Abstract
Diabetic retinopathy (DR) is a complication of long-term diabetes mellitus (DM). Over the last 2 decades lot of work has been on early diagnosis of DR and screening programs have been designed to help the masses. Large numbers of clinical studies have been done for patients of diabetes and DR wherein the role of blood sugar control, metabolic control, role of oral medicines for DR, role of imaging, fluorescein angiography, and retinal photocoagulation has been studied. Newer treatment modalities are being devised and studied for better patient care. We discuss these issues in our review highlight and newer advances over the last few years.
Keywords: Avastin, bevacizumab, diabetic retinopathy, early treatment diabetic retinopathy study, fundus fluorescein angiography, intravitreal steroids, optical coherence tomography, ozurdex, photocoagulation, ranibizumab, retisert, triamcinolone acetonide, screening of diabetic retinopathy, VEGF- TRAP, vitrectomy
INTRODUCTION
Diabetic retinopathy (DR) is a well-known long term complication of diabetes mellitus (DM). DR is a significant cause of blindness and ocular morbidity in developed nations. In India, the number of diabetics is increasing and hence the number of DR patients is bound to increase over the next few decades.[1,2] In patients with DM, the prevalence of any form of DR is about 24%.[2]
The Early Treatment Diabetic Retinopathy Study (ETDRS) was a landmark study done between 1980 and 1985, which laid down the principles of classification of DR and provided guidelines for management by retinal laser treatment in cases of advanced disease, that is, proliferative DR (PDR).[3] In one of the arm of ETDRS designed to study the role of photocoagulation for diabetic macular edema, 3,711 patients were recruited. The results showed that use of focal laser decreased the risk of moderate visual loss (defined as loss of 15 letters of ETDRS chart) by 50%. In another arm, the risk of severe visual loss in severe or very severe nonproliferative DR (NPDR) or early PDR reduced by approximately 50% by panretinal photocoagulation. This formed the basis for further studies and future of DR treatment. The primary outcome in ETDRS was stabilization of vision and prevention of further loss. However, vision improvement was seen in only 3% of patients. Laser photocoagulation was the main intervention in ETDRS apart from systemic control of sugars and other metabolic parameters.[3,4]
ETDRS defined clinically significant macular edema (CSME) in DR which is the commonest cause of decrease in vision and provided for treatment parameters for the same using macular grid retinal laser. However since ETDRS, dramatic changes have occurred in the field of retinal imaging. With new digital high quality stereoscopic fundus camera and optical coherence tomogram, understanding of retinal diseases has increased and newer treatments are now available. In this review newer treatment options will be discussed.
PATHOGENESIS OF DR
The pathogenesis of early DR revolves around damage to the retinal capillary bed caused by persisting high blood glucose levels and high advance glycation end (AGE) products resulting in retinal ischemia. Retinal ischemia in turn leads to production of diffusible angiogenic factor which was earlier called as Factor X by Isaac in 1949.[5] In 1989, Ferrara and Henzel named it as vascular endothelial growth factor (VEGF). Studies have demonstrated the hypoxia could trigger VEGF expression which causes ocular neovascularization.[6]
MANAGEMENT OF DR
Control of systemic metabolic state
One of the aspects of treatment is the management of systemic parameters such as intensive control of blood glucose levels, blood pressure, tight lipid control and management of nephropathy. The United Kingdom Prospective Diabetes Study (UKPDS) showed that reducing glucose exposure (HbA1c 7.0% versus 7.9% over median 10.0 years), with sulfonylurea or insulin therapy, reduced the risk of “any diabetes-related endpoint” by 12% and microvascular disease by 25%, with a 16% trend to a reduced risk of myocardial infarction (P = 0.052). Also it showed that improving blood pressure (142/82 vs 154/87 mmHg over median 8.4 years) with an angiotensin converting enzyme (ACE) inhibitor or a beta blocker, reduced the risk of both microvascular and macrovascular disease.[7] The Diabetes Control and Complications Trial (DCCT) showed that intensive control of blood sugars over the course of diabetes reduces the risk of complication of DM such as retinopathy, nephropathy, and neuropathy by 60%. The benefit of intensive therapy resulted in a delay in the onset and a major slowing of the progression of the above complications.[8]
Management of retinopathy
Early detection and treatment of retinal disease is the second aspect of management of DR. Early diagnosis of DR is possible only with strict screening protocols. In most of the developed nations, all diabetics are routinely screened as a part of national programs. An easy method of screening is the standard retinal photographs taken by trained technicians, optometrists, or specialist.[9,10] These photographs can later be evaluated by specialist and further course of management can be planned.[11] If there is any evidence of DR on standard retinal photographs, the patients are referred to retina specialists. Tests such as fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) can be done as required. FFA is a procedure wherein, sodium fluorescein dye (10%) is injected intravenously through peripheral vein and retinal photographs are taken with help of camera system. The leakage of dye through blood ocular barrier or neovascular fronds is easily diagnosed. Peripheral capillary nonperfusion or ischemic maculopathy can also be diagnosed with the help of FFA.[12,13] OCT is another advancement over the last decade which provides a schematic histology like picture of layers of retina. It provides for thickness of retina to diagnose macular edema. Intraretinal cysts, vitreoretinal traction, epiretinal membranes, and other retinal macular pathologies of DR can be easily diagnosed.[14,15] Ocular treatment revolves around four major strategies: Retinal laser photocoagulation, anti-VEGF drugs, steroids, and surgical intervention.
