Abstract
Background:
Insulinomas are usually solitary; benign and encapsulated small lesions and majority of them measure <2 cm in diameter. They pose a challenge for pre-operative localization. Definitive treatment is surgical excision of the tumor. Intra-operative ultrasonography (IOUS), transhepatic portal venous sampling (THPVS) and positron emission tomography (PET) scan can be done for tumors not localized by conventional imaging modalities.
Materials and Methods:
A retrospective study of patients diagnosed with insulinoma during the period 2004-2012 (8 years) was done. Biochemical diagnostic criteria used were plasma concentrations of glucose <55 mg/dl with corresponding insulin level >3.0 μU/ml (18 pmol/L) and C-peptide of >0.6 ng/ml (0.2 nmol/L). The localization of the tumor was done by various modalities namely computed tomography (CT), magnetic resonance imaging (MRI), IOUS, PET and portal venous sampling. The initial localizing technique in most of these patients were CT or MRI imaging, or both and those who were not localized by the above modalities were subjected to PET CT or THPVS or intra-operative ultrasound depending on the initial imaging results and patient's consent. All the modalities were not used in the same patient, but the modalities were decided as per the imaging results, patient's consent and affordability for the procedure.
Results:
Ninteen cases of insulinoma aged between 10 and 66 years, with a median age of 47 years were included in the analysis. There were 10 males and nine females. Eighty-three percent of patients presented with pre-prandial hypoglycemia (n = 15). Different modalities were employed for pre-operative localization of these patients out of which 5 (26.31%) cases were localized with CT, 5 (26.31) cases with MRI, 5 (26.31%) with THPVS, 1 (5.26%) case with PET CT, 3 (15.78%) of them could not be localized out of which 2 (10.52%) were localized by IOUS and 1 (5.26%) case the lesion could not be localized. Among 19 cases, 12 underwent surgery out of which one patient underwent distal pancreatectomy as tumor was not localized; eight underwent laparoscopic enucleation; three of them required intra-operative exploration and seven of them were not operated, as they did not give consent for surgery. In all the cases, the size of the insulinoma ranged between 1 and 2 cm.
Conclusion:
We report our experience with 19 cases of insulinoma and analyze the role of pre- and intra-operative imaging modalities in the surgical management of insulinomas. Most of our cases were symptomatic, and the most common presentation was with pre-prandial hypoglycemia. THPVS, PET scan and intra-operative ultrasound added to diagnostic sensitivity in some cases not localized by CT or MRI.
Keywords: Hypoglycemia, insulinoma, transhepatic portal venous sampling
INTRODUCTION
Insulinomas present with repeated episodes of hypoglycemia due to endogenous hyperinsulinemia, which occurs mostly in the fasting state but may sometimes occur in the post-prandial period. These are rare tumors with an incidence of 1 in 250,000 patient-years.[1] Sporadic insulinomas are usually solitary, benign and encapsulated small lesions. Ninety percent of them measure <2 cm and with 30% being <1 cm in diameter. Ten percent are multiple, 10% are malignant[2] and 10% are associated with multiple endocrine neoplasia type 1 (MEN-1).[3] Surgical excision is the definitive treatment. Majority of them become symptom-free, there is a recurrence rate of about 7% insulinomas without MEN-1, and 21% in those with MEN-1.[1] Eighty percent of sporadic insulinomas are small and intra- pancreatic making pre-operative localization unsuccessful in 10% to 27%.[4] The present study is a retrospective analysis of insulinoma cases admitted to our center, and our experience with pancreatic venous sampling.
MATERIALS AND METHODS
A retrospective analysis of cases of insulinoma admitted in our hospital during the period of 2004-2012 was done. The variables such as age, sex, type of symptoms, clinical diagnosis, bio-chemical investigations, various invasive and non-invasive imaging modalities used to localize the tumor were obtained from the records. Bio-chemical diagnostic criteria for insulinoma used were plasma concentrations of glucose <55 mg/dl, insulin of at least 3.0 μU/ml (18 pmol/L) and C-peptide of at least 0.6 ng/ml (0.2 nmol/L).[5] 72 h fasting test was done if they did not have spontaneous hypoglycemia. All the cases of insulinoma were screened for MEN syndrome after the diagnosis of insulinoma was confirmed. The sulfonylurea screen was also done. The techniques used to localize the tumor were computed tomography (CT) scan, magnetic resonance imaging (MRI), intra-operative ultrasonography (IOUS), positron emission tomography CT (PET-CT) and transhepatic portal venous sampling (THPVS).
