Abstract
Background:
there are dearths of studies describing the effect of autologous bone marrow derived stem cell transplantation (ABMSCT) through targeted approach in Type 2 Diabetes Mellitus. This study reports the efficacy and safety of super-selective injection of ABMSCT in T2DM.
Materials and Methods:
Ten patients (8 men and 2 women) with T2DM, with duration of disease >5 years and with documented triple drug failure receiving insulin (0.7 U/Kg/day), metformin and pioglitazone underwent super-selective injection of stem cells into superior pancreaticoduodenal artery under fluoroscopic guidance. The primary outcome measure was decrease in insulin requirement by ≥50% (defined as responders), while secondary endpoints were improvement in glucagon stimulated C-peptide levels, changes in weight, HbA1c, lipid profile and quality of life (QOL) at the end of 15 months.
Results:
Six patients (60%) were ‘responders’ at 15 months of follow-up showing a reduction in mean insulin requirement by 74% as compared to baseline and one patient was off-insulin till the end of the study. Mean HbA1c reduction in ‘responders’ was 1.1% (8.1 ± 0.5% to 7.0 ± 0.6%, P = 0.03), accompanied with a significant improvement in glucagon stimulated C-peptide levels (P = 0.03), Homeostasis Model Assessment -β (P = 0.03) and QOL scores. However, ‘non-responders’ did not show any significant alterations in these parameters. No serious adverse events were noted.
Conclusion:
Our observations indicate that ABMSCT is effective in management of T2DM and its efficacy is maintained over a period of 15 months without any adverse events. However, more number of patients and longer duration of follow-up are required to substantiate these observations.
Keywords: β-cells, C-peptide, stem cells
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is characterized by two defects, namely insulin resistance and insulin deficiency. However, insulin resistance alone cannot produce T2DM unless β-cells fail to compensate.[1] Moreover, insulin resistance remains fairly constant after evolution of diabetes in a given individual, while β-cell function/mass progressively declines with advancing duration of disease as evidenced from United Kingdom Prospective Diabetes Study (UKPDS).[2] The functional defects resulting from dual gluco- and lipotoxicity are reversible after correction of respective metabolic abnormalities. However, islet abnormalities that are observed with advancing duration of T2DM including pseudohypertrophy of islets with reversal of β/α-cell ratio and progressive interstitial fibrosis as a result of amylin deposition are irreversible.[3,4] An autopsy series showed a loss of β-cell-mass by 40% in subjects with impaired glucose tolerance (IGT) and by 60% at the time of diagnosis of T2DM.[5] Defects in the entero-incretin insulin axis entail another facet in the pathogenesis of T2DM, where therapeutic interventions can be targeted.[6] Though there are effective options available to target insulin resistance and incretin defects,[5,6] effective therapeutic modalities targeting β-cell dysfunctions are lacking. Early intensive insulin therapy,[7] rosiglitazone[8] and possibly dipeptidyl peptidase inhibitors and glucagon-like peptide-1 analogues[9] have been demonstrated to have some beneficial effects on β-cell function. Pancreatic and islet cell transplantation is in use in both T1DM and T2DM with successful outcome. However, limited availability of cadaveric pancreas and the need for immunosuppression with its associated complications and progressive decline in insulin independence yields a disrepute to it. Stem cell therapy appears to be an attractive option in this regard.[10,11,12] Stem cells can be obtained from various sources like blastocyst (embryonic stem cells), umbilical cord or bone marrow. There is an evidence to suggest that stem cell transplantation can lead to improvement in pancreatic endocrine function and improvement in glycemic control through various mechanisms such as trans-differentiation or differentiation of ductal epithelial cells to islets cells and various growth factors along with stem cells may promote angiogenesis.[14] We describe the effect of autologous bone marrow derived stem cell transplantation (AMSCT) administered through targeted approach in patients with T2DM.
