Abstract
Bone marrow mesenchymal stem cell transplantation has been shown to be therapeutic in the repair of spinal cord injury. However, the low survival rate of transplanted bone marrow mesenchymal stem cells in vivo remains a problem. Neurotrophin-3 promotes motor neuron survival and it is hypothesized that its transfection can enhance the therapeutic effect. We show that in vitro transfection of neurotrophin-3 gene increases the number of bone marrow mesenchymal stem cells in the region of spinal cord injury. These results indicate that neurotrophin-3 can promote the survival of bone marrow mesenchymal stem cells transplanted into the region of spinal cord injury and potentially enhance the therapeutic effect in the repair of spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, cell transplantation, neurotrophin-3, bone marrow mesenchymal stem cells, cell apoptosis, spinal cord anterior horn motor neurons, neural regeneration
Introduction
Transplantation of bone marrow mesenchymal stem cells (BMSCs) can repair injured spinal cord and improve neural function (Ide et al., 2010; Zhang and He, 2014). BMSCs are easy to isolate and culture and can differentiate into both neurons and neurogliocytes in vitro and in vivo (Naghdi et al., 2013). BMSCs exhibit low immunogenicity when they are autologously transplanted (Ide et al., 2010). However, after transplantation into injured spinal cord, stem/neural progenitor cells have a low cell survival rate (Gray et al., 2007).
Neurotrophic factors such as neurotrophins play an important role in neuronal survival (Huang and Reichardt, 2001). These proteins are crucial to the development of the nervous system because they control the considerable loss of excess neurons that fail to adequately connect to their targets (Thomas and Gorassini, 2005; David and Kroner, 2011). Neurotrophin-3 (NT-3) is a key factor in the regenerative environment and exerts a variety of physiological effects on nervous system development.
NT-3 can improve motor neuron survival in either a paracrine or autocrine fashion (Zhang et al., 2012). Additionally, it promotes the formation of neuromuscular junctions and axonal outgrowth (Fan et al., 2010; Yang et al., 2010). Many studies have shown NT-3 can maintain the survival of sensory, motor and sympathetic neurons (Boyce et al., 2012; Thomson et al., 2012; Solev et al., 2013). Treatment with NT-3 has been shown to enhance the sprouting of supraspinal tract axons when injected into the lesion immediately after spinal cord injury (Wood et al., 2009; Zhang et al., 2013). In this study, we investigated the ability of NT-3 gene transfected BMSC transplantation to repair spinal cord injury.
Materials and Methods
Animals
BMSCs were isolated from male Sprague-Dawley rats aged 3 days. Forty-eight healthy female Sprague-Dawley rats, weighing 200–250 g and aged 6–7 weeks were used in the in vivo experiment. All rats were provided by Laboratory Animal Center of Xinxiang Medical College, China with license No. SCXK (Yu) 2008-0001. All rats were housed in plastic cages and subjected to a 12-hour light/dark cycle, and were allowed to move freely. The study protocol was approved by the Institutional Animal Care and Use Committee of Xinxiang Medical College, China.
Rat BMSC culture
Sprague-Dawley rats were decapitated after anesthesia by intraperitoneal injection of pentobarbital (30 mg/kg). The tibiae and femurs were removed from rats and dissected free of muscle. The bones were rinsed in sterilized PBS. The cut surface was placed facing the bottom of the centrifuge tube and the tube was spun at 800 × g for 15 minutes. The bones were removed and bone marrow tissues were washed with PBS. The cells were isolated and re-suspended in culture medium (Sigma, St. Louis, MO, USA) containing alpha minimum essential medium, 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mL glutamine. Cells were cultured at 37°C, 5% CO2 to form colonies. Floating cells were removed with PBS, collected, centrifuged and re-seeded after 2 days. Cells attached on the extracellular cell matrix (SBS Genetech Co., Ltd., Beijing, China) were maintained for an additional 14 days. BMSCs were sub-cultured when they reached 80–90% confluence. The medium was changed every 3 days. Recombinant NT-3 (SBS Genetech Co., Ltd.) was embedded by adding 10 mg NT-3 plasmid to 1 mL 1% fetal bovine serum solution and trypsinized by 0.05% trypsin for 10 minutes. The reaction was stopped by adding DMEM (Sigma) supplemented with 10% fetal bovine serum. 1 × 105 BMSCs were cultured in 20 mL culture medium consisting of DMEM/F12 at a ratio of 1:1, 10% fetal bovine serum, and 50 μg/mL ascorbic acid for 14 days. The culture medium was replaced every 2 days.