Nineteen cases were diagnosed with insulinoma, and all had negative screen for sulfonylurea. Out of these four had to undergo a 72 h fast for diagnosis, while the rest had spontaneous hypoglycemia. All the patients were subjected to a CT scan or MRI. Eleven underwent MRI and nine underwent CT as initial imaging modality for localization, and those who were not localized with CT/MRI or both were planned for THPVS after taking consent from the patient as it is an invasive procedure, or they were planned for PET CT scan as per the imaging results on CT or MRI. The THPVS was performed by percutaneous transhepatic catheterization of splenic, superior mesenteric, inferior mesenteric and pancreaticoduodenal veins.[6] Blood samples were taken from at least 18 to 20 sites according to portal venous chart [Figure 1] for insulin estimation and depending on the regional venous gradient of the insulin levels, insulinomas were localized.
Figure 1.
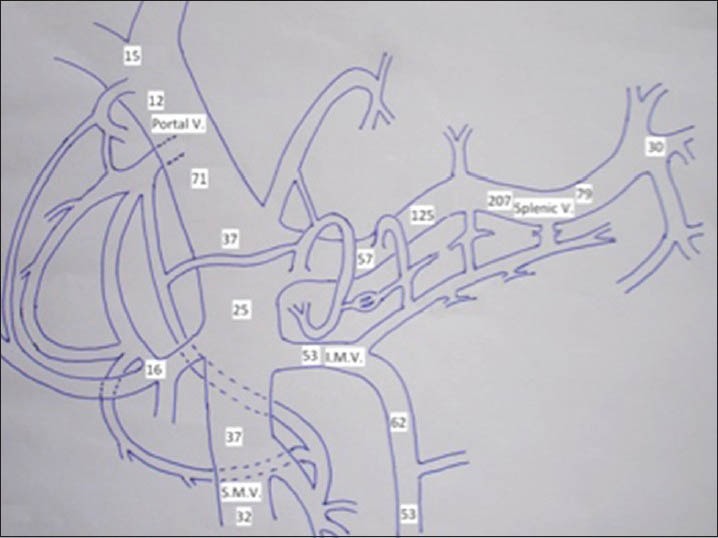
Portal venous chart: Insulinoma in the body of pancreas with high insulin gradient in the region of veins draining the body of pancreas
RESULTS
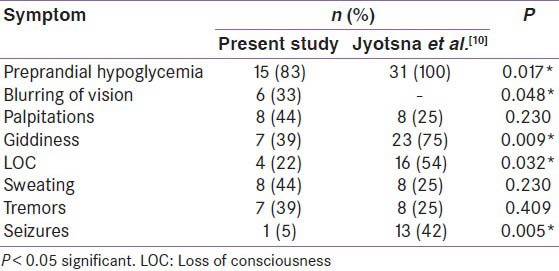
The study cohort comprised of 19 patients of insulinoma, which included 10 male and 9 female patients with a median age of 47 years (10-66 years). The commonest clinical presentation in all these patients was with pre-prandial hypoglycemia (83%, n = 15), [Table 1]. Four of these patients were subjected to 72 h fast. The symptom profile is given in Table 1. Hundred percent of these patients had hypoglycemia within the first 24 h. The range of plasma glucose during hypoglycemia was between 25 and 42 mg/dl. The blood samples of these patients obtained during episodes of hypoglycemia for insulin and C-peptide levels were diagnostic of hyperinsulinemic hypoglycemia. Insulin levels ranged from 60 to 500 μU/ml and C-peptide levels ranged from 3 to 15 ng/ml. The average duration of symptoms was approximately 1.5 years. One of the patients had MEN-1 in whom CT showed four hypodense lesions in the region of the neck of the pancreas measuring 15 × 16 mm, and another lesion was in the uncinate process measuring 15 × 16 mm. This patient also had hyperparathyroidism and prolactinoma.
Table 1.
Presenting complaints of insulinoma patients at a tertiary hospital during 2004-2011
Computed tomography, and MRI, localized 5 cases (26.31%) each. PET CT was used in one case (5.26%) as the CT and MRI did not show any lesion. Five patients had to undergo THPVS (26.31%) A total of three cases (15.78%) could not be localized with routine imaging but were clinically and bio-chemically hyperinsulinemic, and two of these cases (10.52%) were localized intra-operatively by palpation and ultrasonography. The lesions were located in the body and tail. One case remained non-localized underwent distal pancreatectomy [Figure 2].
Figure 2.
