Abstract
Allele and genotype frequency of a genetic variant in ATM gene affecting glycemic response to metformin in South Indian population.
Context:
The novel polymorphism in ATM gene (rs11212617), which is implicated to have association with metformin response, exhibits inter-ethnic variability in the allele and genotype frequency distribution.
Aims and Design:
The objective of the present study is to establish the allele and genotype frequency of rs11212617 single nucleotide polymorphism in ATM gene, in South Indian population and to find if this variant has any role in the etiology of type 2 diabetes mellitus.
Materials and Methods:
The study was performed in 2 cohorts of populations, 112 healthy volunteers and 118 type 2 diabetes mellitus patients. Genomic deoxyribonucleic acid (DNA) was extracted from peripheral blood leucocytes by phenol-chloroform method and genotyping was performed by real-time polymerase chain reaction using TaqMan assay.
Results:
In South Indian population, the frequency of major A allele was 0.65 and the minor C allele was 0.35. AA and CC are the homozygous genotypes with frequency of 0.39 and 0.09 respectively. The frequency of heterozygous genotype AC (0.52) was found to be higher than the homozygotes. There was no significant difference in the frequency distribution in the diabetic population, which implies that this variant does not have any causative role in the disease etiology. The frequency distributions were found to be significantly different from the distributions in other ethnic populations such as Caucasians, Chinese, Japanese and Africans. But there was no significant difference when compared with the Gujarati Indians of Houston.
Conclusion:
The frequency distribution of this novel variant in South Indian population forms a framework for further gene disease association studies to establish the association of this variant with metformin response. Our study could not find any association of this variant with respect to the disease etiology.
Keywords: Ataxia telangiectasia mutated gene, diabetes, metformin
INTRODUCTION
Diabetes mellitus is a chronic progressive multifactorial disorder that demands lifelong optimal control of glycemic status once the diagnosis is made. The International Diabetic Federation estimates that around 61.3 million people in India had diabetes in 2011, which will be projected to 101.2 million in 2030. India is ranked second in the world in diabetes prevalence, just behind China.[1]
Metformin is an oral hypoglycemic agent that is approved as the first-line agent for treatment of type 2 diabetes mellitus as per the American Diabetic Association (ADA), European Association for the Study of Diabetes (EASD) and International Diabetic Federation guidelines.[2,3] Metformin is an insulin sensitizing agent, belonging to the class of biguanides. The glucose lowering response of metformin exhibits a wide inter-individual variability.[4,5] These variations in drug response can be because of phenotypic variation or variation in drug action or distribution. Genetic polymorphisms in drug transporter genes have been largely attributed to the inter-individual variability in clinical response to metformin.[5,6,7,8]
Metformin is an organic hydrophilic cation, which is transported across biological membranes by the organic cationic transporters (OCT). The genetic contribution of inter-individual variability in the clinical response to metformin is largely attributed to OCT1, which is a highly polymorphic transporter. The association of metformin response to the polymorphic variants in SLC22A1 gene coding for OCT1 is established in various populations.[9,10,11] The allele and genotype frequency of the contributing variants in SLC22A1 gene were established in South Indian population as well.[12]
Recently, a novel variant in ataxia telangiectasia mutated (ATM) gene (rs11212617) is strongly implicated to affect the glycemic response of metformin.[13] ATM gene is located on the long arm of chromosome 11q22-23 and belongs to the family of phosphatidyl inositol 3 kinase related kinase that encodes serine-threonine kinases involved in regulation of cell-cycle progression. Mutations in ATM gene are implicated to have a causative role in ataxia telangiectasia, which is an autosomal recessive disorder with manifestations including insulin resistance and type 2 diabetes mellitus.
Though metformin was introduced in clinical practice for over 50 years, the detailed molecular mechanism of action of metformin has not been completely established. Attempts at elucidating the molecular mechanism of metformin have linked adenosine monophosphate-activated protein kinase (AMPK), which is the energy sensor of the cell, to the metformin mediated inhibition of hepatic gluconeogenesis and glucose uptake in muscle and hepatocytes.[14,15] This AMPK in turn can be regulated by ATM gene.[16,17,18] The link between metformin and ATM gene is shown in Figure 1. There were contradictory results about the association of metformin response with rs11212671 polymorphism in ATM gene in different ethnic populations (European[13] and US ethnic population[19]).