RETINAL LASER PHOTOCOAGULATION
Until 1980 there was no effective treatment for DR. ETDRS trial was a landmark which introduced retinal photocoagulation as a treatment modality. The modified grid laser or focal laser was advocated by ETDRS for patients with CSME. The goal was to give mild laser burns in the macular area and help decrease macular edema.[3] For PDR, panretinal photocoagulation was advocated which decreased the hypoxia-induced neovascularization process.[16] However, loss of visual field was a major side effect. The laser treatment is permanent; however, visual gain is modest or low in most cases. With the advancement in bioengineering, now double frequency neodymium-doped yttrium aluminum garnet (Nd-YAG) laser is available which is compact and easy to use. Pattern laser systems are available which give multiple spots in predetermined pattern in fraction of seconds; thereby reducing the time required.[17] Also the risk of inadvertent burns is less. The size, degree, and spacing can be predefined and achieved with newer advance machines. Subthreshold laser and diode laser are also being studied and advocated by retina specialists to reduce collateral damage of healthy tissue and achieve good results.[18,19] Till date retinal laser photocoagulation is the primary and permanent treatment of DR.
VEGF ACTIONS AND INHIBITORS
Over the last decade, extensive research on VEGF molecule has improved our understanding of its prominent role in DR. VEGFs are a subfamily of growth factors that lead to vasculogenesis and angiogenesis. VEGF is primarily secreted from retinal pigmented epithelial cells, pericytes, astrocytes, Muller cells, glial cells, and endothelial cells. VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PGF). VEGF-A is 36-48 kDa glycosylated protein. It has five isoforms: 121, 145, 165, 189, and 206 depending on the number of amino acids present. Among these isoforms, VEGF-A165 isoform plays the most important role in DR. Tissue hypoxia leads to production of hypoxia inducible factor 1 (HIF-1) which upregulates production of VEGF. There are two receptors for VEGF: VEGFR1 and VEGFR2. VEGF receptor 2 is the primary receptor for cellular effects of VEGF-A. The actions of VEGF-A are mediated further through various pathways such as tyrosine kinase pathway, protein kinase C (PKC), and nitric oxide synthesis.[20] [Figure 1].
Figure 1.