Establishment of a rat model of spinal cord injury
Forty-eight rats were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg) and the spinal cord was exposed at T9–10 level by laminectomy. The exposed spinal cord was damaged by a 10 g metal subject dropping from the height of 5 cm and a moderate contusion was induced (Panepucci et al., 2004). The rats with acute spinal cord injury were equally and randomly divided into spinal cord injury, BMSCs and BMSCs + NT-3 groups. 10 μL NT-3-tansfected BMSCs (1 × 105/μL) and 10 μL extracellular matrix gel were injected into the injured spinal cord via a microinjector. All animals were given appropriate antibiotics and analgesic.
Tracing of BMSCs transplanted into injured spinal cord by enhanced green fluorescence protein (EGFP) fluorescence
Sections of injured T10 spinal cord were treated with 5% bovine serum albumin in PBS, 0.02% Triton X-100 (PBS-T) for 4 hours and then incubated in rabbit anti-EGFP (1:200; Sigma) overnight at 4°C. Sections were double labeled with tyrosine hydroxylase (TH). Following three washes in PBS-T, sections were incubated overnight with Alexa 488 goat anti-chicken (1:2,000) and 568 goat anti-rabbit (1:1,000) or 555 goat anti-rat (1:1,000) secondary antibodies. When using chicken anti-TH, Alexa 568 goat anti-chicken (1:1,000) and 488 goat anti-rabbit (1:200) were used. Sections were observed using a fluorescence microscope (Olympus, Tokyo, Japan).
TUNEL for apoptosis in spinal cord anterior horn motor neurons
Four weeks after transplantation, spinal cord was cut at 5 mm away from the contused site and fixed in a 10% formalin solution for 2 hours, embedded in paraffin, and consecutively sliced into sections at 5 μm thickness. Apoptosis was detected by TUNEL assay (Boster, Wuhan, China). The TUNEL reaction mixture was dropped onto the spinal cord sections and incubated for 1 hour at 37°C under parafilm. TUNEL positive cells (apoptotic spinal cord anterior horn motor neurons) were analyzed under a light microscope (Olympus, Tokyo, Japan). Five damaged areas per section were randomly selected under non-overlapping × 400 field of view. The number of total apoptotic spinal cord anterior horn motor neurons stained with yellow dye was calculated. Spinal cord anterior horn motor neuron apoptotic rate (%) = number of apoptotic cells/number of total cells × 100%.
Statistical analysis
Statistical data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. Differences between groups were compared using two-sample t-test, and a level of P < 0.05 value was considered statistically significant.
Results
Characteristics of BMSCs transfected with NT-3
The cultured BMSCs appeared flattened and spindle-shaped. In culture, BMSCs adhered to the culture flask within 1–3 hours. Adherent cells were round, and small cytoplasmic projections were seen after 24 hours. At 72 hours, adherent cells multiplied and developed toward single fiber cells and cell clumps were observed. After transfection with NT-3, BMSCs visibly proliferated, grew rapidly and had stretched single long spindles. Three days later, the number of NT-3 transfected BMSCs was significantly greater than that of BMSCs cultured alone (P < 0.05; Figure 1).
Figure 1.