MATERIALS AND METHODS
Patients
Ten patients who fulfilled the inclusion criteria were enrolled for the study. The inclusion criteria were: age between 30 to 75 years, known duration of T2DM > 5 years, failure to achieve glycemic targets while receiving triple oral anti-diabetic drugs thereby requiring insulin for optimal glycemic control in doses ≥ 0.7 U/kg/day for at least one year and having negative glutamic acid decarboxylase (GAD) antibody status. All the patients were on stable doses of insulin, metformin (2 gm per day) and pioglitazone (30 mg per day) for the past 3 months. Exclusion criteria included serum creatinine >1.5 mg/dl, abnormal liver function tests, active infections, malignancy or acute coronary syndrome or any cardiovascular event in the previous three months. Written informed consent was obtained from all the study subjects.
Before commencing the study, a detailed baseline clinical examination and biochemical investigations were carried out. HbA1c level was measured using high performance liquid chromatography by Bio- Rad D10 (Bio-Rad Laboratories Inc, Hercules, CA, USA) (normal range: 3.8-5.9%). Glucagon stimulated C-peptide levels were estimated in the fasting state after intravenous administration of 1 mg glucagon (GlucaGen, Novo Nordisk, Denmark). Blood samples were drawn at -15, 0 (mean taken as fasting value) and 6 minutes (stimulated level) after injection. It was measured by immunochemiluminiescence (Elecsys 2010 Roche Diagnostics, GmbH, Mannheim, Germany) (normal range: 1.1-4.4 ng/ml). Fasting plasma insulin was estimated after omitting NPH insulin for at least 36 h and regular insulin for at least 18 h. Homeostatic model assessment was applied for the assessment of HOMA-IR (insulin resistance) and HOMA-β (β-cell function).
Stem cells harvesting and transplantation
Approximately 150 ml of bone marrow was aspirated through the posterior superior iliac spine under local anesthesia from each patient. Autologous mononuclear cells (MNC) were separated by ultracentrifugation after layering on density gradient medium (Ficoll-Hypaque), washed using phosphate buffer saline and resuspended in heparinized normal saline (final volume: 5 ml). Aliquots were taken from the above sample for total nucleated cell count, MNC count, viability testing using trypan blue, CD 34+ cell count by flow cytometry and sterility testing.
A 5F catheter was navigated through a trans-femoral route into the gastro-duodenal artery beyond the origin of the cystic artery. After confirmation of the position of the catheter, a super-selective injection of stem cells was carried out at the origin of the superior pancreaticoduodenal artery under fluoroscopic guidance. Post stem cell injection, a diagnostic run was taken to look for the patency of gastro-duodenal artery. After the procedure, patients were observed for the next 72 h for any procedure related complications (local bleeding, hematoma formation, and examination of distal pulses). Five-point profile of blood glucose values and any alteration in insulin requirement was recorded.
Follow-up
The patients were followed up at two weekly intervals for the first two months, monthly for the next four months, and thereafter every three monthly. The patients were asked to self monitor their blood glucose (at least 15 values in a month including a 5 point profile) on various days. Glycemic targets included fasting plasma glucose between 70 to 130 mg/dl, post meal plasma glucose <180 mg/dl and HbA1c <7%. The patients were asked to contact a single investigator (Vimal Upreti) by phone for insulin dose adjustment. Standard counseling regarding the diet and regular exercise was given to all the patients during each visit.
sitagliptin, a dipeptidyl peptidase 4, inhibitors became available in the Indian market six months after the onset of the study. For the sake of uniformity, it was added on to all patients after six months as sitagliptin has been shown to improve survival of transplanted islet cells and causes neogenesis of pancreatic islets in animal models.[9,12,13,14] Self monitoring of blood glucose record was analyzed at each visit, HbA1c and lipid profile were estimated at three monthly intervals, while glucagon stimulated C-peptide, fasting plasma insulin and plasma glucose were assayed at the end of 12 months. Quality of life was assessed using World Health Organization Quality of Life WHOQOL-BREF (WHO, Dec 1996) and Treatment Related Impact Measure-Diabetes (TRIM-D) questionnaire, with permission from Novo Nordisk company at baseline and after 15 months of the follow-up. WHOQOL- BREF assesses QOL related to any chronic disease and is evaluated under the following heads: physical health, psychological health, social relationship, environmental health and general well being. TRIM-D, on the other hand, is a diabetes specific questionnaire that assesses treatment related impact measures of diabetes under treatment burden, daily life, diabetes management, compliance and psychological health.