Figure 1.
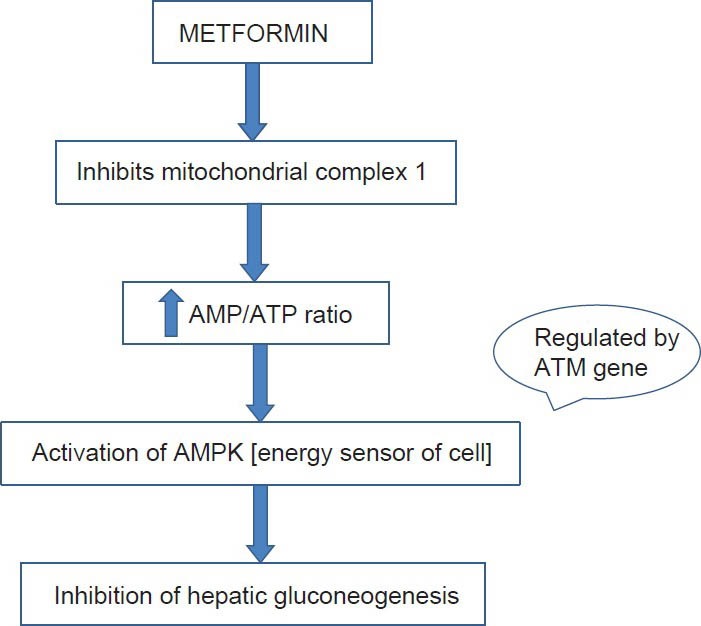
Schematic representation of the molecular mechanism of metformin linking ATM gene. AMPK: 5’ Adenosine monophosphate activated protein kinase, ATM gene: Ataxia telangiectasia mutated gene
In the current scenario of diabetic prevalence, considering the robust use of metformin by the diabetic population, effective utilization of the advances made in the field of personalized medicine to establish the frequency of this variant affecting clinical response will contribute to rational therapeutics.
As there is a possible role for ethnic variations in ATM gene polymorphism to contribute to the inter-individual variability in metformin treatment response, this study was intended to establish the allele and genotype frequency of single nucleotide polymorphism (SNP) rs11212617 in ATM gene affecting metformin response in the South Indian population and also to assess if the polymorphism has a role in the etiology of type 2 diabetes.
MATERIALS AND METHODS
Study settings
The study was performed in Department of Pharmacology in collaboration with Department of Medicine, JIPMER, Puducherry. Institute ethics committee approval was obtained before the commencement of the study. Informed consent was obtained from all healthy volunteers and type 2 diabetes mellitus patients who were willing to participate in the study.
Study subjects
The study was done in 2 cohorts of population including healthy volunteers and patients with type 2 diabetes mellitus, visiting the diabetic out-patient department, JIPMER. Around 112 unrelated healthy volunteers and 118 type 2 diabetes mellitus patients from Southern states of the country (Tamil Nadu, Kerala, Karnataka and Andhra Pradesh) of Dravidian descendants were recruited into the study. All these subjects were residents of South India for at least three successive generations and were in the age group between 18 and 60 years, of either sex. The volunteers did not have any other concomitant illness and were not on any other concomitant medications. Of the 112 healthy volunteers, 66 (58.9%) were females and 46 (41.1%) were males. Of the 118 type 2 diabetes mellitus patients included in the study, 90 (76.3%) were females and 28 (23.7%) were males. All subjects were informed about the details of the study in their native language and informed consent was obtained as per the format given in the Institute ethical guidelines, JIPMER, Puducherry. Blood sample of around 5 ml was collected in ethylenediaminetetraacetic acid (EDTA) containing tubes (100 μl of 10% EDTA as anti-coagulant) for genotyping the patients. After separating the plasma by centrifugation, deoxyribonucleic acid (DNA) was extracted from the peripheral blood leucocytes by phenol-chloroform method. The extracted DNA was then diluted in Tris-EDTA buffer and the samples were stored at 4°C. The DNA samples were quantified using the multianalyzer (TECAN Infinite M200, Switzerland) and stored at −20°C until genotyping.