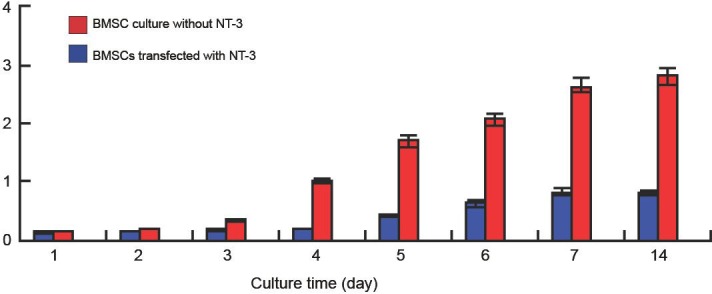
The number of bone marrow mesenchymal stem cells (BMSCs) transfected with or without neurotrophin-3 (NT-3) during culture.
After 3 days, the number of BMSCs transfected with NT-3 was significantly higher than that of non-transfected with NT-3 (P < 0.05). The data were expressed as mean ± SD and analyzed using two-sample t-test.
NT-3 transfection increased the number of BMSCs transplanted into injured spinal cord
Four weeks after transplantation, fluorescence microscopy showed that the NT-3 + BMSCs group had a greater number of BMSCs in the injured spinal cord compared with the spinal cord injury and BMSCs groups (P < 0.05; Table 1). The morphology of cells in the BMSCs + NT-3 group was regular, while the shape of cells in the other two groups was irregular (Figure 2).
Table 1.
Number (× 105) of bone marrow mesenchymal stem cells (BMSCs) in the injured spinal cord in rats
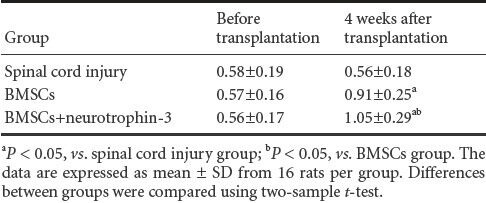
Figure 2.
Expression of enhanced green fluorescent protein (EGFP; green fluorescence) in the bone marrow mesenchymal stem cells (BMSCs) transplanted into the injured spinal cord at 4 weeks after transplantation (fluorescence microscope, × 200).
EGFP expression was significantly greater in the BMSCs + neurotrophin-3 group (C) than in the spinal cord injury (A) and BMSCs groups (B). The expression was lowest in the spinal cord injury group.
NT-3 transfection decreased apoptosis of motor neurons in anterior horn spinal cord
TUNEL staining showed that at 4 weeks after transplantation, the number of apoptotic motor neurons in the spinal cord anterior horn in the BMSCs + NT-3 and BMSCs groups was significantly reduced compared with the spinal cord injury group (19.84 ± 0.63% and 30.14 ± 1.02% vs. 41.67 ± 1.48%, P < 0.05; Figure 3).
Figure 3.

Apoptosis of motor neurons in spinal cord anterior horn at 4 weeks after transplantation (TUNEL staining, × 400).
(A–C) Spinal cord injury, bone marrow mesenchymal stem cells (BMSCs), and BMSCs + neurotrophin-3 (NT-3) groups. Apoptotic cells in the spinal cord anterior horn exhibited yellow-brown nuclei. The number of apoptotic cells was lowest in the BMSCs + NT-3 group but highest in the spinal cord injury group.
Discussion
BMSCs transplanted into the injured spinal cord can enhance axonal regeneration and promote functional recovery in animals. BMSCs exhibit multi-differentiation potential, and autologous transplantation of BMSCs can overcome problems of ethics and immune rejection (Alto et al., 2009; Smith et al., 2009; Oda et al., 2014). Thus, BMSCs have been used in cell therapy and gene therapy of many diseases. There is evidence that BMSCs can be induced to differentiate into nerve cells in vitro and they can promote nerve fiber regeneration in the injured spinal cord after transplantation (Langworthy and Appel, 2012; Saito et al., 2012; Lin et al., 2013; Vawda and Fehlings, 2013). BMSCs express substrate matrix metalloproteinases and play an important role in the repair of spinal cord injury (Alexanian et al., 2008; Yip and Malaspina, 2012; Dasari et al., 2014). Previous studies have demonstrated that BMSCs can promote local angiogenesis and vascular remodeling, phagocytosis and immune regulation to inhibit apoptosis (Alexanian et al., 2008; Yip and Malaspina, 2012; Dasari et al., 2014).
