Abstract
Objective:
To assess bone mineral density (BMD) in type 2 diabetes mellitus (T2DM) patients and its relation, if any, to clinical, hormonal and metabolic factors.
Materials and Methods:
A prospective evaluation of 194 T2DM patients (97 men and 97 women) was carried out. BMD was done with dual energy X-ray absorptiometry (DXA) at the lumbar spine and total hip. Physical activity, nutritional intake and sunlight exposure were calculated. Biochemical and hormonal tests included serum 25 hydroxy vitamin D [25(OH) D], parathyroid hormone, estrogen, testosterone and urinary calcium-creatinine ratio. Glycosylated hemoglobin and complete lipid profiles were done in patients with diabetes. Five hundred and seventy one non-diabetic controls (262 males and 309 females) were evaluated for BMD alone.
Results:
BMD was normal (Z score > -2) in 156 (80.5%) and low (Z score ≤ -2) in 38 (19.5%) patients in the diabetes study group. BMD in the diabetes group was significantly higher than the control group in both sexes at the hip and spine. The difference was no longer significant on analysis of a BMI matched control subgroup. Weight and BMI showed significant correlation to BMD. Duration of T2DM, degree of glycemic control, use of drugs like statins and thiazolidinediones, 25(OH) D levels, calcium intake, sunlight exposure and physical activity did not significantly affect BMD in this cohort of individuals with diabetes.
Conclusions:
Bone mineral density of Asian Indian T2DM subjects was similar to that of healthy volunteers in this study.
Keywords: Asian Indian, bone mineral density, obesity, type 2 diabetes mellitus, vitamin D deficiency
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is rising globally, especially in Asian Indians. Decreased bone mineral density (BMD) is an established complication of type 1 diabetes mellitus and has been attributed to early age at diagnosis leading to decreased bone accrual, long duration, prolonged poor glycemic control and high insulin doses. However, contradictory results have been reported regarding BMD of T2DM patients. Several studies have shown that BMD at the lumbar spine and femoral neck were either similar,[1,2] increased[3,4,5] or rarely decreased in comparison with healthy controls.[6,7,8]
The exact pathophysiology behind the BMD changes in T2DM has not been well elucidated. Bone mineral density in diabetics is influenced by factors like calcium balance,[9] osteoblast function,[10] advanced glycation end products in bone collagen, inflammatory cytokines like leptin,[11] peroxisome proliferator-activated receptors (PPAR) isoform status and vitamin D status. High BMD in most of the recent studies with T2DM is secondary to lower bone volume[12,13] and functional hypoparathyroidism.[14]
There are no studies regarding BMD status in T2DM without known complications in the Asian Indian population or of the various factors affecting it: Viz sunlight, vitamin D status and lifestyle. Hence, we studied the BMD profile and its relation if any, to clinical, hormonal and metabolic factors in Asian Indian T2DM patients.
MATERIALS AND METHODS
It is a cross sectional prospective observational study comparing the BMD of T2DM patients with that of sex matched healthy volunteers who attained peak bone mass (PBM). Diagnosis of T2DM was based on American Diabetic Association (ADA) criteria, 2008.[15] Subjects with T2DM were recruited from outpatient Endocrinology department of tertiary care hospital in India. Sex matched healthy control subjects who had crossed the age for PBM were recruited from community based health camps. The study was approved by the institutional ethics committee and a written informed consent was obtained from all participants. Two hundred and fourteen T2DM patients were screened for the study of which 21 refused to participate in the study. One hundred and ninety four (97 men and 97 women) T2DM patients and 571 healthy volunteers (262 men and 309 women) aged between 30 to 50 years formed the study participants. Postmenopausal status, smoking (>1 year), chronic alcoholism, serum creatinine >1.4 mg/dl, pre-existing hypogonadism, hypopituitarism, thyrotoxicosis, past or present drug intake that may alter bone metabolism (e.g., vitamin D supplements, steroids, bisphosphonates, cytotoxics, anticonvulsants, thyroxine) and evidence of diabetic end organ complications constituted the exclusion criteria.