Outcome measures
The primary outcome measure was a reduction in insulin requirement by ≥50% (responders), while secondary endpoints were improvement in glucagon stimulated C-peptide levels and change in weight, HbA1c, lipid profile and quality of life at the end of the study as compared to the baseline.
Statistical analysis
The statistical program for Social Sciences (Release 16, PC Windows; SPSS Inc., Chicago IL) was used for the data analysis. Data was expressed as mean + SD, unless otherwise specified. Baseline and post treatment data were compared using Friedman test and Wilcoxon signed ranks test for tests of significance. Linear regression analysis was used to find correlation between independent variables. A probability P < 0.05 was regarded statistically significant.
RESULTS
Ten patients (8 men and 2 women) satisfying the inclusion criteria underwent autologous bone marrow derived stem cell transplantation (ABMSCT). The mean ± SD age, duration of diabetes and body mass index were 57.5 + 5.9 years, 14.6 ± 7.5 years and 26.5 ± 3.4 kg/m2 respectively. The mean ± SD duration of insulin therapy in these patients was 5.6 ± 3.0 years and the mean ± SD dose of insulin required was 69.4 ± 20.8 units per day. The mean ± SD dose of bone marrow aspirate harvested was 156.0 ± 4.9 ml, which yielded a mean ± SD 3.1 ± 1.4 × 106 of CD-34 positive hematopoietic stem cells, which were infused into the superior pancreaticoduodenal artery.
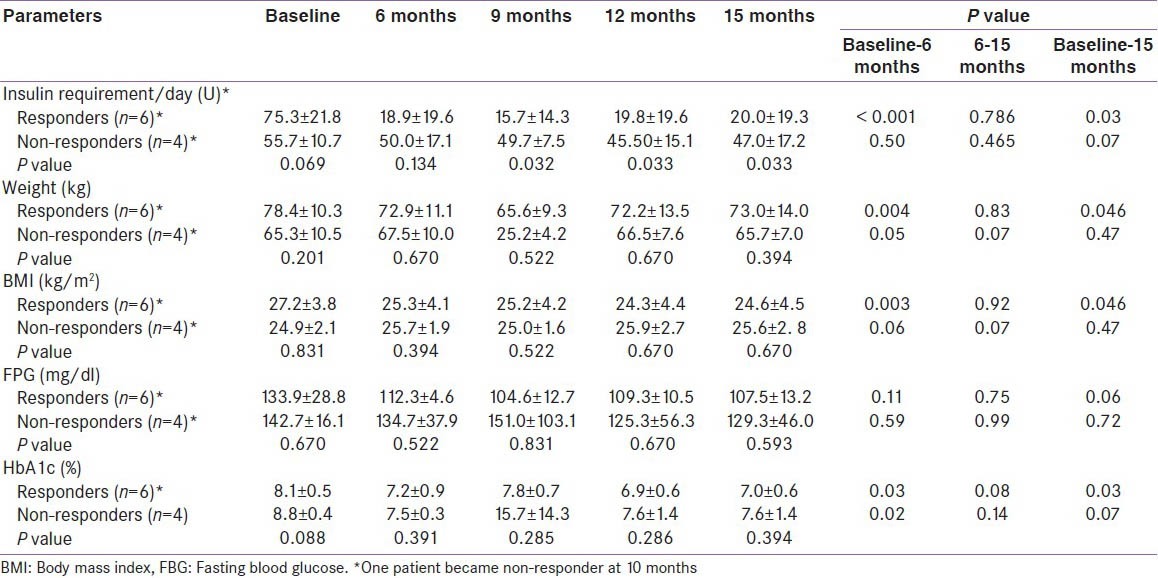
The primary outcome (decrease in insulin requirement by ≥ 50%) was observed in 7 (70%) patients at the end of six months, and it persisted in 6 (60%) patients after 15 months of follow-up. The mean ± SD insulin requirement decreased significantly from 75.3 ± 21.8 units per day to 18.9 ± 19.6 units per day at six months (P < 0.001) and the decrement was