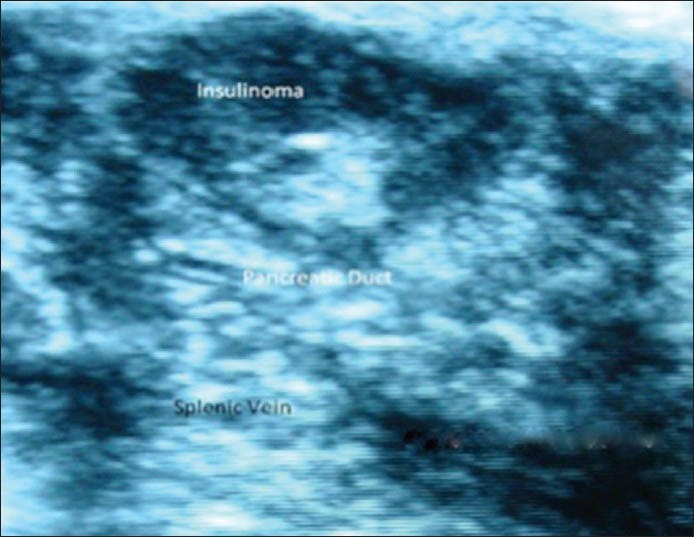
Intraoperative ultrasonography: Insulinoma at the junction of head and body of pancreas close to pancreatic duct
Surgery was advised in all 19 cases, but only 12 gave consent. Out of the 12 operated cases, eight underwent laparoscopic enucleation, and three underwent exploration, as they were not localized pre-operatively. Out of these three cases two cases were enucleated, and one patient had to undergo distal pancreatectomy, as it remained non-localized even with IOUS. The size of the tumor in these cases ranged from 1 to 2 cm and the largest was 2 cm. Histopathology was consistent with islet cell tumor in 11 patients. On the follow-up for 8 years out of 12 operated cases 10 are asymptomatic, and two patients had recurrence of symptoms and were found to be biochemically hyperinsulinemic but as they were not willing for repeat surgery, they were started on diazoxide. Six cases were not operated and they were lost to follow-up.
DISCUSSION
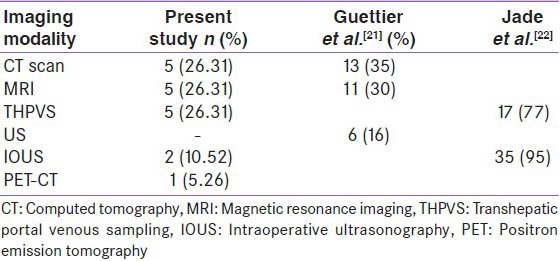
In a non-diabetic individual with fasting hypoglycemia, insulinoma should be considered in the differential diagnosis. Clinical presentation can vary, they may present with adrenergic symptoms like palpitations, tremor, anxiety, warmth and dry mouth, cholinergic symptoms comprising of hunger, sweating and paresthesias.[7] Neuroglycopenic symptoms start as a blood glucose falls to approximately 50-55 mg/dL (2.8-3.1 mmol/L), which include slurred speech, blurred vision, confusion, drowsiness and difficulty in concentrating. A strong clinical suspicion to diagnose an insulinoma with varied clinical presentation is needed. These patients tend to eat more to avoid symptoms of hypoglycemia and thus they become overweight and obese. Prolonged hypoglycemia may also be associated with symptoms and signs of neuropathy. In cases with milder symptoms or in patients in whom hypoglycemia is not documented, a 72 h prolonged fasting is advocated to induce hypoglycaemia.[8,9] In our series four cases underwent prolonged fasting and developed hypoglycemia within 24 h, compared to another series in which during 72 h prolonged fast all cases attained hypoglycemia within 48 h.[10] Our experience in localizing insulinomas with different imaging modalities is similar to other series.[11,12,13,14,15,16] In a series of 31 insulinoma patients, the symptom profile of patients was similar to our cases.[10] All our patients presented with pre-prandial hypoglycemia (100%), the other symptoms included were, palpitations (25%), tremors (25%), and loss of consciousness (54%). Pre-operative localization in the present series was successful in 80% of cases, different modalities were employed for pre-operative localization [Table 2]; 5 (26.31%) cases were localized with CT, 5 cases (26.31%) with MRI, 5 (26.31%) with portal venous sampling (THPVS), 1 (5.26%) case with PET CT, 3 (15.78%) of them could not be localized. Among those 3 cases 2 (10.52) were localized by intra-operative palpation and ultrasonography [Figure 1] and 1 (5.26%) case was not localized. Thus, more percentage of cases were localized preoperatively in our series as compared to the data by Jyotsna et al., where preoperative localization of insulinoma was successful in 71% with various imaging modalities, with CT angiography, 83%, intra-arterial digital subtraction angiography, 65%, dual-phase CT, 57%, and conventional MRI in 31% and intra-operative palpation and ultrasonography localized 76% and 92%, respectively.[10] In Kaczirek et al. series, insulinoma was localized by conventional CT in 33%, single-slice helical CT in 58%, multi-detector CT in 100%, MRI in 85% and invasive angiography in 65%[8] and with a combination of different modalities, the sensitivity rate was up to 88%. When conventional imaging modalities cannot localize, invasive procedure can be utilized. The sensitivity of THPVS reported in the literature is 77-100%.[17,18,19] In our experience with five cases, the sensitivity of THPVS was 100%. In one of the series pre-operative localization with THPVS was 77% with no false positive results.