Genotype study
Genotyping for rs11212617 was carried out using real-time thermo cycler (ABI Prism 7300, Foster city, CA, USA) using TaqMan SNP genotyping assay method. The SNP genotyping assay ID for the SNP rs11212617 located in the intron region of chromosome 11 was C_1314213_10 (Applied Biosystems, Foster city, CA, USA). The genomic DNA was diluted (50 ng/50 μl) and incubated at 37°C for 24 h before subjecting to real-time polymerase chain reaction (PCR). The PCR reaction was carried out using 15 μl final volumes that contained 7.5 μl of Universal master mix (2X), 0.375 μl of 40X working stock of TaqMan genotyping assay, 3.75 μl of 50 ng genomic DNA diluted in autoclaved DNase free milliQ water and 3.375 μl of deionized water. The thermo cycler conditions were as follows: For 2 min at 50°C; for 10 min at 95°C to activate the polymerase AmpliTaq Gold and then 40 cycles of denaturation for 15 s at 92°C and annealing and extension for 1 min at 60°C.
Statistical analysis
Graph Pad InStat 3.06 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com is used for data analysis. The allele and genotype frequencies of South Indian population were determined by direct gene counting. The observed frequencies were compared with expected frequencies and tested for Hardy-Weinberg equilibrium. Differences in allele and genotype frequencies of South Indian and other ethnic population were measured using Chi-square test and Fischer exact test. P < 0.05 was considered statistically significant.
RESULTS
Around 112 healthy volunteers and 118 type 2 diabetes patients from South Indian states were genotyped for rs11212617 polymorphism in ATM gene. The frequencies were in Hardy Weinberg equilibrium. A allele is the major allele and C allele is the minor allele. AA is the normal genotype, AC is the heterozygous mutant and CC is the homozygous mutant. The allele and genotype frequencies are given in the Table 1. [P <0.05 is considered statistically significant].
Table 1.
Comparison of allele and genotype frequencies of ATM gene polymorphism (rs11212617) in healthy volunteers and diabetic South Indian population
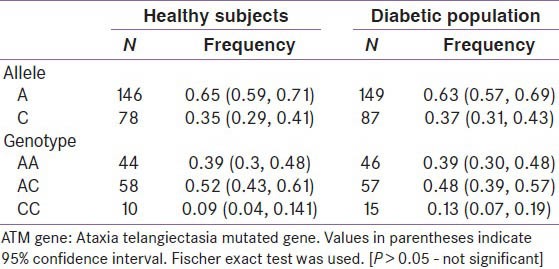
The allele frequencies were compared between the healthy volunteers and diabetic population by Fischer exact test and they were not statistically significant (P > 0.05) [Table 1]. Odds ratio was found to be 1.093 (95% confidence interval-0.7463-1.601).
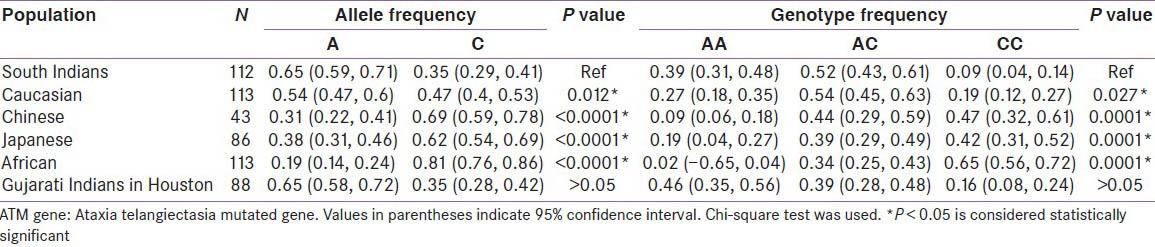
The allele and genotype frequencies of South Indians were compared with allele and genotype frequencies of other ethnicities. All alleles and genotypes were in Hardy Weinberg equilibrium. A allele is the normal allele and C allele is the variant allele. AA is the normal genotype, AC is the heterozygous mutant and CC is the homozygous mutant. The allele and genotype frequencies of different ethnicities are given in the Table [Table 2].
Table 2.