BMSCs can be induced to differentiate into neurons and neuroglia that can migrate to the damaged region and generate many neurotrophic factors and receptors, which can promote the repair of injured spinal cord neurons and inhibit the formation of nerve regeneration cicatrix (Fan et al., 2011; Saito et al., 2012). Some studies have reported that BMSCs have the ability to regenerate and repair the spinal cord injury (Gou et al., 2010; Harvey and Chopp, 2013).
Owing to the presence of a growth-inhibitory environment associated with reactive astrocytes and myelin on the host side of the graft-host interface, axon regeneration fails when axons depart from the transplants and back into the host spinal cord (Zhang et al., 2009; Kan et al., 2010; Song et al., 2014). Thus, strategies emphasizing additional treatments within the caudal host spinal cord, including providing attractive cues, are essential to reconstruct new functional circuits across the spinal cord injury (Awad et al., 2013). The present results show that transfection with NT-3 can increase the number of BMSCs transplanted into the injured spinal cord and reduce the apoptotic cells in the spinal cord anterior horn compared with those cells without NT-3 transfection.
Acknowledgments
We are grateful to Dr. Zhang XZ, Xinxiang Medical College in China, for his generous help in this research and Dr. Tong L, the First Affiliated Hospital of Xinxiang Medical College in China, and Zhang HQ, English Laboratory of Xinxiang Medical College in China for revising our manuscript.
Footnotes
Funding: This study was supported by Scientific Research Fund of Xinxiang Medical University, No. 2013ZD120; Science and Technology Innovation Talents in Universities in Ministry of Education of Henan Province in 2010, No. 2010HASTIT036.
Conflicts of interest: None declared.
Copyedited by Paul P, Haase R, Li CH, Song LP, Zhao M
References
- 1.Alexanian AR, Maiman DJ, Kurpad SN, Gennarelli TA. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev. 2008;17:1123–1130. doi: 10.1089/scd.2007.0212. [DOI] [PubMed] [Google Scholar]
- 2.Alto LT, Havton LA, Conner JM, Hollis ER, 2nd, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad BI, Carmody MA, Steinmetz MP. Potential role of growth factors in the management of spinal cord injury. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.01.042. doi:10.1016/j.wneu.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35:221–232. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells. 2014;6:120–133. doi: 10.4252/wjsc.v6.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 7.Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev Rep. 2011;7:195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Xiao Z, Zhang H, Chen B, Tang G, Hou X, Ding W, Wang B, Zhang P, Dai J, Xu R. Linear ordered collagen scaffolds loaded with collagen-binding neurotrophin-3 promote axonal regeneration and partial functional recovery after completespinal cord transection. J Neurotrauma. 2010;27:1671–1683. doi: 10.1089/neu.2010.1281. [DOI] [PubMed] [Google Scholar]
- 9.Gou S, Wang C, Liu T, Wu H, Xiong J, Zhou F, Zhao G. Spontaneous differentiation of murine bone marrow-derived mesenchymal stem cells into adipocytes without malignant transformation after long-term culture. Cells Tissues Organs. 2010;191:185–192. doi: 10.1159/000240246. [DOI] [PubMed] [Google Scholar]
- 10.Gray M, Palispis W, Popovich K, Popovich PG, van Rooijen N, Gupta R. Macrophage depletion alters the blood-nerve barrier without affecting Schwann cell function after neural injury. J Neurosci Res. 2007;85:766–777. doi: 10.1002/jnr.21166. [DOI] [PubMed] [Google Scholar]
- 11.Harvey RL, Chopp M. The therapeutic effects of cellular therapy for functional recovery after brain injury. Phys Med Rehabil Clin N Am. 2003;14:S143–151. doi: 10.1016/s1047-9651(02)00058-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ide C, Nakai Y, Nakano N, Seo T-B, Yamada Y, Endo K, Noda T, Saito F, Suzuki Y, Fukushima M, Nakatani T. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Kan EM, Ling EA, Lu J. Stem cell therapy for spinal cord injury. Curr Med Chem. 2010;17:4492–4510. doi: 10.2174/092986710794182971. [DOI] [PubMed] [Google Scholar]
