in adult DA neurons in vivo, which is dependent on mTORC1 activity and contributes to the protection of the nigrostriatal DA projection (Nam et al., 2014a). The results describe the relationships between GDNF reproduction and mTORC1 activation. To ascertain whether naringin mediates mTORC1 activity, we further examined the effects of naringin with or without mTORC1-specific inhibitor rapamycin on the activation of mTORC1 (Figure 1). Rats received daily intraperitoneal injection of rapamycin (5 mg/kg) or naringin (80 mg/kg), or co-injection of rapamycin and naringin for 4 days. Similar to the previous results (Leem et al., 2014), our results showed that treatment with naringin alone increased mTORC1 substrate phospho-4E-BP1 expression in the substantia nigra of rat brain compared to the intact controls, as shown by the results of western blot analysis (Figure 1). Rapamycin did not alter the baseline activity of mTORC1 in the substantia nigra. However, its treatment decreased the level of naringin-increased phospho-4E-BP1 (Figure 1), suggesting that naringin mediated mTORC1 activity for neurotrophic effects similar to the effects induced by the active Rheb in the substantia nigra (Nam et al., 2014a).
Figure 1.
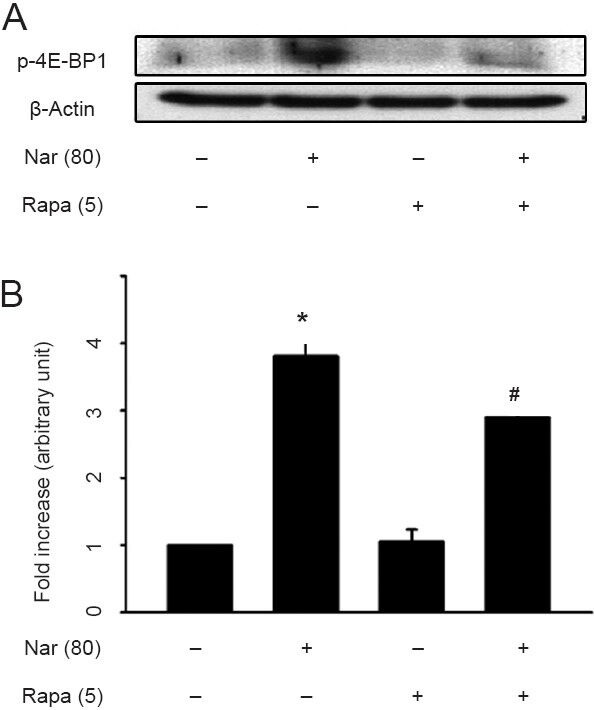
Western blot analysis of phospho-4E-BP1 (p-4E-BP1).
To investigate the levels of p-4E-BP1, rats received daily intraperitoneal injection of rapamycin [5 mg/kg, Rapa (5)] or naringin [80 mg/kg, Nar (80)], or co-injection of rapamycin and naringin for 4 days. (A) Representative Western blots of p-4E-BP1 in the rat substantia nigra. (B) The results show that treatment with naringin induces an increase in the level of mTORC1 substrate p-4E-BP1 in the substantia nigra (*P < 0.001, vs. intact controls), and treatment with 5 mg/kg rapamycin alone indicates no alteration on the basic level of p-4E-BP1 compared to the intact controls. However, its treatment attenuated the level of naringin- increased p-4E-BP1 [#P = 0.024, vs. Nar (80) alone]. All values on the optical density of each band represent mean ± SEM of three pooled samples (one-way analysis of variance and Student-Newman-Keuls analysis).
Another potential effect of naringin to protect the nigrostriatal DA projection is its anti-inflammatory activities. Brain and immune system are complicatedly connected and engage in crosstalk to maintain homeostasis. A number of evidences from human and animal studies have suggested that neuroinflammation is an important contributor to the neuronal loss in Parkinson's disease, and that long-term use of anti-inflammatory agents reduce the risk for Parkinson's disease (Gao et al., 2005; Chen et al., 2005). Parkinson's disease exhibits the activation of microglia, a resident immune cell of the brain, and activated microglia is involved in the loss of dopaminergic neurons in Parkinson's disease (Mount et al., 2007). Numerous microglial-derived neurotoxic factors, such as reactive oxygen species, inducible nitric oxide synthase and proinflammatory cytokines, can induce neuron death (Block and Hong, 2005; Colton et al., 2006; Yu et al., 2008; Kim et al., 2010; Nam et al., 2014b). Among them, TNF-α is one of proinflammatory cytokines which has received much attention due to its ability to promote the progressive degeneration of the DA projection (McCoy et al., 2006). A deficiency of TNF-α decreases dopamine content loss in the striatum after neurotoxin administration (Ferger et al., 2004). Null mice of TNF-α also showed protection against DA neurotoxicity (Sriram et al., 2002), suggesting an overall detrimental effect of TNF-α on the nigrostriatal DA system. In addition to the neurotrophic effects, we have found that naringin attenuates the level of TNF-α in the microglia increased by MPP+-induced neurotoxicity, which indicates the anti-inflammatory activity of naringin against inflammation in the substantia nigra (Leem et al., 2014).