Comparison of frequency of ATM gene variant (rs11212617) in South Indian population with allele and genotype frequencies of other ethnicities
DISCUSSION
In the South Indian population, the frequency of major A allele was 65.2% and the frequency of minor C allele was found to be 35.8%. Though the frequency of C allele is lower than A allele, the minor allele has been implicated to be associated with the treatment success with metformin therapy.[13] This may be because this allele is a dominant allele and the presence of even single allele in heterozygotes can contribute to metformin response. It was observed that the frequency of heterozygotes in healthy subjects of South Indian population is higher (AC genotype 51.8%) than homozygotes and similar frequency trend was also seen in the diabetic cohort (AC genotype 48.4%). This indicates that the same trend can be expected to reflect in the metformin treatment response in our population.
The association of a variant in ATM gene (rs11212617) to metformin response[20,21,22] is a relatively a new concept and is based on stronger evidences given by the reports from the first genome wide association study on metformin response in European diabetic population by the GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group and The Wellcome Trust Case Control Consortium 2 (WTCCC2).[13] In this study, the minor allele frequency was reported to be 44% and this allele was said to be associated with treatment success (HbA1c < 7%) with Odds ratio of 1.64. The study also included two replication cohorts from UKPDS study, which also showed that there is an association of the minor C allele with metformin response with odds ratio of 1.21 (95% CI, 1.05-1.38) and 1.37 (95% CI, 1.10-1.72) respectively. The combined meta-analysis of the three cohorts confirmed this association with odds ratio of 1.35 (95% CI, 1.22-1.49). This association was later replicated in 3 cohorts of population (West-Friesland, Netherlands and UK) by van Leeuwen et al.,[20] which confirmed the association. The authors have concluded that the SNP rs11212617 in ATM gene is the first robustly replicated variant associated with metformin treatment response. In contrary, the Diabetic Prevention Program (DPP) study group[19] (5 US ethnic groups) has reported that there was no association of the minor C allele with metformin response. The authors have concluded that this contradictory result may be because of the ethnic variation and the pre-diabetic cohort that was chosen for the study. The role of ethnic variation is thus strongly confirmed by these studies.
Since ATM gene mutation is linked with ataxia-telangiectasia with manifestations of insulin resistance, there is a possibility that this polymorphism can have a causative role in type 2 diabetes mellitus. The role of the studied SNP rs11212617 in the etiology of diabetes was assessed by comparing the allele and genotype frequencies of healthy volunteers with that of type 2 diabetes patients. The observed frequencies in the diabetic cohort was compared with the expected frequencies and confirmed to be in Hardy-Weinberg equilibrium. The frequency distribution in diabetic cohort is then compared with the allele and genotype distribution in healthy volunteers. There was no statistically significant difference between the two cohorts, which infers that the SNP rs11212617 in ATM gene does not contribute to the causation of type 2 diabetes mellitus.
The allelic distribution of South Indian healthy subjects was found to be significantly different from other ethnicities such as Caucasians (A = 53.3%, C = 46.5%), Chinese (A = 31.4%, C = 68.6%), Japanese (A = 38.4%, C = 61.6%), and Africans (A = 18.6%, C = 81.4%). There was no significant difference in allele and genotype frequency distribution between the South Indians and Gujarati Indians in Houston (P > 0.05). The genotype frequencies of South Indians also differ significantly from all other ethnic populations compared. To the best of our knowledge the frequency distribution of SNP rs11212617 in ATM gene has not been established in South Indian population.
CONCLUSION
Diabetes mellitus is a chronic disorder that is ever growing in prevalence and complications. Considering the widespread use of metformin and the considerable economic burden that each patient has to bear for the lifelong management of diabetes and its complications it becomes very much essential for physicians to individualize the therapy for each patient and to keep a proper watch on the control. Indians being more prone to diabetes, understanding the trends in the frequency distribution of genes affecting the treatment response of one of the most widely prescribed drug becomes highly important.
This study gives the pattern of frequency distribution of the robustly studied variant in ATM gene in the South Indian population. This can form a framework for further studies to find if there is an association of metformin treatment response with the variant in ATM gene. Our study could not find any difference in the frequency distribution of this polymorphism in ATM gene between healthy volunteers and diabetic cohorts.
Footnotes
Source of Support: Intramural funding, JIPMER
Conflict of Interest: None declared.