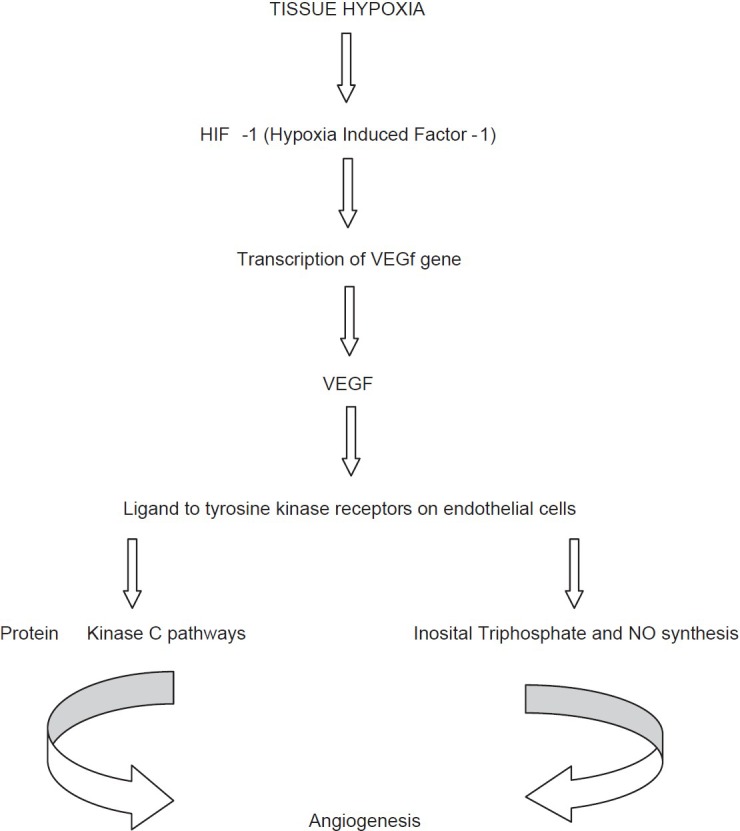
Retinal angiogenesis pathway
The flow chart below depicts the various steps in the synthesis and action of VEGF. All these steps are amenable to intervention. The production and actions of VEGF can be inhibited. If VEGF actions are inhibited angiogenesis can be prevented. Further complications of PDR such as vitreous hemorrhage and tractional retinal detachment can be prevented.[20,21,22]
VEGF actions also cause dilatation of blood vessels through release of NO and transudation, which accentuates macular edema. Inhibition of VEGF will inhibit transudation and decrease macular edema.[23]
Inhibition of VEGF at various steps
Gene transcription stage- A new small interfering RNA (siRNA) named bevasiranib is being developed to inhibit the genes that produce VEGF. In RNAi assessment of bevasiranib in diabetic macular edema (RACE) trial, a phase 2 pilot study, preliminary safety and efficacy was proved. It was a double masked multicentric randomized clinical trial which showed positive results in decreasing macular edema in patients with diabetes macular edema (DME).[24]
Inhibition of extracellular VEGF
Bevacizumab (Avastin, Genentech Inc, San Francisco, CA) is monoclonal antibody against all isoforms of VEGF-A and commonly used off-label drug given by intravitreal injection. It was originally approved for use in colon cancer. Good results with off-label use for ocular use of bevacizumab. Dose is 0.05 ml (1.25 mg) given intravitreally under aseptic precautions. The Diabetic Retinopathy Clinical Research (DRCR) phase 2 and bevacizumab or laser therapy (BOLT) study showed favorable results with use of bevacizumab along with laser therapy rather than laser therapy alone.[25,26,27]
Ranibizumab (Lucentis, Genetec Inc, San Francisco, CA) is a humanized monoclonal antibody fragment (fragment antigen antibody (Fab)) designed for ocular use. It is Food and Drug Administration (FDA)-approved for decreasing neovascularization due to age-related macular degeneration. It is found to be effective for decreasing macular edema and PDR. Dose is 0.05 ml (0.5 mg) given intravitreally under aseptic precautions. In three well-designed trials – RESOLVE, RIDE and RISE, and READ-2 – ranibizumab was found to be more effective than sham injections and/or focal/grid laser in improving visual acuity and reduce macular thickness. Newer trial like RESTORE and DRCT.net showed that 1-year treatment of ranibizumab along with laser therapy was more effective that laser therapy alone.[28,29,30,31]
Pegaptanib sodium (Macugen, Eyetech Pharmaceuticals Inc, New York, NY and Pfizer Inc, New York, NY) is a RNA aptamer and was approved for neovascular age related macular degeneration (AMD). The phase 2 randomized control trial done by Macugen Diabetic Retinopathy Study Group favored the use of Macugen with laser photocoagulation for better results.[32,33]
Inhibitor of VEGF receptor
Aflibercept (VEGF-Trap, Regeneron Pharmaceuticals Inc, Tarrytown, NY and Sanofi Aventis Inc, Paris, France) is human recombinant protein that behaves like a decoy receptor and binds with all isoforms of VEGF-A and neutralizes their effects. The DA VINCI study showed favorable results. The visual improvement was accompanied with decrease in retinal thickness on OCT. Phase 3 trials of VEGF-Trap are underway.[34]
Inhibition of intracellular signaling pathways
Midostaurin (Fermntek Biotechnology, Israel) is an inhibitor of multiple isoforms of PKC. It did show some positive result in animal models, however was found to have significant adverse effects in humans.[35,36]
Ruboxistaurin (Eli Lilly and Co., Delaware, Indiapolis, USA) is an orally available PKC-beta isoform selective inhibitor. It is being used for NPDR. Two studies done are PKC-Diabetic Retinopathy Study (PKC-DRS) and PKC-DME study (PKC-DMES). Both the studies have showed encouraging results.[37,38]
STEROIDS
Corticosteroids were the first intravitreal treatment for DME. Corticosteroids reduce vascular permeability. Their exact mode of action is not well-understood; however, steroids reduce production of arachidonic acid derivatives, prostaglandins, intercellular adhesion molecule (ICAM), tumor necrosis factor (TNF)-alpha, and VEGF. Triamcinolone acetonide is the most widely used steroids used for DME. Intravitreal triamcinolone is widely used against DME for a number of years. The effect lasts for 2-6 months and requires retreatment with major side effects being cataract and glaucoma. Doses 1, 2, or 4 mg given intravitreally under aseptic precautions.[39,40,41,42]