As described in the preceding text, although GDNF has beneficial effects in models of Parkinson's disease, its direct infusion into the brain is necessary to achieve a therapeutic purpose. In addition, although gene therapy with viral vectors inducing neurotrophic effects such as mTORC1 activation similar to GDNF-mediated effects is still being investigated to treat Parkinson's disease patients, there are concerns about live virus administration and genetically modifications of host neuronal cells. Thus, alternative therapeutic approaches such as natural compounds or chemical drugs can be useful to develop a protector of the nigrostriatal DA system in the adult brain, and many researchers have currently proposed intervention strategies using phytochemicals as an alternative approach against neurodegenerative diseases. In particular, phytochemicals are usually considered to be harmless to health with less toxic and fewer side effects than synthetic drugs.
Among various phytochemicals, naringin, which has found in grapefruits and citrus fruits in high concentrations (Jagetia and Reddy, 2002), has not only pharmacological anti-inflammatory and antioxidant activities but also neuroprotective effects against neurodegenerative disorders (Golechha et al., 2011; Gopinath et al., 2011; Chanet et al., 2012). Recently, we have also demonstrated the ability of naringin to prevent neurodegeneration (Leem et al., 2014). In a neurotoxin model of Parkinson's disease, treatment with naringin protects the nigrostriatal DA projection by increasing GDNF expression and decreasing TNF-α expression in DA neurons and microglia, respectively, which suggests that naringin can impart to the adult DA neurons the ability to reproduce GDNF as a therapeutic agent against Parkinson's disease with anti-inflammatory effects on brain inflammation. Thus, naringin may be a promising natural compound with neuroprotective activity and safety. However, there is lack of evidence for the neuroprotective roles of naringin in clinical trials, and there is still insufficient mechanisms of naringin-mediated effects on Parkinson's disease. Therefore, further studies are needed to evaluate detailed mechanisms of naringin-induced effects in the adult brain, except the GDNF-mediated pathway, and to investigate whether naringin can prevent and improve parkinsonism in humans. In addition, it may be worthwhile to test whether post-treatment with naringin can restore the function of DA neurons in the adult brain.
Footnotes
Funding: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government, No. 2008-0061888 and 2012R1A1A1039140.
References
- 1.Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Bäckman CM, Shan L, Zhang YJ, Hoffer BJ, Leonard S, Troncoso JC, Vonsatel P, Tomac AC. Gene expression patterns for GDNF and its receptors in the human putamen affected by Parkinson's disease: a real-time PCR study. Mol Cell Endocrinol. 2006;252:160–166. doi: 10.1016/j.mce.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Burke RE, O’Malley K. Axon degeneration in Parkinson's disease. Exp Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanet A, Milenkovic D, Manach C, Mazur A, Morand C. Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 7.Cheng HC, Kim SR, Oo TF, Kareva T, Yarygina O, Rzhetskaya M, Wang C, During M, Talloczy Z, Tanaka K, Komatsu M, Kobayashi K, Okano H, Kholodilov N, Burke RE. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YJ, Li WY, Moon GJ, Lee PH, Ahn YH, Lee G, Bang OY. Enhancing trophic support of mesenchymal stem cells by ex vivo treatment with trophic factors. J Neurol Sci. 2010;298:28–34. doi: 10.1016/j.jns.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci U S A. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- 12.Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci. 2005;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 13.Golechha M, Chaudhry U, Bhatia J, Saluja D, Arya DS. Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol Pharm Bull. 2011;34:360–365. doi: 10.1248/bpb.34.360. [DOI] [PubMed] [Google Scholar]
- 14.Gopinath K, Prakash D, Sudhandiran G. Neuroprotective effect of naringin, a dietary flavonoid against 3-nitropropionic acid-induced neuronal apoptosis. Neurochem Int. 2011;59:1066–1073. doi: 10.1016/j.neuint.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience. 2012;227:134–143. doi: 10.1016/j.neuroscience.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Jagetia GC, Reddy TK. The grapefruit flavanonenaringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutat Res. 2002;519:37–48. doi: 10.1016/s1383-5718(02)00111-0. [DOI] [PubMed] [Google Scholar]