Diabetes and other medical conditions were ruled out with appropriate investigations in controls and their baseline demographic data with BMD were used for this study. All patients and controls gave written informed consent. The diabetic study group underwent biochemical and hormonal evaluation along with BMD and study related procedures.
A detailed clinical evaluation of the diabetes patients with respect to its treatment (sulfonylureas, metformin, thiazolidinediones and statins) and presence of complications was carried out. Subjects with body mass index (BMI) >25 kg/m2 were considered obese.[16] Sunlight exposure (to exposed areas especially face and hands) in terms of duration of outdoor activity, weekly schedule and outdoor attire was documented. The “rule of nine” was adapted to estimate the fraction of body surface area exposed to sunlight by each subject's usual outdoor attire.[17] Physical activity was evaluated by global physical activity questionnaire developed by world health organization (WHO). Nutritional intake was calculated using a questionnaire with specific reference to dietary intake of energy, protein, calcium and phosphorous.[18]
Biochemical investigations included serum calcium, phosphorus, alkaline phosphatase (Normal range 30-150 IU/l), albumin, creatinine, glycosylated hemoglobin (HbA1c), fasting lipid profile and urine calcium creatinine ratio (Ur.Cr/Ca). Hormonal investigations included parathyroid hormone (PTH) (Intra assay coefficient of variation (CV): 5.5-6.3%, inter assay coefficient of variation (CE): 7.9-8.6% and analytical sensitivity (S): 3 pg/ml), serum 25-hydroxy vitamin D (25(OH) D) (CV: 8.2-11%, CE: 9.6-12.5%, S: 1.5 ng/ml), Follicular stimulating hormone (FSH) (CV: 2.3-3.7%, CE: 5.4-6.7%, S: 0.1 IU/L), leutinising hormone (LH) (CV: 4.8-6.5%, CE: 7.2-26%, S: 0.1 IU/L), estradiol (E2) (CV: 6.3-15%, CE: 6.4-16%, S: 15 pg/ml), testosterone (T) (CV: 4.9-17%, CE: 6.0-12% and S: 15 ng/dl), sex hormone-binding globulin (SHBG) (CV: 4.1-7.7%, CE: 5.8-13% and S: 0.2 nmol/L), total tri-iodothyronine (T3) (CV: 5.4-13.2%, CE: 7.7-15.6% and S: 35 ng/dl), total tetra-iodothyronine (T4) (CV: 6.3-8.4%, CE: 6.7-9.8% and S: 0.4 μg/dl), thyroid stimulating hormone (TSH) (CV: 3.9-13.8%, CE: 8-17.5% and S: 0.004 μIU/ml) and urine free deoxypyridinoline (Ur.DPD) (CV: 8-15%, CE: 8-20% and S: 6 nM).
We estimated calculated free testosterone (cFT) from total testosterone and SHBG, using the method of Vermeulen et al., by a computer program (Free and Bioavailable Testosterone calculator, developed at the Hormonology Department, University Hospital, Ghent, Belgium and available at http://www.issam.ch/freetesto.htm).[19] All hormonal investigations except 25(OH) D were done by chemiluminescent immunometric assay with Immulite 1000 system of Diagnostic Products Corporation, Los Angeles, USA. Serum level of 25(OH) D was assayed with 125I RIA using DiaSorin kit. Serum 25(OH) D levels of > 30 ng/ml, 20-30 ng/ml and < 20 ng/ml were taken as suggestive of vitamin D sufficiency, insufficiency and deficiency respectively.[20]
BMD measurement
Assessment of BMD was performed with the dual energy X-ray absorptiometry (DXA) (Hologic inc. USA model Delphi W 70460). Measurements included DXA of the total hip, left femoral neck, intertrochanter, Ward's area and lumbar spine (posterior anterior projection). All the above sites were used to divide the diabetes study group into groups I [normal BMD (z score > -2)] and II [low BMD (z score < -2)], but only the total hip and lumbar spine were used to compare BMD in the study group and the controls. BMI matched controls were selected with BMI criteria of 22-30 kg/m2 in men and 24-30 kg/m2 in women. The short term in vivo precision (CV%) of DXA unit were as follows: Lumbar spine-1.09%, femoral neck-3.29% and total hip-1.26%, intertrochanter-3.09%, wards area-2.04%. BMD was reported in terms of g/cm2 and also in z-scores. Z-scores were based on the Caucasian normative database in the absence of normative data in Asian Indians.