Due to high efficacy of steroids, sustain release implants of steroids have been designed for intraocular use. Retisert is intravitreal implant of fluocinolone acetonide (0.59 mg) which is surgically implanted. It is FDA-approved for use in chronic noninfectious uveitis. However in phase 2 clinical trials, 33% patients required glaucoma surgery and 90% required cataract surgery.[43,44,45]
Ozurdex is biodegradable injectible implant to be placed intravitreally which releases dexamethasone. The results are encouraging and it is studied for future use.[46,47,48]
Intravitreal or subtenon steroids are good, efficacious, and are widely used. However, continuous monitoring for rise is intraocular pressure is necessary. They should be used with caution and under explained risks.
SURGICAL INTERVENTION
Vitreous humor has been found to have all the angiogenic factor that lead to DR. Elevated inflammatory markers, VEGF, and AGE are known to be present in vitreous. In addition; vitreous traction, thick posterior hyloid, and epiretinal membranes are known to produce retinal traction and possible macular edema in some cases. In selected cases, pars plana vitrectomy may be required to treat DME. For patients with vitreous hemorrhage and retinal detachment, vitreoretinal surgery is required. Newer adjuvancts such as intravitreal injection of autologous plasmin enzyme (APE) are being developed for chemical vitreolysis which help in resolution of vitreous hemorrhage and may find place in standard care in years to come.[49] Advancement in machines, better instrumentation and visualization along with adjuncts such as perfluorocarbon liquids (PFCL), vitreoretinal surgery is much safer today with good results in expert hands. Sutureless vitrectomy using 23 or 25 gauge is being advocated for minimum intervention and optimum results.[50,51,52,53]
Newer drugs
Certain newer drugs have dual role and are now known to improve the retinopathy. Fenofibrate, a common drug used for dyslipidemia has been found to be useful in DR in the FIELD study. Patients treated with fenofibrate required less photocoagulation in PDR by 30% and DME by 31%.[54] Fenofibrate reduces the progression of retinopathy by 22% in all patients and 79% in patients with preexisting retinopathy. This result was unrelated to serum lipid levels, which were statistically similar in both, the group treated with fenofibrate and the control group. Retinal endothelial cells have peroxisome proliferator-activated receptor (PPAR)-α receptors and fenofibrate is known to be a PPAR-α agonist. Various mechanisms proposed are activation of PPAR-α receptors of retinal endothelial cells, which in turn inhibits VEGF. It also induces expression and activation of antioxidant enzymes, such as superoxide dismutase, glutathione, and induces apoptosis of human monocyte-derived macrophages, providing neuroprotective effects.[54,55]
To summarize, we can conclude that prevention and treatment of DR requires long-term approach. Proper control of blood sugar levels, blood pressure, lipids, and renal parameters form a major part of treatment of DR which should not be ignored. Ocular intervention requires a combined approach of intravitreal injections, retinal laser photocoagulation and vitreoretinal surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Heriot WJ, Borger JP, Zimmet P, King H, Taylor R, Raper LR. Diabetic retinopathy in a natural population. Aust J Ophthalmol. 1983;11:175–9. [PubMed] [Google Scholar]
- 3.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 4.Patz A, Smith RE. The ETDRS and Diabetes 2000. Ophthalmology. 1991;98:739–40. doi: 10.1016/s0161-6420(13)38007-5. [DOI] [PubMed] [Google Scholar]
- 5.Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc UK. 1948;68:137–80. [Google Scholar]
- 6.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–8. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 7.King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643–8. doi: 10.1046/j.1365-2125.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Hautala N, Aikkila R, Korpelainen J, Keskitalo A, Kurikka A, Falck A, et al. Marked reductions in visual impairment due to diabetic retinopathy achieved by efficient screening and timely treatment. Acta Ophthalmol. 2014;92:582–7. doi: 10.1111/aos.12278. [DOI] [PubMed] [Google Scholar]
- 10.Bhargava M, Cheung CY, Sabanayagam C, Kawasaki R, Harper CA, Lamoureux EL, et al. Accuracy of diabetic retinopathy screening by trained non-physician graders using non-mydriatic fundus camera. Singapore Med J. 2012;53:715–9. [PubMed] [Google Scholar]