REFERENCES
- 1.5th ed. Brussels, Belgium: International Diabetes Federation; 2011. [Accessed 2013 May 20]. International Diabetes Federation. IDF Diabetes Atlas. Available from: http://www.idf.org/diabetesatlas . [Google Scholar]
- 2.Global Guideline for Type 2 Diabetes. International Diabetes Federation. 2012. [Accessed 2013 May 20]. Available from: http://www.idf.org/guidelines .
- 3.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florez JC. Does metformin work for everyone? A genome-wide association study for metformin response. Curr Diab Rep. 2011;11:467–9. doi: 10.1007/s11892-011-0220-0. [DOI] [PubMed] [Google Scholar]
- 5.Manolopoulos VG, Ragia G, Tavridou A. Pharmacogenomics of oral antidiabetic medications: Current data and pharmacoepigenomic perspective. Pharmacogenomics. 2011;12:1161–91. doi: 10.2217/pgs.11.65. [DOI] [PubMed] [Google Scholar]
- 6.Zolk O. Current understanding of the pharmacogenomics of metformin. Clin Pharmacol Ther. 2009;86:595–8. doi: 10.1038/clpt.2009.144. [DOI] [PubMed] [Google Scholar]
- 7.Zolk O. Disposition of metformin: Variability due to polymorphisms of organic cation transporters. Ann Med. 2012;44:119–29. doi: 10.3109/07853890.2010.549144. [DOI] [PubMed] [Google Scholar]
- 8.Avery P, Mousa SS, Mousa SA. Pharmacogenomics in type II diabetes mellitus management: Steps toward personalized medicine. Pharmgenomics Pers Med. 2009;2:79–91. doi: 10.2147/pgpm.s5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shikata E, Yamamoto R, Takane H, Shigemasa C, Ikeda T, Otsubo K, et al. Human organic cation transporter (OCT1 and OCT2) gene polymorphisms and therapeutic effects of metformin. J Hum Genet. 2007;52:117–22. doi: 10.1007/s10038-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 10.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–31. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J. 2009;9:242–7. doi: 10.1038/tpj.2009.15. [DOI] [PubMed] [Google Scholar]
- 12.Umamaheswaran G, Praveen RG, Arunkumar AS, Das AK, Shewade DG, Adithan C. Genetic analysis of OCT1 gene polymorphisms in an Indian population. Indian J Hum Genet. 2011;17:164–8. doi: 10.4103/0971-6866.92094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou K, Bellenguez C, Spencer CC, Bennett AJ, et al. GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group, Wellcome Trust Case Control Consortium 2. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117–20. doi: 10.1038/ng.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas LB, Gomes MB. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viollet B, Foretz M. Revisiting the mechanisms of metformin action in the liver. Ann Endocrinol (Paris) 2013;74:123–9. doi: 10.1016/j.ando.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Yee SW, Chen L, Giacomini KM. The role of ATM in response to metformin treatment and activation of AMPK. Nat Genet. 2012;44:359–60. doi: 10.1038/ng.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: A translational research approach to understanding a potential new therapeutic target. Diabet Med. 2010;27:1097–106. doi: 10.1111/j.1464-5491.2010.03098.x. [DOI] [PubMed] [Google Scholar]
- 19.Florez JC, Jablonski KA, Taylor A, Mather K, Horton E, White NH, et al. The C allele of ATM rs11212617 does not associate with metformin response in the diabetes prevention program. Diabetes Care. 2012;35:1864–7. doi: 10.2337/dc11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen N, Nijpels G, Becker ML, Deshmukh H, Zhou K, Stricker BH, et al. A gene variant near ATM is significantly associated with metformin treatment response in type 2 diabetes: A replication and meta-analysis of five cohorts. Diabetologia. 2012;55:1971–7. doi: 10.1007/s00125-012-2537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glazer NL. Variation in the ATM gene may alter glycemic response to metformin. Circ Cardiovasc Genet. 2011;4:210–1. doi: 10.1161/CIRCGENETICS.111.960047. [DOI] [PubMed] [Google Scholar]
- 22.Tkáč I. Replication of the association of gene variant near ATM and response to metformin. Pharmacogenomics. 2012;13:1331–2. [PubMed] [Google Scholar]