Statistical analysis
Statistical analysis was done using SPSS Version 16 software. All results are expressed as mean ± standard deviation of the mean (SD) and median. The statistical significance between means was calculated by Student's t-test, analysis of variance or Mann-Whitney U test when appropriate. Differences between proportions were assessed by the Chi-square test. Multivariate linear regression analysis was used to determine independent associates of BMD in the diabetes group. A value of P < 0.05 was considered significant.
RESULTS
The diabetes study group consisted of 97 M and 97 F each, respectively (age range: 30-50 years) with T2DM without micro or macrovascular complications. Table 1 shows the baseline characteristics of the study group. Ninety eight (58: Fand 40: M) patients (51%) were obese.
Table 1.
The general characteristics of the study group
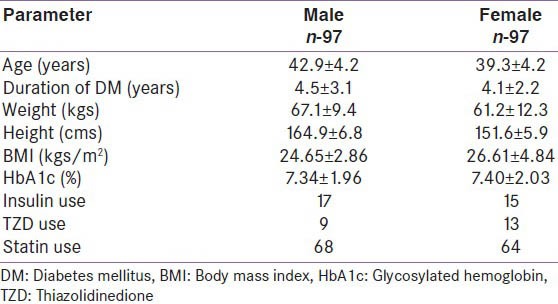
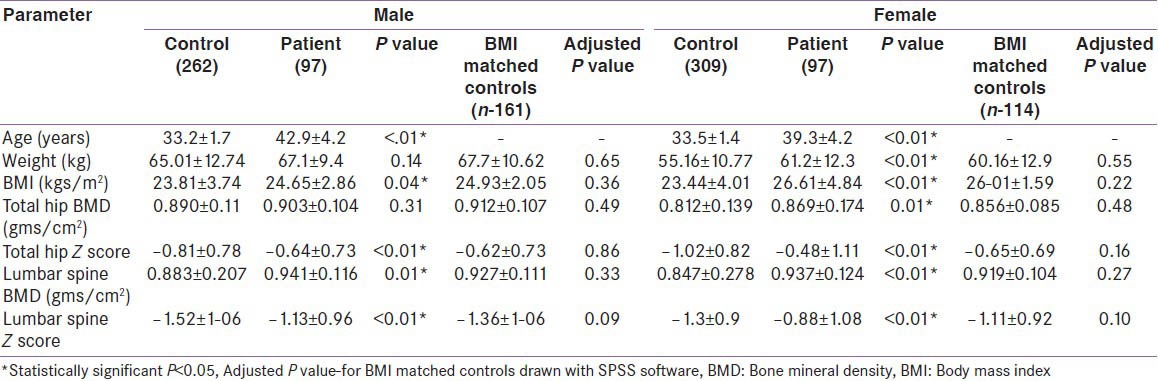
In the diabetes study group, 156 (76: Mand 80: F) had normal BMD, i.e., group I. BMD greater than the mean of the age and sex matched Caucasian controls (z score > 0) was seen in 75 of 156 with normal BMD (48%). Low BMD was present in 38 (21 M and17 F), i.e., group II. The control group was significantly younger and had a lower BMI than the study group. A comparison of baseline characteristics in T2DM showed a significant increase in weight and BMI in group I (M and F) [Table 2]. The mean duration of T2DM (41.1 vs 41.3 years) was not significantly different in groups I and II, respectively. Glycemic control was similar in both groups I and II (mean HbA1c: 7.26 vs 7.44). BMD in T2DM patients was significantly higher than that of the control group in all regions of interest except in total hip in men [Table 3]. In a subgroup of controls with matched BMI (M-161; F-114), the difference in BMD and z-scores between the diabetes group and the controls was no longer significant at both lumbar spine and total hip.