continued to be maintained (20.0 ± 19.3 units per day) even at 15 months (P = 0.03) in responders. Responders had significant reduction in insulin requirement as compared to non responders at 9, 12 and 15 months (P = 0.032, 0.033 and 0.033, respectively). The decrease in insulin requirement observed in the responders was accompanied with significant weight loss during the first six months and later, weight remained virtually stable throughout the study. However, the correlation between the initial weight loss and decrease in insulin requirement was not statistically significant (r = 0.59 P = 0.07). Moreover, the 50% decrease in insulin requirement preceded the weight loss in 4 patients (within 10 days in 3 patients). Moreover, the decrease in insulin requirement in responders at the end of the study was significant even after adjusting for weight (P = 0.000). There was a significant improvement in HbA1c (8.1 ± 0.5 to 7.0 ± 0.6%, P = 0.03), glucagon stimulated C-peptide (1.2 ± 0.1 to 2.3 ± 0.3 ng/ml, P = 0.03) and HOMA-β (37.7 ± 18.4 to 148.5 ± 0.6, P = 0.03). The improvement in HOMA-β and HOMA-IR in responders was significant as compared to non-responders at 12 months (P = 0.03, P = 0.05 respectively) while there was no alteration in HOMA-IR (P = 0.17) in ‘responders’ at the end of the study. The above described parameters for both the ‘responders’ and ‘non-responders’ are summarized in Tables 1-4. Four of the 6 ‘responders’ and 1 of the 4 ‘non-responders’ achieved HbA1c ≤ 7%. Two patients were off-insulin at the end of 6 months, however one of them required reintroduction of insulin at 10 months because of increasing HbA1c, though his requirement was still less than 50% of the baseline.
Table 1.
Baseline and follow-up clinical characteristics of the study population
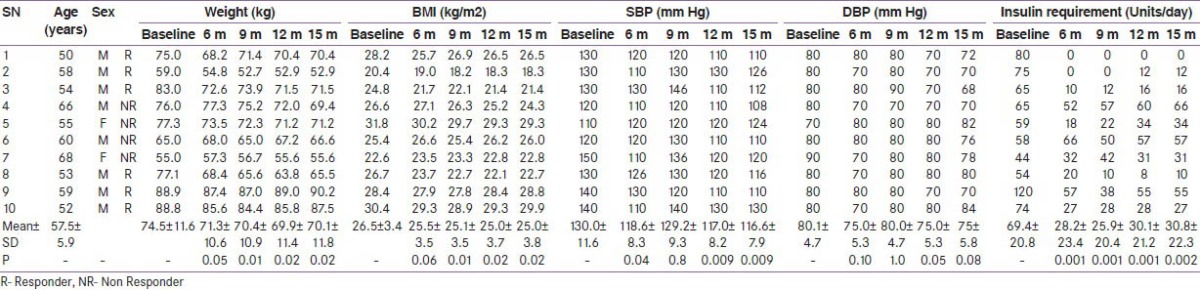
Table 4.