- 15.Langworthy MM, Appel B. Schwann cell myelination requires Dynein function. Neural Dev. 2012;7:37. doi: 10.1186/1749-8104-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin WP, Chen XW, Zhang LQ, Wu CY, Huang ZD, Lin JH. Effect of neuroglobin genetically modified bone marrow mesenchymal stem cells transplantation onspinal cord injury in rabbits. PLoS One. 2013;8:e63444. doi: 10.1371/journal.pone.0063444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naghdi M, Tiraihi T, Mesbah-Namin SA, Arabkharadmand J, Kazemi H, Taheri T. Improvement of contused spinal cord in rats by cholinergic-like neuron therapy. Iran Red Crescent Med J. 2013;15:127–135. doi: 10.5812/ircmj.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oda Y, Tani K, Isozaki A, Haraguchi T, Itamoto K, Nakazawa H, Taura Y. Effects of polyethylene glycol administration and bone marrow stromal cell transplantation therapy in spinal cord injury mice. J Vet Med Sci. 2014;76:415–421. doi: 10.1292/jvms.13-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panepucci RA, Siufi JL, Silva WA, Jr, Proto-Siquiera R, Neder L, Orellana M. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;14:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 20.Saito F, Nakatani T, Iwase M, Maeda Y, Murao Y, Suzuki Y, Fukushima M, Ide C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci. 2012;30:127–136. doi: 10.3233/RNN-2011-0629. [DOI] [PubMed] [Google Scholar]
- 21.Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solev IN, Balabanyan VY, Volchek IA, Elizarova OS, Litvinova SA, Garibova TL, Voronina TA. Involvement of BDNF and NGF in the mechanism of neuroprotective effect of human recombinant erythropoietin nanoforms. Bull Exp Biol Med. 2013;155:242–244. doi: 10.1007/s10517-013-2123-3. [DOI] [PubMed] [Google Scholar]
- 23.Song Q, Xu R, Zhang Q, Ma M, Zhao X. Therapeutic effect of transplanting bone mesenchymal stem cells on the hind limbs’ motor function of rats with acute spinal cord injury. Int J Clin Exp Med. 2014;7:262–267. [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 25.Thomson SR, Nahon JE, Mutsaers CA, Thomson D, Hamilton G, Parson SH, Gillingwater TH. Morphological characteristics of motor neurons do not determine their relative susceptibility to degeneration in a mouse model of severe spinal muscular atrophy. PLoS One. 2012;7:e52605. doi: 10.1371/journal.pone.0052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vawda R, Fehlings MG. Mesenchymal cells in the treatment of spinal cord injury: current & future perspectives. Curr Stem Cell Res Ther. 2013;8:25–38. doi: 10.2174/1574888x11308010005. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Li Y, Gao Y, Chen X, Yao J, Lin W, Chen Y, Liu J, Yang Y, Wang X. Combined use of spinal cord-mimicking partition type scaffold architecture and neurotrophin-3 for surgical repair of completely transected spinal cord in rats. J Biomater Sci Polym Ed. 2013;24:927–939. doi: 10.1080/09205063.2012.727267. [DOI] [PubMed] [Google Scholar]
- 28.Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009;5:959–968. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Duan H, Mo L, Qiao H, Li X. The effect of the dosage of NT-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2010;31:4846–4854. doi: 10.1016/j.biomaterials.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, He X. A meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PLoS One. 2014;9:e93487. doi: 10.1371/journal.pone.0093487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YJ, Zhang W, Lin CG, Ding Y, Huang SF, Wu JL, Li Y, Dong H, Zeng YS. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2012;313:64–74. doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]