Table 2.
Comparison of different baseline characteristics in T2DM patients based on BMD
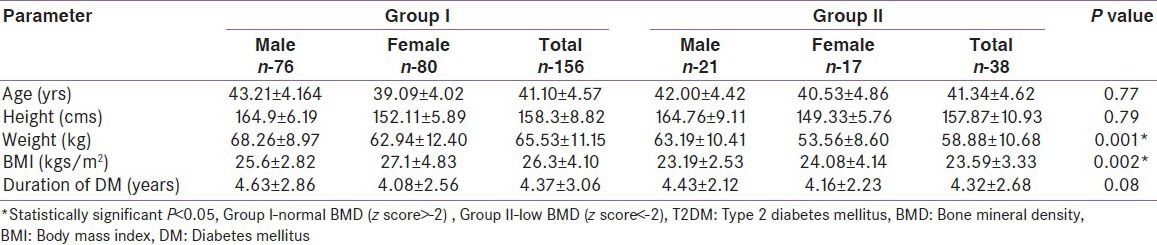
Table 3.
Comparison of BMD of control and diabetes study cohorts
Patients on statin (Atorvastatin 10-40 mg, mean of 12.7 mg) treatment for a minimum of 6 m were compared in group I (n-109) and group II (n-23). There was no significant difference in the use of statins between them in our study. Patients exposed to thiazolidinedione (Rosiglitazone (n-6,2-8 mg, mean: 4.7 mg); Pioglitazone (n-16,15-45 mg, mean: 28.8 mg)) were evaluated for any correlation with BMD. There was no significant difference in use of thiazolidinediones between groups I and II (M and F). This could be due to the small subgroup size (only 22 patients were on thiazolidinediones).
Dietary parameters
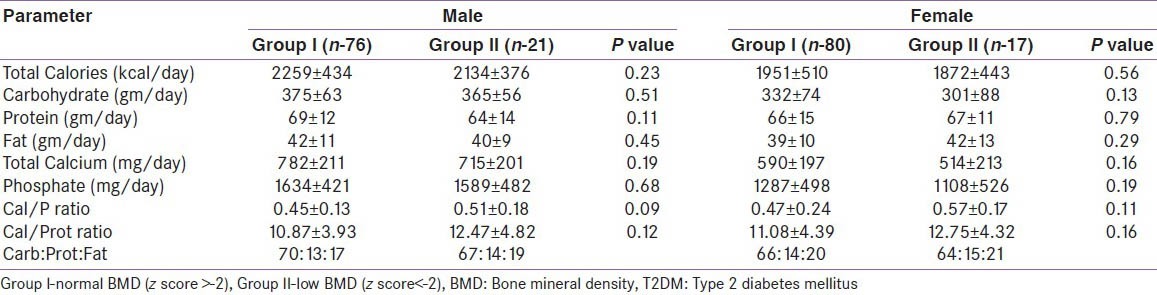
Diabetes study subjects were on dietary advice as per ADA guidelines.[15] The mean calorie intake was similar in groups I and II (M and F). In group I the calcium intake was 782 mg/d and 590 mg/d in males and females respectively, while in group II it was 715 mg/d and 514 mg/d, respectively [Table 4]. The calcium/phosphorus ratio was 0.45 and 0.47 in group I males and females respectively, while in group II it was 0.53 and 0.57 respectively. Calcium/protein ratio (mg/g) was also low (<13) in groups I and II (M and F).
Table 4.