Glucose-insulin homeostasis parameters at baseline and follow-up of the study population
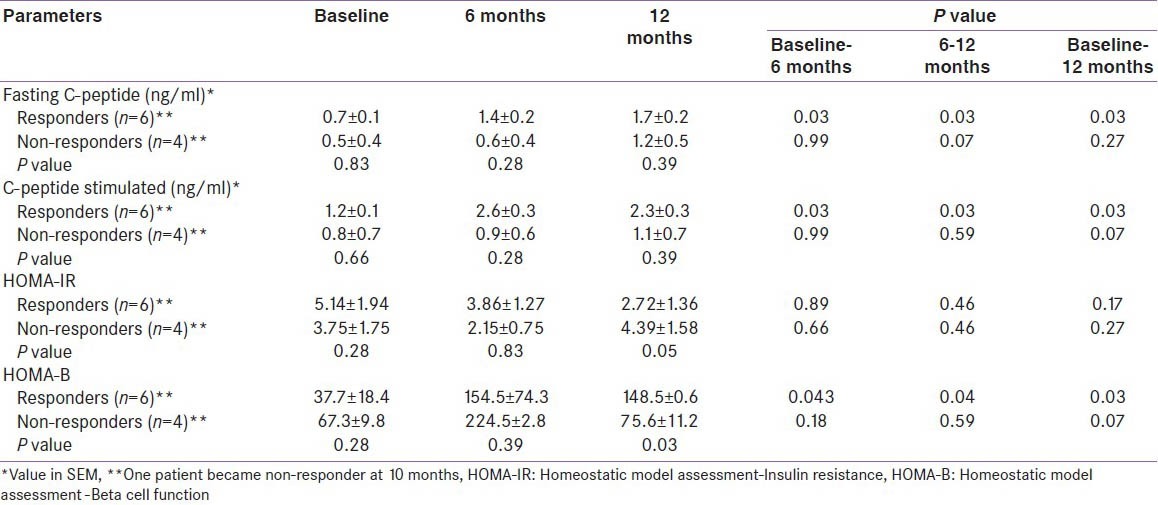
Table 3.
Clinical and biochemical characteristics of the study population
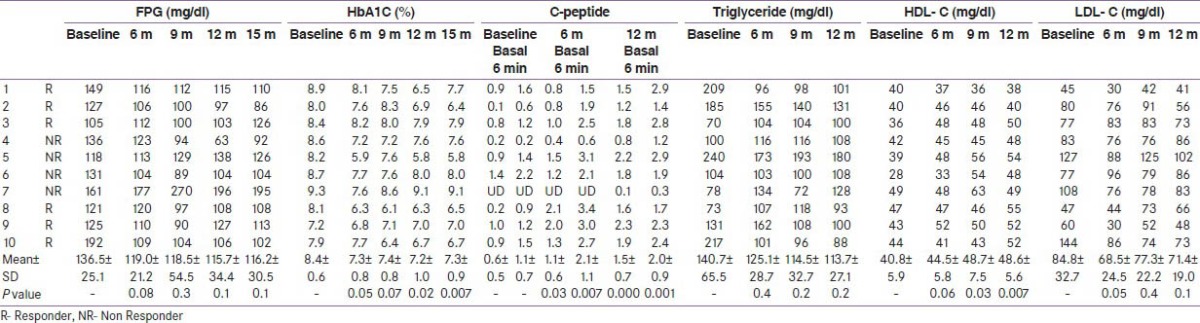
Significant reduction in systolic BP and improvement in HDL- cholesterol was recorded at the end of six months and this was maintained till the last follow-up [Table 2]. However, diastolic BP or other parameters of lipid profile did not show any change during the study period.
Table 2.
Baseline and follow-up biochemical parameters of the study population
As regards to QOL, there was a significant improvement in the general well being of the whole group, while environmental health parameters improved only in the ‘responders’ as assessed by WHOQOL-BREF after 15 months of follow-up as compared to baseline. However, other parameters of QOL did not show any significant change. Using the TRIM- D questionnaire, improvement was seen in all domains, except for daily life, however statistical significance was reached only for the treatment burden in the ‘responders’ (P = 0.05), while no change was seen in the ‘non-responders’.