- 11.Aptel F, Denis P, Rouberol F, Thivolet C. Screening of diabetic retinopathy: Effect of field number and mydriasis on sensitivity and specificity of digital fundus photography. Diabetes Metab. 2008;34:290–3. doi: 10.1016/j.diabet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Color photography vs fluorescein angiography in the detection of diabetic retinopathy in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Arch Ophthalmol. 1987;105:1344–51. doi: 10.1001/archopht.1987.01060100046022. [DOI] [PubMed] [Google Scholar]
- 13.Ivanisević; M, Stanić; R. Importance of fluorescein angiography in the early detection and therapy of diabetic retinopathy. Ophthalmologica. 1990;201:9–13. doi: 10.1159/000310117. [DOI] [PubMed] [Google Scholar]
- 14.Koleva-Georgieva DN, Sivkova NP. Optical coherence tomography for the detection of early macular edema in diabetic patients with retinopathy. Folia Med (Plovdiv) 2010;52:40–8. [PubMed] [Google Scholar]
- 15.Cavallerano JD, Aiello LP, Cavallerano AA, Katalinic P, Hock K, Kirby R, et al. Joslin Vision Network Clinical Team. Nonmydriatic digital imaging alternative for annual retinal examination in persons with previously documented no or mild diabetic retinopathy. Am J Ophthalmol. 2005;140:667–73. doi: 10.1016/j.ajo.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 16.Fong DS, Ferris FL, 3rd, Davis MD, Chew EY. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no.24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1999;127:137–41. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- 17.Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91:251–8. doi: 10.1111/j.1755-3768.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- 18.Venkatesh P, Ramanjulu R, Azad R, Vohra R, Garg S. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: A prospective, randomized study using multifocal electroretinography. Photomed Laser Surg. 2011;29:727–33. doi: 10.1089/pho.2010.2830. [DOI] [PubMed] [Google Scholar]
- 19.Sivaprasad S, Dorin G. Subthreshold diode laser micropulse photocoagulation for the treatment of diabetic macular edema. Expert Rev Med Devices. 2012;9:189–97. doi: 10.1586/erd.12.1. [DOI] [PubMed] [Google Scholar]
- 20.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–71. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 22.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 23.Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151–69. doi: 10.1177/2042018813512360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Last accessed on 2012 May 15]. Available from: http://www.retinalphysician.com/articleviewer.aspx?articleid=1018; 98 .
- 25.Stefanini FR, Arevalo JF, Maia M. Bevacizumab for the management of diabetic macular edema. World J Diabetes. 2013;4:19–26. doi: 10.4239/wjd.v4.i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ababneh OH, Yousef YA, Gharaibeh AM, Abu Ameerh MA, Abu-Yaghi NE, Al Bdour MD. Intravitreal bevacizumab in the treatment of diabetic ocular neovascularization. Retina. 2013;33:748–55. doi: 10.1097/IAE.0b013e3182721153. [DOI] [PubMed] [Google Scholar]
- 27.Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012;12:CD007419. doi: 10.1002/14651858.CD007419.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Stewart MW. Critical appraisal of ranibizumab in the treatment of diabetic macular edema. Clin Ophthalmol. 2013;7:1257–67. doi: 10.2147/OPTH.S36443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, et al. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. Am J Ophthalmol. 2013;156:502–10e2. doi: 10.1016/j.ajo.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Evoy KE, Abel SR. Ranibizumab: The first vascular endothelial growth factor inhibitor approved for the treatment of diabetic macular edema. Ann Pharmacother. 2013;47:811–8. doi: 10.1345/aph.1S013. [DOI] [PubMed] [Google Scholar]
- 32.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 33.Pacella E, La Torre G, Impallara D, Malarska K, Turchetti P, Brillante C, et al. Efficacy and safety of the intravitreal treatment of diabetic macular edema with pegaptanib: A 12-month follow-up. Clin Ter. 2013;164:e121–6. doi: 10.7417/CT.2013.1543. [DOI] [PubMed] [Google Scholar]
- 34.Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, et al. da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–65. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Ryan GJ. New pharmacologic approaches to treating diabetic retinopathy. Am J Health Syst Pharm. 2007;64:S15–21. doi: 10.2146/ajhp070332. [DOI] [PubMed] [Google Scholar]
