Abstract
The pharmacological actions of the glucagon-like peptide-1 receptor agonists (GLP-1RA) are largely predictable as they interact directly with GLP-1 receptors on beta cells to mediate their glucose lowering effects by increasing GLP-1 in pharmacological range and not at all dependent upon endogenous GLP-1 secretion. The mechanism of action of dipeptidyl peptidase-4 inhibitors (DPP-4I) are relatively less clear although classical mechanism is to inhibit the endogenous GLP-1 metabolism and thereby increasing GLP-1 level in the physiological range. DPP-4I also increase the half-life of GLP-1 to some extent by inhibiting their quick degradation by DPP enzyme ubiquitously present in the body. Interestingly, even with the effective blockade with currently existing DPP-4I, the half-life of GLP-1 only increases from 1 min to 5 min and therefore its residual time in plasma still remains pretty short. Intriguingly, this GLP-1 rise is so modest and so short-lived that it may be difficult to believe that this would sufficiently engage and activate the GLP-1 receptor in beta cell to produce significant insulinotropic effect. However, in clinical trials as well as in real life scenario, the anti-glycemic efficacies seen with DPP-4I are quite satisfactory and sometime very much competitive to GLP-1RA as evident from their head-to-head trials including meta-analysis. This efficacy outcome challenges the “only” GLP-1 dependent mechanism of glucose lowering and provokes an insight that other neuro-endocrine pathway may be playing a second fiddle. This review will collate those emerging concept and put a perspective as to how DPP-4I might be working though other pathway besides direct GLP-1 mediated receptor activation.
Keywords: Dipeptidyl peptidase-4 inhibitors, glucose-dependent intestinal polypeptide, glucagon-like peptide-1, glucagon-like peptide-1 agonist, incretin, type 2 diabetes
INTRODUCTION
It is increasingly clear now that the entero-insular axis plays a major role in glucose homeostasis. Glucose-dependent intestinal polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) together termed “incretins” account for approximately 70% of beta cell insulin secretion and both peptides are necessary for normal glucose tolerance.[1] GLP-1 is the incretin hormone arises from the post-translational processing of pro-glucagon in intestinal L cells and secreted in two major forms: GLP-1 (7-36) and GLP-1 (7-37 amide) often termed “active” GLP-1. The main biological action of GLP-1 depends on their two N-terminal amino acid which are primarily removed by an enzymes dipeptidyl peptidase-4 (DPP-4) into truncated “inactive” GLP-1 (9-36, 9-37 amide). GLP-1 is responsible for glucose-dependent insulin secretion, suppression of glucagon secretion and delayed gastric emptying. Interestingly, ubiquitous distribution of DPP-4 results in GLP-1 having half-life of approximately 1 min only in the circulation.[1,2]
Consequently, to exploit this gluco-metabolic effect of GLP-1 and to preserve and harness its characteristics two approaches were considered. The first approach included the development of GLP-1 receptor agonist (GLP-1RA) with closest possible homology to native GLP-1 structure, but resistant to DPP-4 and therefore capable of binding and stimulating GLP-1 receptor for a longer time. Another approach included the development of a molecule which can inhibit DPP-4 and thereby increases endogenous GLP-1 in circulation for a longer time.[2] Although, DPP-4 inhibitors (DPP-4I) prevents the degradation of active GLP-1, it does not significantly increase the levels of circulating total GLP-1 and does not prevent the kidney from rapidly clearing GLP-1.[3] An experimental animal studies demonstrated that although the levels of intact GLP-1 and GIP are preserved and increased following treatment with the DPP-4I (NVP-DPP728), the levels of total circulating incretins (degraded plus intact peptides) actually decreased for both GLP-1 and GIP. The mechanism underlying this observation remains unclear, but feedback inhibition on the K or L cell remains a possibility.[3] Another study by Dai et al. in healthy adults also demonstrated that although DPP-4I (PF-00734200) increases active GLP-1 concentration by 2-3-fold (over placebo), this does not occur in a linear fashion. The increase in GLP-1 was non-linear and not directly proportional to the glycemic efficacy. Moreover, even with near complete inhibition of DPP-4 for over 24 h with the highest possible dose of DPP-4I, the GLP-1 levels actually declined during the night compared with post-dinner levels.[4] Furthermore, with the use of currently available DPP-4I which typically exhibits effective (80–97%) DPP-4 inhibition, half-life of intact GLP-1 only increases from approximately 1 min to 5 min.[5,6]
It is yet unclear whether DPP-4 is the only enzyme that contributes to the degradation of GLP-1. Although, some studies have implicated a role for neutral endopeptidase (NEP) 24.11 in the endoproteolysis of GLP-1, currently, there is little evidence from the use of inhibitors or genetic studies to ascertain the relative importance of NEP 24.11 for GLP-1 biology in vivo.[7,8]
Taken together, it is now increasingly clear that the increase in circulating intact GLP-1 levels after DPP-4 inhibition seems to be modest or minimal as well as very short-lived. Consequently, it appears difficult to believe that this trivial GLP-1 rise would engages the GLP-1 receptors in beta cell accounting for effective glycemic control seen with the current uses of DPP-4I. Perhaps, this has raised serious doubt about a purely endocrine mechanism for DPP-4I ability to control blood glucose.[6]
This review will attempt to find out what could be a possible pathway apart from direct GLP-1 receptor-mediated mechanism by which DPP-4I may be lowering blood glucose.