No serious adverse events were noted. Minor events included nausea and vomiting after glucagon for stimulated C-peptide assessment and mild pain during the bone marrow aspiration which lasted for 24 h. No major hypoglycemia was observed. Minor hypoglycemic episodes occurred more often in the ‘non-responders’ than the ‘responders’ at the end of six months (58 vs. 18 episodes, P = 0.012) as well as at the end of the study (68 versus 4 episodes, P = 0.018).
DISCUSSION
This study shows the efficacy of autologous bone marrow derived stem cell transplantation in patients with T2DM in terms of reduction in insulin requirement with sustenance of HbA1c < 7%, which persisted for at least 15 months in almost two third of the patients. It was accompanied by the improvement in stimulated C-peptide levels and HOMA-β. No serious adverse events were observed.
Bone marrow derived stem cell therapy has opened new vistas in the management of diabetes. insulin independence and improvement in C-peptide have been shown in patients of T1DM after peripheral administered of hematopoietic stem cells.[10] Similarly in patients with T2DM, Estrada et al.,[12] showed progressive and sustained improvement in FPG and HbA1c over a period of one-year follow-up, after ABMSCT through targeted approach along with hyperbaric oxygen. However, the details about the infusate were not revealed and the stem cell were injected into dorsal pancreatic artery (a branch of splenic artery), which feeds the body of the pancreas with limited density of β cells. In our study, a super-selective injection of stem cells was administered into superior pancreaticoduodenal artery which is feeding the head and part of the body of the pancreas with relatively higher density of β-cells as compared to the body of the pancreas. Maximal decrease in FPG and HBA1c was observed at 12 months as compared to our study where it was observed in the first three months with the achievement of primary outcome measure and it was sustained during follow-up period of 15 months. Almost two third of the patients were lost to follow-up in the above described study, as compared to no attrition in our study [Table 5].
Table 5.
Summarization of previous studies on ABMSCT in diabetic patients
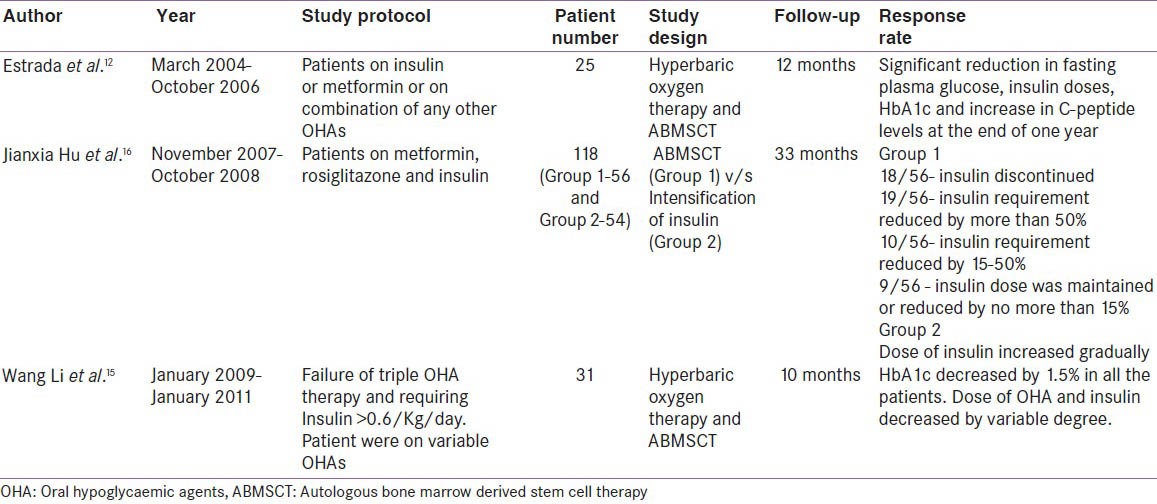
In a study from China, Li et al.,[15] used combined ABMSCT and hyperbaric oxygen therapy on 31 patients in T2DM and showed HbA1c decrease of >1.5% as quickly as one month after therapy and it was maintained over the study period of one year. The C-peptide increased significantly at three months but returned to baseline at one year. The author concluded that combined ABMSCT and HBO therapy led to improvement in glucose control, decrease in requirement of insulin and oral hypoglycemic agents and a transient improvement in β-cell function. In a recent study, Hu et al., demonstrated the long term efficacy and safety of autologous bone marrow mononuclear cells (ABMNC) in comparison to intensive insulin therapy in patients with T2DM.[16] One hundred and eighteen patients were followed up for a period of 33 months and showed ABMNC group could achieve significantly lower HbA1c with reduction in oral hypoglycemic drugs and insulin doses as compared to the control group on intensive insulin therapy. However, the study was not appropriately designed as patients were open to choose their mode of treatment and it was not oriented with intention-to-target HbA1c < 7%. Moreover, the control group was not appropriately matched for the operative procedure.[16] In our previous study, we showed ≥50% reduction in the insulin requirement from the baseline in three fourth of the patients with a reduction in HbA1c from 8.4 ± 0.6% to 7.3 ± 0.8% and a significant increase in stimulated C-peptide after ABMSCT.[11]