Comparison of dietary parameters in T2DM patients based on BMD
Biochemical parameters
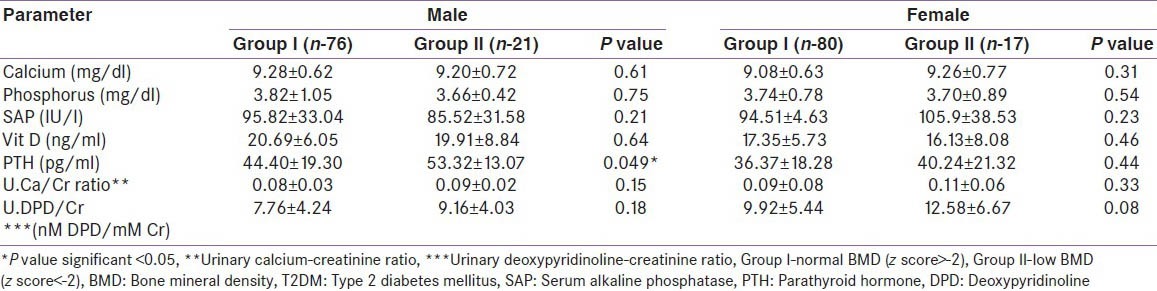
Parathyroid hormone was normal in group I (M and F) but elevated in male patients in group II [Table 5]. A similar observation was not found in female patients in group II. Serum calcium, phosphorus, alkaline phosphatase, U.Ca/Cr and U.DPD/Cr ratios were not significantly different among groups I and II (M and F). There was also no correlation in levels of E2 and cFT with BMD (M and F).
Table 5.
Comparison of biochemical parameters in patients with T2DM based on BMD
Vitamin D status
There was no significant statistical difference in 25(OH) D levels between groups I and II (M and F) [Table 6]. In the diabetes study group as a whole, 8.2%, 42.3% and 49.5% of men had normal, insufficient and deficient 25(OH) D levels respectively. Similarly, 2.1%, 34% and 63.9% of women had normal, insufficient and deficient 25(OH) D levels respectively [Figure 1]. The mean 25(OH) D level of our diabetes subjects was 20.3 ng/ml (M) and 17.1 ng/ml (F). There was no correlation with the 25(OH) D levels and BMD in our cohort.
Table 6.
Distribution of vitamin D status In T2DM patients based on BMD
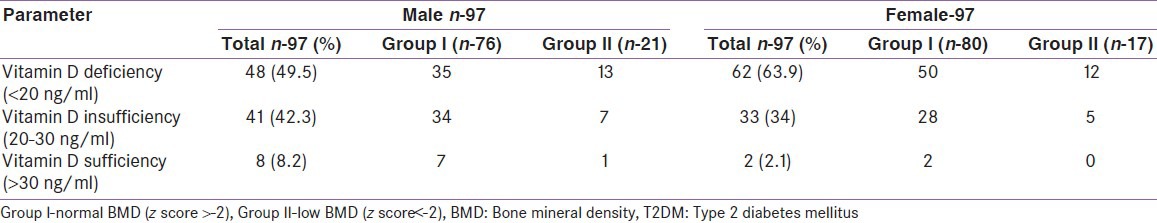
Figure 1.
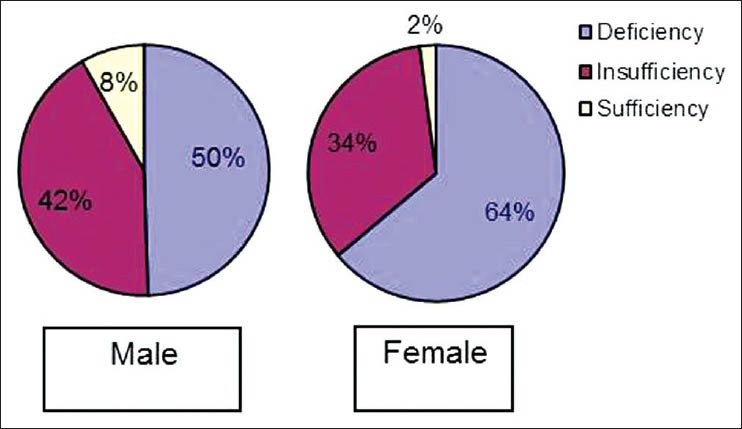
Distribution of vitamin D status in Type 2 DM patients
Physical activity and sunlight exposure
Physical activity was found to be moderate in groups I and II (M and F) and there was no significant difference between them. Similarly, the sunlight exposure was comparable in both groups I and II (M and F), (143.54 vs 132.05 minutes per week in males and 108.19 vs 92.06 min per week in females respectively).