- 17.Jagetia GC, Reddy TK. Modulation of radiation-induced alteration in the antioxidant status of mice by naringin. Life Sci. 2005;77:780–794. doi: 10.1016/j.lfs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Song JY, Park HJ, Park HK, Yun DK, Chung JH. Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol. 2009;13:281–285. doi: 10.4196/kjpp.2009.13.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Kim JY, Lee HJ, Lim HJ, Lee da Y, Kim do H, Ryu JH. Suppression of inducible nitric oxide synthase expression by furfuran lignans from flower buds of Magnolia fargesii in BV-2 microglial cells. Phytother Res. 2010;24:748–753. doi: 10.1002/ptr.3028. [DOI] [PubMed] [Google Scholar]
- 20.Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, During M, Kholodilov N, Burke RE. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70:110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SR, Kareva T, Yarygina O, Kholodilov N, Burke RE. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol Ther. 2012;20:275–286. doi: 10.1038/mt.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjær S, Ibáñez CF. Identification of a surface for binding to the GDNF-GFR α1 complex in the first cadherin-like domain of RET. J Biol Chem. 2003;278:47898–47904. doi: 10.1074/jbc.M309772200. [DOI] [PubMed] [Google Scholar]
- 23.Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Leem E, Nam JH, Jeon MT, Shin WH, Won SY, Park SJ, Choi MS, Jin BK, Jung UJ, Kim SR. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson's disease. J Nutr Biochem. 2014;25:801–806. doi: 10.1016/j.jnutbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 26.Manfredsson FP, Okun MS, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson's disease. Curr Gene Ther. 2009;9:375–388. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- 27.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam JH, Leem E, Joen MT, Jeong KH, Park JW, Jung UJ, Kholodilov N, Burke RE, Jin BK, Kim SR. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: Neuroprotective mechanisms of hRheb (S16H) in a model of Parkinson's disease. Mol Neurobiol. 2014a doi: 10.1007/s12035-014-8729-2. DOI: 10.1007/s12035-014-8729-2. [DOI] [PubMed] [Google Scholar]
- 30.Nam JH, Leem E, Joen MT, Kim YJ, Jung UJ, Choi MS, Maeng S, Jin BK, Kim SR. Inhibition of prothrombin kringle-2-induced inflammation by minocycline protects dopaminergic neurons in the substantia nigra in vivo. Neuroreport. 2014b;25:489–495. doi: 10.1097/WNR.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 31.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 33.Peterson AL, Nutt JG. Treatment of Parkinson's disease with trophic factors. Neurotherapeutics. 2008;5:270–280. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 35.Recchia A, Rota D, Debetto P, Peroni D, Guidolin D, Negro A, Skaper SD, Giusti P. Generation of a alpha-synuclein-based rat model of Parkinson's disease. Neurobiol Dis. 2008;30:8–18. doi: 10.1016/j.nbd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Rong W, Wang J, Liu X, Jiang L, Wei F, Hu X, Han X, Liu Z. Naringin treatment improves functional recovery by increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem Res. 2012;37:1615–1623. doi: 10.1007/s11064-012-0756-7. [DOI] [PubMed] [Google Scholar]
- 37.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 38.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 41.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 42.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 43.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 44.Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumäe U, Sariola H, Saarma M, báñez CF. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- 45.Uesaka T, Nagashimada M, Enomoto H. GDNF signaling levels control migration and neuronal differentiation of enteric ganglion precursors. J Neurosci. 2013;33:16372–16382. doi: 10.1523/JNEUROSCI.2079-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu HH, Wu FL, Lin SE, Shen LJ. Recombinant arginine deiminase reduces inducible nitric oxide synthase iNOS-mediated neurotoxicity in a coculture of neurons and microglia. J Neurosci Res. 2008;86:2963–2972. doi: 10.1002/jnr.21740. [DOI] [PubMed] [Google Scholar]