Sustained reduction in insulin dosage and decrease in HbA1c at the end of follow-up of 15 months may be attributed to functional improvement in β-cell due to improved metabolic milieu (glucotoxicity) and/or to β-cell regeneration caused by SCT or both, through various mechanisms as described later. The improvement in glucose profile which was accompanied with significant reduction in insulin doses could not have been sustained for an extended period of time, if it would have only been due to amelioration of glucotoxicity. Therefore, a possibility of improvement in β-cell mass is likely, however this has not been examined in the present study but animal data supports this view.[13,14] The improvement in β-cell function/mass in the present study was evidenced by the increase in stimulated C-peptide levels and HOMA-β. These observations are similar to that reported in patients with T1DM and T2DM in earlier studies.[10,12] Sitagliptin, a DPP IV inhibitor has been shown to induce β-cell neogenesis in the mice model.[9,17,18,19] However, the available data in humans regarding the efficacy of sitagliptin on glycemic durability are not encouraging.[20] In our study, the addition of sitagliptin did not result in further improvement in HbA1c or stimulated C-peptide levels.
The probable determinants of response to SCT include baseline C-peptide levels, dose of stem cells infused and possibly targeted vs. peripheral infusion of stem cells. these parameters have not been studied earlier. Stimulated C-peptide levels represent β-cell reserve and it is likely that patients with higher C-peptide levels may respond better, as was observed in the present study. The reduction in insulin dosage correlated with the dose of infused stem cells (r = 0.57, P = 0.04) in the present study. However, the dose of infused stem cells in our study was much lower as compared to the dose used by Voltarelli et al.,[21] The empiric dose of autologous bone marrow derived hematopoietic stem cells (CD 34 positive) stated to achieve therapeutic effect is 10 to 40 × 106 cells.[9,17,18,19] Hence, a higher dose might have resulted in further reduction in insulin requirement. Most of the available studies have injected stem cells through peripheral intravenous route whereas the targeted approach of direct injection of stem cells into the celiac axis, used in one animal study[13] has shown to be effective because of direct ‘homing in’ of stem cells into the pancreas. We and others (unpublished observation) have shown[11,12,22] good results following a targeted approach which has a theoretical advantage of delivering a bolus dose of stem cells and various growth factors to the affected site, thereby providing maximum stimulation to the various regenerative mechanisms. However, at present, it is not clear whether peripherally administered stem cells or targeted approach is superior?
We also observed an improvement in both systolic BP and HDL-cholesterol at the end of six months which was sustained during follow-up, though this study was not designed to look into these effects. These could be ascribed to the weight loss and the overall improvement in the metabolic milieu. Minor hypoglycemic episodes were substantially less in the responders as compared to the non-responders, suggesting an improvement in endogenous β-cell functions. Because of the decrease in hypoglycemic episodes, inter-prandial snacking was probably curtailed, which resulted in weight loss, as was observed in this study.