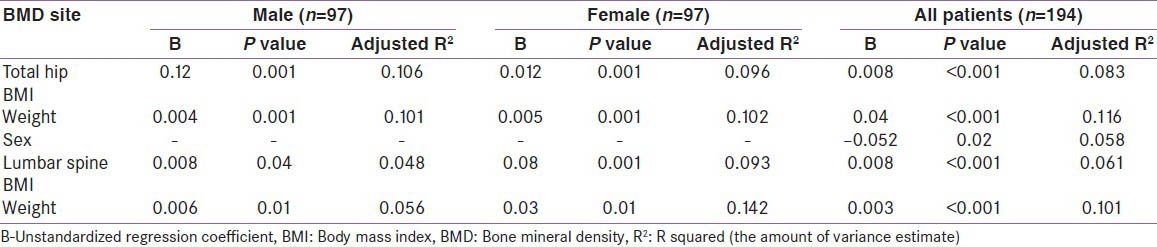
A multiple linear regression analysis of affecting factors in the diabetes study group (both men and women taken together and separately), showed BMI and weight to be independent predictors of BMD at both the spine and hip [Table 7]. Sex was an independent predictor of BMD at the total hip in the whole study group. Other variables like age, estradiol, free testosterone and calcium intake, when evaluated, were not independently associated with BMD.
Table 7.
Multiple linear regression analysis of independent associates of spine and hip BMD
DISCUSSION
BMD in T2DM is determined by various pathogenetic factors and several acquired conditions pertaining to diabetes. Circulating insulin/insulin growth factors and estrogen levels are the most important among the pathogenetic factors. A majority of studies on BMD in T2DM have documented increased or normal BMD in different areas of the body using different modalities. Fremantle diabetes study (FDS) by Rakic et al.[4] documented an increase in BMD in T2DM in both sexes. The BMD in the femoral neck were 0.851 and 0.808 gms/cm2 in males and females respectively, and in the total spine was 1.117 and 1.031 gms/cm2 and was greater than the age and sex matched non-diabetic controls. BMD in T2DM in FDS is much higher than that in our study. This could be largely due to the fact that Indians typically have BMD which is approximately 5% lower than the Caucasian population.[21] Tuominen et al.,[1] and Sosa et al.,[2] documented normal BMD in T2DM.
Decreased BMD was documented by Guven et al.,[6] and Al-Maatouq et al.[7] Guven et al., documented a femoral neck BMD of 0.769 and 0.716 gms/cm2 in men and women respectively and lumbar spine BMD of 0.912 and 0.845 gms/cm2 in the same cohort, respectively. Both the studies, done in a predominantly Muslim population had control groups with higher BMD. A majority of studies show no correlation of BMD to glucose control.[22] However, the Fremantle study of 194 T2DM patients showed that HbA1c was independently associated with BMD at the hip and femoral neck.[4] The diabetes study group when compared to the non-diabetic control group had a higher BMI and this was statistically significant in both sexes in our study. Studies done by Strotmeyer et al.,[12] have uniformly shown BMI to be an important variable affecting BMD. Obesity itself is known to be associated with an increased bone mass in most studies. Since T2DM is preceded by a period of insulin resistance, it is postulated that hyperinsulinemia may confer a protective effect on BMD, either directly through elevated fasting insulin or indirectly through BMI. Obesity could have the additional favorable effect of decreasing SHBG levels and increasing the production of metabolically active free estrogen. Leptin is an important peptide responsible for protective effects on bone. It leads to increased osteoblastic differentiation and may also reduce osteoclastogenesis by altering the expression of RANKL and osteoprotegerin in stromal cells.[23] The aromatization of androgens to estrogens in adipose tissue represents an important source of estrogen, which is anabolic for bone.