AN INSIGHT ON ANTI-GLYCEMIC EFFICACY OF DIPEPTIDYL PEPTIDASE-4 INHIBITOR VERSUS GLUCAGON LIKE PEPTIDE-1 RECEPTOR AGONISTS THROUGH HEAD-TO-HEAD TRIALS
The pharmacological actions of the GLP-1RA are largely predictable as these agents are not susceptible to inactivation by DPP-4 and increase GLP-1 to near supra-physiological levels (50-60 pmol) in the plasma for several hours depending upon the pharmacokinetic and pharmcodynamic profile and duration of action of the GLP-1RA in question. Typically, longer acting agents would exhibit more sustained GLP-1 rise compared to shorter acting agents. Thus, it is quite clear that GLP-1RA interact directly with GLP-1 receptors on beta cells to mediate their anti-glycemic effects. However, the mechanisms of action of DPP-4I in relation to glycemic efficacy are relatively less clear as mentioned earlier.
Interestingly, head-to-head clinical trials comparing glycemic efficacy of DPP-4I with GLP-1RA revealed a thought provoking insights. Although, head-to-head trials of DPP-4I versus longer acting GLP-1RA suggested a clear superiority of longer acting GLP-1RA (once weekly) in all glycemic parameters, results with shorter acting GLP-1RA (exenatide twice daily and lixisenatide) are not very convincing when compared to DPP-4I [Table 1].[9,10,11,12,13,14,15,16,17,18] Notably, Glycosylated hemoglobin and fasting glucose did not differ significantly between DPP-4I versus shorter acting GLP-1RA.[9,10,11,12] Liraglutide (LIRA) was also superior to sitagliptin in one study, however another study yielded non-inferiority.[13,14] Moreover, a meta-analysis comparing GLP-1RA versus DPP-4I done from some of these trials also suggested a clear anti-glycemic superiority of longer acting GLP-1RA over DPP-4I, although no significant difference observed with shorter acting GLP-1RA.[19] It is worthwhile to mention that consistent weight loss seen with GLP-1RA and possibly some other extra-glycemic benefit including blood pressure reduction may be a clear advantage with existing GLP-1RA compared to DPP-4I.[19]
Table 1.