Quality of life (QOL) score showed improvement in general well being using WHOQOL- BREF and treatment burden using TRIM-D questionnaire in the responders. It can be explained by an increased sense of well being due to the overall improvement in metabolic profile, reduction in insulin dosage, and antihypertensive medications and improved lipid profile. However, psychological benefits incurred due to an invasive procedure with incentive of cure cannot be ruled out.
Mechanisms by which SCT results in improvement in β-cell function/mass are conjectural. There are data from animal models that clearly demonstrate the regeneration of islets after SCT.[14,23] Mechanisms attributed for improvement in β-cell function/mass include: a) secretion of various growth factors such as hepatocyte growth factor, vascular endothelial growth factors by the injected stem cells resulting in angiogenesis and stimulation of growth differentiation and survival of β-cells. This may be due to expression of transcription factors such as PDX-1, b) trans-differentiation of stem cell into β-cell and, c) regeneration of small islets from pancreatic stem cells around the pancreatic ducts.[14,23] At present, these hypotheses cannot be substantiated in humans due to lack of morphometric studies in vivo. However, hyperglycemic clamp studies can be a surrogate marker of improvement in β-cell function as shown in animal studies.
Limitations of our study include small number of patients, lack of a control arm, inability to demonstrate ‘homing in’ of stem cells in the pancreas and/or morphometric evidence of regeneration of pancreatic islets. Further larger, multicenter studies are required to substantiate these observations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. HOMA-beta in the UKPDS and ADOPT. Is the natural history of type 2 diabetes characterised by a progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diab Vasc Dis Res. 2009;6:133–8. doi: 10.1177/1479164109336038. [DOI] [PubMed] [Google Scholar]
- 3.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–8. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 4.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–8. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 5.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 9.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55:1695–704. doi: 10.2337/db05-1602. [DOI] [PubMed] [Google Scholar]
- 10.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 11.Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407–16. doi: 10.1089/scd.2009.0164. [DOI] [PubMed] [Google Scholar]
- 12.Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008;17:1295–304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- 13.Perin EC, Geng YJ, Willerson JT. Adult stem cell therapy in perspective. Circulation. 2003;107:935–8. doi: 10.1161/01.cir.0000057526.10455.bd. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez SS, Jimenez LM, Murillo AZ, Gomez IG, Ligero JM, Gomez-Pineda A, et al. A new approach for bone marrow-derived stem cells intrapancreatic autotransplantation in diabetic rats. Microsurgery. 2006;26:539–42. doi: 10.1002/micr.20283. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhao S, Mao H, Zhou L, Wang ZJ, Wang HX. Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin Med J (Engl) 2011;124:3622–8. [PubMed] [Google Scholar]
- 16.Hu J, Li C, Wang L, Zhang X, Zhang M, Gao H, et al. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J. 2012;59:1031–9. doi: 10.1507/endocrj.ej12-0092. [DOI] [PubMed] [Google Scholar]
- 17.Fung M, Thompson D, Shapiro RJ, Warnock GL, Andersen DK, Elahi D, et al. Effect of glucagon-like peptide-1 (7-37) on beta-cell function after islet transplantation in type 1 diabetes. Diabetes Res Clin Pract. 2006;74:189–93. doi: 10.1016/j.diabres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Gallwitz B. Sitagliptin: Profile of a novel DPP-4 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc) 2007;43:13–25. doi: 10.1358/dot.2007.43.1.1043909. [DOI] [PubMed] [Google Scholar]
- 19.Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract. 2006;73:107–10. doi: 10.1016/j.diabres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Riche DM, East HE, Riche KD. Impact of sitagliptin on markers of beta-cell function: A meta-analysis. Am J Med Sci. 2009;337:321–8. doi: 10.1097/MAJ.0b013e31818eb721. [DOI] [PubMed] [Google Scholar]
- 21.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–9. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 22.Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203–5. doi: 10.1016/S0140-6736(04)16635-X. [DOI] [PubMed] [Google Scholar]