Statins are reportedly associated with an increase in bone morphogenic protein-2, a protein that plays an important role in osteoblast differentiation and bone formation. Chung et al.[24] documented an increase in BMD in the femoral neck and total hip but not at the lumbar spine in patients on statin therapy. PPAR-γ is essential for normal adipocyte differentiation and proliferation as well as fatty acid uptake and storage. Thiazolidinediones are commonly used to increase insulin sensitivity for the treatment of T2DM. Additionally, PPARγ activation leads to preferential inhibition of osteoblastogenesis. Schwartz et al.,[25] documented increased bone loss at total body, trochanter and lumbar spine in women with the use of thiazolidinediones. Kahn et al.[26] noted increased incidence of limb fractures in women on rosiglitazone.
The calcium intake was adequate in both groups I and II in both sexes. This is in accordance with a study by Tandon et al.,[21] among the paramilitary forces in North India. It is in contrast however, to the study by Harinarayan et al.[27] from South India where the calcium intake was less than the Indian Council of Medical Research recommendations of 400 mg/d[28] across socioeconomic status and vitamin D levels. There was a significant difference in PTH levels in group II in men as compared to group I. This cannot be explained by vitamin D deficiency, as the vitamin D levels were comparable in groups I and II (M and F).
The mean 25(OH) D level of our study group was lower than that in FDS[4] which could be attributed to increased skin pigmentation, crowded housing with limited sunlight exposure, smog, and the angle of ultraviolet rays. There was no correlation of vitamin D status with BMD in this study. Duration of sunlight exposure is positively correlated with bone density and 25(OH) D levels in various studies.[29,30] There was no significant difference in sun exposure between groups I and II (M and F) subjects with T2DM. Vitamin D deficiency and insufficiency was seen in 50% and 42% of male diabetes study subjects and 64% and 34% of female diabetes study subjects, respectively, despite adequate sunlight exposure.
Cross sectional studies have shown a positive correlation between BMD and exercise while interventional studies suggest that high impact exercises are better at increasing BMD.[31,32] Moderate physical activity has also been documented to lead to better bone health in studies in T2DM in contrast to heavy physical activity. Physical activity was found to be moderate in groups I and II (M and F) in our study and there was no significant difference between them.
Most recent studies regarding bone mineral changes in T2DM have shown increased density.[22,33] In our study, more than 80% of subjects with diabetes had normal BMD. Life style measures including adequate sunlight exposure, good calcium intake and moderate physical activity seen in our subjects could be significant factors contributing to normal BMD.
Ours was a cross sectional study with all its inherent limitations. There is no established normative BMD data for Indians. Another limitation is the lack of age matched controls. However, the effect of age is likely to be minimal once it has crossed the age for peak bone mass. The BMD of T2DM patients is moreover, greater than the controls, who recently attained peak bone mass. Comparison with the Caucasian database could have resulted in overestimation of low BMD and underestimation of normal BMD in our study. A comparative study of factors affecting BMD in the non-diabetic control group would have added strength to our study.