Head-to-head trials of GLP-1 RA versus DPP-4 inhibitors
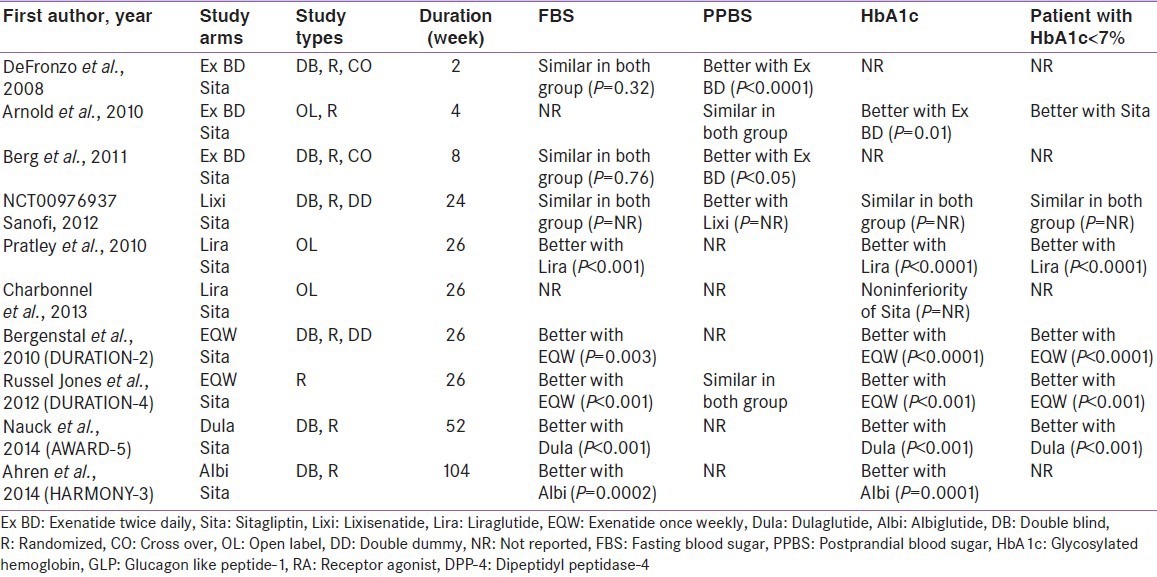
However, this near-competitive result from head-to-head studies represents a clinical conundrum and warrants a clear explanation as to why shorter acting GLP-1RA (in particular) is not substantially superior considering the enormous pharmacological GLP-1 rise with GLP-1 agonist compared to the trivial rise of GLP-1 with DPP-4I. Furthermore, this also excites to find out other possible neuro-endocrine mediated mechanism associated with DPP-4I.
Subcutaneous glucagon-like peptide-1 receptor agonist: Possible reason for less than expected response
When native GLP-1 is administered as a short-term intravenous (IV) infusion, a full normalization of glucose concentrations has been observed without any risk of gastro-intestinal (GI) side effects. In contrast, subcutaneous (SC) GLP-1RA reduces glucose to a clinically meaningful extent, however not completely able to bring it to normoglycemic range in spite of substantial and almost equal GLP-1 rise like native GLP-1. It is puzzling to know that studies with IV and SC GLP-1 agonist reported similar circulating steady-state plasma concentrations for both total and intact GLP-1, while the degree of normalization in glucose was significantly different. The reasons for this discrepancy are not yet fully clear, but following mechanistic explanation is currently being proposed.[20,21]
The short-lived peak in GLP-1 plasma concentration does not last longer than 60–90 min with SC route of administration[20,21]
Approximately 20% or even lower bioavailability of GLP-1 through SC administration[21]
Inability to use a larger dose of SC GLP-1RA because of associated GI side effects-nausea and vomiting are observed at much lower GLP-1 doses than are necessary to display the full glucose-lowering effect[21]
Possibility of GLP-1RA molecular modification, when it is exposed to the S.CSCsubcutaneous environment, cannot be ruled out[21]
Possible interaction between higher local concentrations of GLP-1 with GLP-1 receptors in adipose tissue.[21]
It is worthwhile to note that peptidergic branches of autonomic nervous system also innervates the adipose tissue and exposure of GLP-1 receptors on these nerve endings to higher local GLP-1 can potentially trigger nausea and vomiting. If this GI side effect occurs at doses that are not sufficient to elevate systemic GLP-1 or GLP1-RA into the truly therapeutic range, this might explain both the issues of GI side effects and inability to reach normoglycemia as seen with IV GLP-1.[21]
Dipeptidyl peptidase-4 inhibitors: Possible reason for more than expected response
Dipeptidyl peptidase-4 inhibitors (DPP-4I) augment circulating concentrations of intact, biologically active, endogenously secreted GLP-1, and it is widely believed that the effects of DPP-4I are largely mediated by the physiological rise of endogenous GLP-1, although inconsistencies on such mechanism exist in the literature as mentioned earlier. GIP is also a substrate of DPP-4, but its insulinotropic effect is reduced in patients with type 2 diabetes.