- 36.Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, et al. PKC412--a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15:17–28. [PubMed] [Google Scholar]
- 37.Njie-Mbye YF, Kulkarni-Chitnis M, Opere CA, Barrett A, Ohia SE. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front Physiol. 2013;4:366. doi: 10.3389/fphys.2013.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamsi HN, Masaud JS, Ghazi NG. Diabetic macular edema: New promising therapies. World J Diabetes. 2013;4:324–38. doi: 10.4239/wjd.v4.i6.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Ma J, Meng N, Li H, Qu Y. Comparison of intravitreal triamcinolone acetonide with intravitreal bevacizumab for treatment of diabetic macular edema: A meta-analysis. Curr Eye Res. 2013;38:578–87. doi: 10.3109/02713683.2013.767351. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Wu X, Geng J, Yuan Z, Chen L. IVTA as adjunctive treatment to PRP and MPC for PDR and macular edema: A meta-analysis. PLoS One. 2012;7:e44683. doi: 10.1371/journal.pone.0044683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi HP, Bi S, Wei SQ, Cui H, Zhao JB. Intravitreal versus subtenon triamcinolone acetonide injection for diabetic macular edema: A systematic review and meta-analysis. Curr Eye Res. 2012;37:1136–47. doi: 10.3109/02713683.2012.705412. [DOI] [PubMed] [Google Scholar]
- 42.Sheth S, Rush R, Natarajan S, Gillies M. Intravitreal triamcinolone acetonide versus combined intravitreal bevacizumab and dexamethasone in diffuse diabetic macular oedema. Clin Experiment Ophthalmol. 2011;39:673–81. doi: 10.1111/j.1442-9071.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 43.Messenger WB, Beardsley RM, Flaxel CJ. Fluocinolone acetonide intravitreal implant for the treatment of diabetic macular edema. Drug Des Dev Ther. 2013;7:425–34. doi: 10.2147/DDDT.S44427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campochiaro PA, Nguyen QD, Hafiz G, Bloom S, Brown DM, Busquets M, et al. sFAMOUS Study Group. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120:583–7. doi: 10.1016/j.ophtha.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz SG, Flynn HW., Jr Fluocinolone acetonide implantable device for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:347–51. doi: 10.2174/138920111794480651. [DOI] [PubMed] [Google Scholar]
- 46.Herrero-Vanrell R, Cardillo JA, Kuppermann BD. Clinical applications of the sustained-release dexamethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–46. doi: 10.2147/OPTH.S15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zucchiatti I, Lattanzio R, Querques G, Querques L, Del Turco C, Cascavilla ML, et al. Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012;228:117–22. doi: 10.1159/000336225. [DOI] [PubMed] [Google Scholar]
- 48.Pacella E, Vestri AR, Muscella R, Carbotti MR, Castellucci M, Coi L, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex ®) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;7:1423–8. doi: 10.2147/OPTH.S48364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Llopis M, Udaondo P, Millán JM, Arevalo JF. Enzymatic vitrectomy for diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:319–23. doi: 10.4239/wjd.v4.i6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JY, Jeong HS, Lee DY, Sohn HJ, Nam DH. Early postoperative intraocular pressure stability after combined 23-gauge sutureless vitrectomy and cataract surgery in patients with proliferative diabetic retinopathy. Retina. 2012;32:1767–74. doi: 10.1097/IAE.0b013e3182475ad6. [DOI] [PubMed] [Google Scholar]
- 51.Park DH, Shin JP, Kim SY. Comparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Retina. 2010;30:1662–70. doi: 10.1097/IAE.0b013e3181d95261. [DOI] [PubMed] [Google Scholar]
- 52.Sato T, Emi K, Bando H, Ikeda T. Faster recovery after 25-gauge microincision vitrectomy surgery than after 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Clin Ophthalmol. 2012;6:1925–30. doi: 10.2147/OPTH.S37864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenberger SD, Miller DM, Riemann CD, Foster RE, Sisk RA, Hutchins RK, et al. Outcomes of 25-gauge pars plana vitrectomy in the surgical management of proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging. 2011;42:474–80. doi: 10.3928/15428877-20110901-02. [DOI] [PubMed] [Google Scholar]
- 54.Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomized controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 55.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomized controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]