SUMMARY AND CONCLUSIONS
BMD of T2DM Asian Indian subjects following up in a tertiary care hospital in India was similar to that of the general population. Weight and BMI had the most significant impact on BMD in both men and women.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 2.Sosa M, Dominguez M, Navarro MC, Segarra MC, Hernández D, De Pablos P, et al. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1996;10:201–5. doi: 10.1016/1056-8727(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, et al. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med. 1995;122:409–14. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rakic V, Davis WA, Chubb SA, Islam FM, Prince RL, Davis TM. Bone mineral density and its determinants in diabetes: The Fremantle Diabetes Study. Diabetologia. 2006;49:863–71. doi: 10.1007/s00125-006-0154-2. [DOI] [PubMed] [Google Scholar]
- 5.Shan PF, Wu XP, Zhang H, Cao XZ, Yuan LQ, Liao EY. Age-related bone mineral density, osteoporosis rate and risk of vertebral fracture in mainland Chinese women with type 2 diabetes mellitus. J Endocrinol Invest. 2011;34:190–6. doi: 10.1007/BF03347065. [DOI] [PubMed] [Google Scholar]
- 6.Guven M, Colak R, Tutus A. The evaluation of bone mineral Density in male and postmenopausal female patients with type 2 Diabetes Mellitus. Turk J Endocrinol Metab. 1999;4:169–72. [Google Scholar]
- 7.Al-Maatouq MA, El-Desouki MI, Othman SA, Mattar EH, Babay ZA, Addar M. Prevalence of osteoporosis among postmenopausal females with diabetes mellitus. Saudi Med J. 2004;2510:1423–7. [PubMed] [Google Scholar]
- 8.Yaturu S, Humphrey S, Landry C, Jain SK. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit. 2009;15:CR5–9. [PubMed] [Google Scholar]
- 9.Thalassinos NC, Hadjiyanni P, Tzanela M, Alevizaki C, Philokiprou D. Calcium metabolism in diabetes mellitus: Effect of improved blood glucose control. Diabet Med. 1993;10:341–4. doi: 10.1111/j.1464-5491.1993.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 10.Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, et al. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 11.Gordeladze JO, Reseland JE. A unified model for the action of leptin on bone turnover. J Cell Biochem. 2003;88:706–12. doi: 10.1002/jcb.10385. [DOI] [PubMed] [Google Scholar]
- 12.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The health, aging, and body composition study. J Bone Miner Res. 2004;19:1084–91. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson IH, Fazzalari NL. Interrelationships between structural parameters of cancellous bone reveal accelerated structural change at low bone volume. J Bone Miner Res. 2003;18:2200–5. doi: 10.1359/jbmr.2003.18.12.2200. [DOI] [PubMed] [Google Scholar]
- 14.Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: Effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab. 2006;91:3355–63. doi: 10.1210/jc.2006-0460. [DOI] [PubMed] [Google Scholar]
- 15.Standards of medical care in diabetes – 2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 16.Misra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722–9. doi: 10.1038/sj.ijo.0801748. [DOI] [PubMed] [Google Scholar]
- 17.Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxy vitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952–6. doi: 10.1210/jc.2002-020636. [DOI] [PubMed] [Google Scholar]
- 18.Gopalan C, Rama Sastri BV, Balasubramanian SC. Indian Council of Medical Research. 1st ed. Hyderabad: National Institute of Nutrition; 1971. Nutritive value of Indian foods. [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Tandon N, Marwaha RK, Kalra S, Gupta N, Dudha A, Kochupillai N. Bone mineral parameters in healthy young Indian adults with optimal vitamin D availability. Natl Med J India. 2003;16:298–302. [PubMed] [Google Scholar]
- 22.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 23.Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17:1563–9. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- 24.Chung YS, Lee MD, Lee SK, Kim HM, Fitzpatrick LA. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2000;85:1137–42. doi: 10.1210/jcem.85.3.6476. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–54. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 27.Harinarayan CV, Ramalakshmi T, Venkataprasad U. High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pac J Clin Nutr. 2004;13:359–64. [PubMed] [Google Scholar]
- 28.Park K. Park's Textbook of Preventive And Social Medicine. 19th ed. Jabalpur: Banarsidas Bhanot Publishers; 2007. Summary of RDA for Indians. Nutritional and Health; p. 506. [Google Scholar]
- 29.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxy vitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 30.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxy vitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51:1075–81. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 31.De Luis Román DA, Aller R, Perez Castrillon JL, De Luis J, Gonzalez Sagrado M, Izaola O, et al. Effects of dietary intake and life style on bone density in patients with diabetes mellitus type 2. Ann Nutr Metab. 2004;48:141–5. doi: 10.1159/000078376. [DOI] [PubMed] [Google Scholar]
- 32.Todd JA, Robinson RJ. Osteoporosis and exercise. Postgrad Med J. 2003;79:320–3. doi: 10.1136/pmj.79.932.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