Initial studies in mice with disruption of single incretin receptor with either GLP-1R-/- or GIP-R-/- could not abolish the glucose reducing property of DPP-4I.[22] This glucose lowering ability of DPP-4I in the absence of GLP-1 receptors led to an initial impression of non-GLP-1 mediated pathway and possible mediation of other incretins or neuropeptides in lowering glucose with DPP-4I. This was also thought to happen because of up-regulatory response of other receptors if one is knocked out. Nevertheless, with the simultaneous disruption of both the receptors GLP-1R-/-/GIP-R-/- in double incretin receptor knockout (DIRKO) mice, the glucose reducing properties of DPP-4I almost abolished thereby suggesting a major role of GLP-1 and GIP receptors as dominant mediators for anti-diabetic effect of DPP-4I.[23] In fact, these observations seen in DIRKO mice suggested GLP-1 as the sole (or major) mediator of the therapeutic effect of DPP-4 inhibition.[24] Ironically, there are emerging arguments that oppose this view. An earlier review by Nauck and El-Ouaghlidi suggested that the therapeutic actions of DPP-4I are not mediated by GLP-1 and cited following thought provoking reasons.[25]
DPP-4I causes little increase in endogenous GLP-1
Meal-stimulated levels of GLP-1 fall in response to DPP-4 inhibition
DPP-4I have little effect on gastric emptying
DPP-4I have delayed effects on glucose homeostasis.
This hypothesis was one of the first arguments that have led many to question whether the effects of DPP-4I are mediated solely through preserving intestinally secreted intact GLP-1. Subsequently, few recent studies have demonstrated that alpha cells also express and secrete GLP-1 and GIP and these islet alpha cell secreted incretins exert their insulinotropic effect directly on adjacent beta cells. Interestingly, a recent study confirms that DPP-4I might exert some of their effects on insulin secretion by preserving intact GLP-1 and GIP secreted from alpha cells.[26]
Second, a considerable number of gluco-regulatory peptides in addition to GLP-1 and GIP have been identified as exogenous substrates susceptible to DPP-4 cleavage, of which pituitary adenylate cyclase activating peptide (PACAP), gastrin-releasing peptide (GRP) and possibly oxyntomodulin (OXM) seems to possess an important role in glucose metabolism and deserve special mention:
Animals lacking DPP-4 exhibited a significantly slower clearance of circulating PACAP, with virtually complete suppression of the DPP-4 metabolite, PACAP [3-38].[27] Study with exogenous infusion of PACAP38 and GRP evoked differential metabolic effects in wild versus DPP-4-/- mice and simultaneous DPP-4 inhibition potentiate their insulinotropic response thereby suggesting a plausible role of this substrate in reducing glucose during acute and chronic DPP-4 inhibition.[28] This exploratory experimental study, looked whether DPP-4 inhibition (by valine-pyrrolidide) affects the insulin and glucose responses to IV glucose together with IV GLP-1, GIP, PACAP38 or GRP and suggested that the acute (1-5 min) insulin response to GLP-1 was augmented by val-pyr by 80%, that to GIP by 40%, that to PACAP38 by 75% and that to GRP by 25% (all P < 0.05). This was also associated with enhanced glucose elimination rate after GLP-1 and PACAP38 (both P < 0.01), but not after GIP or GRP and interestingly, the augmented insulin response to GRP by val-pyr was prevented by the GLP-1R antagonist exendin (9-39), raising the possibility that GRP effects may occur secondary to stimulation of GLP-1 secretion. Hence, this study concluded that the DPP-4 inhibition augments the insulin response not only to GLP-1, but also to GIP, PACAP38, and GRP[28]
Oxyntomodulin is a gut peptide in the pre-pro-glucagon family, which appeared to have acute gluco-regulatory effects and weight loss in preclinical models, attributed in part to GLP-1 receptor activation in non-diabetic humans. Experiments using mass spectroscopy identified OXM and growth hormone [1-43] fragment as a new candidate in vivo DPP-4 substrates. A very recent study in type 2 diabetes for the first time suggested its acute gluco-regulatory role comparable to LIRA, which is independent of weight loss. This study (N = 12) hypothesized that OXM has glucoregulatory effects in type 2 diabetes independent of weight loss and compared acute changes in pancreatic beta cell function in response to a single dose of either OXM (continuous IV infusion at 3 pmol/kg/min) or LIRA (0.6 mg, SC) in a setting of a randomized, double-blind, placebo-controlled, three-period crossover trial. Study revealed that the effects of OXM and LIRA on blunting of glycemic excursion were comparable (P = NS). This finding demonstrate for the first time that OXM may have significant direct acute glucoregulatory effects in type 2 diabetes, independent of weight loss. Hence, it can be postulated that DPP4-I may influence glucose control through OXM metabolism being its substrate.[29]
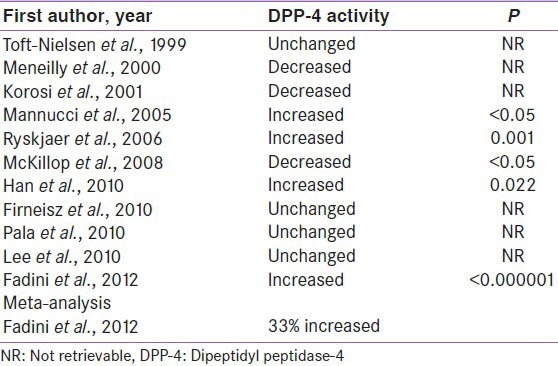
Third, enhanced DPP-4 activities in type 2 diabetes have been observed by many researchers; nevertheless these findings are discordant amongst individual studies.[30,31,32,33,34,35,36,37,38,39,40] Some studies suggested increased DPP-4 activity, some showed unchanged and some revealed decrease DPP-4 activity [Table 2]. However, a recent meta-analysis by Fadini et al. suggested a 33% enhanced DPP4 activity in type 2 diabetes.[40] Therefore, it can be assumed that the reduction of postprandial active GLP-1 in type 2 diabetes could possibly be attributable to either impairment in GLP-1 secretion or an increase of its degradation (because of enhanced DPP-4 activity), or both. If latter mechanism is substantiated through further studies as the dominant mechanism, than DPP-4I may play a further role in managing type 2 diabetes.
Table 2.
DPP-4 activity in type 2 diabetes
Finally, some recent animal studies suggested other possible mechanism by which DPP-4I might be lowering blood glucose independent of endogenous GLP-1. Following theories have been proposed pending further substantiation in human studies.[41,42,43,44,45,46]
Gut-to-brain-to-cell axis theory
Few experimental studies initially showed that a vagal hepato-pancreatic reflex is initiated by activation of the hepato-portal glucose sensor to control peripheral glucose utilization. This pathway required the simultaneous activation of brain GLP-1 signaling to trigger control of glucose-regulated insulin secretion, muscle blood flow and insulin sensitivity. These findings for the first time convincingly raises the possibility that the gluco-regulatory actions of DPP-4 may involve local regulation of the GLP-1-dependent gut-to-brain-to-periphery axis.[41,42] Thus, to test this hypothesis, Waget et al. conducted an experimental study and inhibited DPP-4 activity in the intestine using very low oral dose of sitagliptin and concluded that selective local reduction in intestinal DPP-4 activity is sufficient for activation of the neurally mediated gut-to-brain-to-periphery axis.[43] Subsequently, few other studies also demonstrated that DPP-4I prandial glycemic control in part could be mediated via inhibition of intestinal DPP-4, proximal to the site of GLP-1 secretion which could lead to activation of the neural gut-to-brain-to-cell axis through a mechanism that does not require direct actions of circulating GLP-1 on islet cells.[43,44,45]
Portal sensing theory or neural theory or gut-to-cell axis theory
The dominant mechanism through which DPP-4 inhibition controls glycaemia may involve enteric GLP-1 signaling as a component of the gut-to-cell axis. Since the portal vein carries highest GLP-1 concentration of any major vessel in the circulation and the same visceral afferents that serve the portal vein also innervate the intestine including the L-cells that produce GLP-1, it is likely that local neural mechanism plays a dominant role in glucose balance. As 50% of GLP-1 is inactivated by DPP-4 in the capillaries of the gut before it reaches the portal vein, thus the potential for intestinal nerves to mediate GLP-1 action is more plausible explanation for GLP-1 action of DPP-4I.[43,44,45]
Enteral theory or portal: Systemic glucagon-like peptide-1 gradient theory
Higher GLP-1 concentration in Portal:Systemic circulation with DPP-4 inhibition might be doing some trick for DPP-4I.[43,44,45]
Dia-peptide theory
New evidence suggest a potential biological role for bioactive dipeptides “his-ala” and “tyr-ala” which is a metabolic product after breakdown of GLP-1 and GIP respectively by DPP-4. Recent studies suggest worsening of glycemia with this diapeptide and it is likely that this dipeptide may also regulate glucose metabolism. Reduced liberation of these bioactive diapeptides with the use of DPP-4I could also contribute to the therapeutic effects of DPP-4 inhibition.[43,44,45]
Paracrine theory
The mechanism through which GIP acts locally in the gut on glucose homeostasis is unknown but might be related to its role on intestinal glucose absorption by reducing intestinal motility through a somatostatin mediated pathway.[43,44,45]
Direct glucagon like peptide-1 secretagogue
Possible direct effects on the intestinal L cell, unexpectedly revealing a novel action for sitagliptin as a DPP-4-independent GLP-1 secretagogue.[46]
Taken together, these arguments cast doubt on the assumption that GLP-1 is the only, or at least the major, mediator of the clinical effects seen with DPP-4 inhibition. It should be noted that these emerging mechanism of action with DPP-4I have been primarily observed with either experimental DPP-4I or Sitagliptin, nonetheless; it is highly likely to be a class effect. These emerging theories also need to be substantiated through many more human studies as well as with other existing DPP-4I before any conclusion can be made.
CONCLUSION
Although, SC GLP-1RA shows overall better glucose lowering than DPP-4I, it does not achieve near normoglyemia like native IV GLP-1. Unfortunately, SC GLP-1RA is associated with lower bioavailability and nagging gastrointestinal side effect at relatively lower dose, limiting its incremental dose-effect response. If these limitations with SC approach can be further confirmed in experimental studies; there could be possibilities of some improvement with available GLP-1RA. If a molecular modification occurs in adipose tissue lowering its bioavailability, peptides resistant to these modifications could be developed, or the enzymes responsible could be inhibited by appropriate agents. If SC nerves mediate adverse GI events, other routes of administration may be helpful and currently inhaled, and oral route of these agents are targeted.
The action of the DPP-4I appears to be only partially dependent on overall endogenous GLP-1, and other factors like pitutary adenylate cyclase activating peptide (PACAP), OXM, portal neural sensing, portal GLP-1 gradient, GIP, His-Ala dipeptide, alpha cell-mediated response and possibly some other yet unidentified substrates may also be involved in mediating the glucose lowering effects. Therefore, more effort should be put into elucidating the role of other pathway or other potential incretin hormones or neuropeptides to know the DPP-4I mechanism of action.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 3.Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. J Endocrinol. 2002;172:355–62. doi: 10.1677/joe.0.1720355. [DOI] [PubMed] [Google Scholar]
- 4.Dai H, Gustavson SM, Preston GM, Eskra JD, Calle R, Hirshberg B. Non-linear increase in GLP-1 levels in response to DPP-IV inhibition in healthy adult subjects. Diabetes Obes Metab. 2008;10:506–13. doi: 10.1111/j.1463-1326.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- 5.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7-36) NH2, GLP-1-(7-37), and GLP-1- (9-36) NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;88:1772–9. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 6.Bock G, Dalla Man C, Micheletto F, Basu R, Giesler PD, Laugen J, et al. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–96. doi: 10.1111/j.1365-2265.2009.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Göke R, Göke B, Thole H, et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1 (7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149–56. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- 8.Hupe-Sodmann K, Göke R, Göke B, Thole HH, Zimmermann B, Voigt K, et al. Endoproteolysis of glucagon-like peptide (GLP)-1 (7-36) amide by ectopeptidases in RINm5F cells. Peptides. 1997;18:625–32. doi: 10.1016/s0196-9781(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–52. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 10.Arnolds S, Dellweg S, Clair J, Dain MP, Nauck MA, Rave K, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: A proof-of-concept study. Diabetes Care. 2010;33:1509–15. doi: 10.2337/dc09-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: A randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13:982–9. doi: 10.1111/j.1463-1326.2011.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Last accessed on 2014 Jun 20]. Available from: http://www.en.sanofi.com/img/content/study/EFC10780_summary.pdf .
- 13.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: A 26.week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 14.Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: A randomised clinical trial. Diabetologia. 2013;56:1503–11. doi: 10.1007/s00125-013-2905-1. [DOI] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): A randomised trial. Lancet. 2010;376:431–9. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 16.Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): A 26-week double-blind study. Diabetes Care. 2012;35:252–8. doi: 10.2337/dc11-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5) Diabetes Care. 2014;37:2149–58. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–8. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- 19.Deacon CF, Mannucci E, Ahrén B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab. 2012;14:762–7. doi: 10.1111/j.1463-1326.2012.01603.x. [DOI] [PubMed] [Google Scholar]
- 20.Nauck MA, Wollschläger D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, et al. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–53. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 21.Nauck MA, Baranov O, Ritzel RA, Meier JJ. Do current incretin mimetics exploit the full therapeutic potential inherent in GLP-1 receptor stimulation? Diabetologia. 2013;56:1878–83. doi: 10.1007/s00125-013-2953-6. [DOI] [PubMed] [Google Scholar]
- 22.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–9. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–35. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 24.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: Preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–43. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 25.Nauck MA, El-Ouaghlidi A. The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia. 2005;48:608–11. doi: 10.1007/s00125-005-1704-8. [DOI] [PubMed] [Google Scholar]
- 26.Omar B, Ohlsson L, Yamada Y, Seino Y, Ahren B. Direct enhancement of insulin secretion by dipeptidyl peptidase 4 inhibitors in pancreatic islets: Studies in incretin receptor deficient mice. Diabetologia. 2013 Abstract 44, EASD Barcelona. [Google Scholar]
- 27.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38) J Biol Chem. 2003;278:22418–23. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 28.Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055–9. doi: 10.1210/en.2004-1174. [DOI] [PubMed] [Google Scholar]
- 29.Shankar SS, Shankar R, Mixson L, Pramanik B, Stoch S, Steinberg HO, et al. Oxyntomodulin has significant acute glucoregulatory effects comparable to liraglutide in subjects with type 2 diabetes. Diabetolgia. 2014 Abstract 48, EASD Barcelona. [Google Scholar]
- 30.Toft-Nielsen M, Damholt M, Hilsted J, Hughes TE, Krarup T, Madsbad S, et al. GLP-1 secretion is decreased in NIDDM patients compared to matched control subjects with normal glucose tolerance. Diabetologia. 1999;A40:143. [Google Scholar]
- 31.Meneilly GS, Demuth HU, McIntosh CH, Pederson RA. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med. 2000;17:346–50. doi: 10.1046/j.1464-5491.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 32.Korosi J, McIntosh CH, Pederson RA, Demuth HU, Habener JF, Gingerich R, et al. Effect of aging and diabetes on the enteroinsular axis. J Gerontol A Biol Sci Med Sci. 2001;56:M575–9. doi: 10.1093/gerona/56.9.m575. [DOI] [PubMed] [Google Scholar]
- 33.Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–72. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 34.Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst J, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155:485–93. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 35.McKillop AM, Duffy NA, Lindsay JR, O’Harte FP, Bell PM, Flatt PR. Decreased dipeptidyl peptidase-IV activity and glucagon-like peptide-1 (7-36) amide degradation in type 2 diabetic subjects. Diabetes Res Clin Pract. 2008;79:79–85. doi: 10.1016/j.diabres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Han SJ, Kim HJ, Choi SE, Kang Y, Lee KW, Kim DJ. Incretin secretion and serum DPP-IV activity in Korean patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:e49–52. doi: 10.1016/j.diabres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Firneisz G, Varga T, Lengyel G, Fehér J, Ghyczy D, Wichmann B, et al. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: A novel liver disease biomarker. PLoS One. 2010;5:e12226. doi: 10.1371/journal.pone.0012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pala L, Ciani S, Dicembrini I, Bardini G, Cresci B, Pezzatini A, et al. Relationship between GLP-1 levels and dipeptidyl peptidase-4 activity in different glucose tolerance conditions. Diabet Med. 2010;27:691–5. doi: 10.1111/j.1464-5491.2010.03010.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Yabe D, Nohtomi K, Takada M, Morita R, Seino Y, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;57:119–26. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 40.Fadini GP, Albiero M, Menegazzo L, de Kreutzenberg SV, Avogaro A. The increased dipeptidyl peptidase-4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin-treated patients. Diabetes Obes Metab. 2012;14:518–22. doi: 10.1111/j.1463-1326.2011.01550.x. [DOI] [PubMed] [Google Scholar]
- 41.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–63. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Pénicaud L, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577–87. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–29. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- 44.D’Alessio DA. What if gut hormones aren’t really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152:2925–6. doi: 10.1210/en.2011-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vella A. Mechanism of action of DPP-4 inhibitors: New insights. J Clin Endocrinol Metab. 2012;97:2626–8. doi: 10.1210/jc.2012-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sangle GV, Lauffer LM, Grieco A, Trivedi S, Iakoubov R, Brubaker PL. Novel biological action of the dipeptidylpeptidase-IV inhibitor, sitagliptin, as a glucagon-like peptide-1 secretagogue. Endocrinology. 2012;153:564–73. doi: 10.1210/en.2011-1732. [DOI] [PubMed] [Google Scholar]