[14] THPVS needs special skills and experience, so it's not a widely used procedure and it also associated with morbidity. When invasive procedures fail to localize the lesion IOUS can be used as a diagnostic modality. In few series IOUS could localize insulinoma in 86% of cases.[19,20] Somatostatin (SST) labeled analogues are also being increasingly used in the diagnosis and therapy for neuroendocrine tumors expressing SST receptors (SSTR) on their surface. Indium-111-octreotide is used for imaging neuroendocrine tumors. SSTR scintigraphy is based on the expression of SSTR. One-third of insulinomas do not express SSTR2 and SSTR5.[23] Scintigraphy has low sensitivity (20-50% NETs) in localizing small tumors lacking SSTR.[24] Newer compounds gallium-68-DOTA (68Ga-DOTA) have improved PET resolution and newer peptides have high affinity for SSTR with improved sensitivity. Modifications of octreopeptide molecule give important SSTR analogs like TOC, NOC and TATE. Gallium DOTA has three components, Ga68 is a radioisotope, DOTA is chelate and TOC, NOC and TATE are peptides that bind to SSTR, these peptides differ in their binding ability to subtypes of SST.[25] All these peptides can bind SSTR2; DOTA-NOC also binds to SSTR3 and SSTR5 with greater affinity. 68Ga-DOTA-TATE has predominant affinity for SSTR2.[26] Gallium-68-DOTA-TOC sensitivity is about 97%.[27] One case in the present series that was not localized with MRI/CT was localized with Ga68-DOTA-NOC. In our experience, IOUS succeeded in localizing insulinoma in 66.67% of the cases. Intra-operative ultrasound helps in avoiding blind surgical procedures and may aid to identify any additional lesions. As majority of sporadic insulinomas are small and intra-pancreatic, even with all available imaging modalities it may not be localized in 10-27% of cases.[4]
Table 2.
Various modalities used for successful preoperative localization techniques
CONCLUSION
In a non-diabetic patient with hypoglycemia, the diagnosis of insulinoma should be kept in mind. The commonest presentation in our series was pre-prandial hypoglycemia. Transhepatic portal venous sampling or PET CT scan are important diagnostic modalities in patients not localized with the conventional diagnostic techniques of CT and MRI. The use of invasive procedure like THPVS should be considered in centers where PET CT may not be available or affordable. IOUS should be performed wherever feasible as it gives extra information about any additional lesion. A proper diagnosis and management can have a great impact on outcome and survival so it is important to address this.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Functioning insulinoma – incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin Proc. 1991;66:711–9. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 2.Klöppel G, Heitz PU. Pancreatic endocrine tumors. Pathol Res Pract. 1988;183:155–68. doi: 10.1016/S0344-0338(88)80043-8. [DOI] [PubMed] [Google Scholar]
- 3.Callender GG, Rich TA, Perrier ND. Multiple endocrine neoplasia syndromes. Surg Clin North Am. 2008;88:863–95. doi: 10.1016/j.suc.2008.05.001. viii. [DOI] [PubMed] [Google Scholar]
- 4.Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–98. doi: 10.1016/j.bpg.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Tseng HC, Yao CZ, Zhong SX, Zhang JX, Zhu Y. Percutaneous transhepatic portal vein catheterization for localization of insulinoma. World J Surg. 1984;8:575–82. doi: 10.1007/BF01654940. [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and management of adult hypoglycemic disorders: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:709–28. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 7.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes. 1993;42:1233–7. doi: 10.2337/diab.42.9.1233. [DOI] [PubMed] [Google Scholar]
- 8.Czupryniak L, Strzelczyk J, Drzewoski J. Diagnostic difficulties in long-standing insulinoma with near-normal plasma insulin levels. J Endocrinol Invest. 2005;28:170–4. doi: 10.1007/BF03345362. [DOI] [PubMed] [Google Scholar]
- 9.Wiesli P, Brändle M, Zapf J, Seiler H, Zwimpfer C, Spinas GA, et al. Assessment of hyperinsulinaemia at the termination of the prolonged fast. Clin Chim Acta. 2004;342:227–31. doi: 10.1016/j.cccn.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Jyotsna VP, Rangel N, Pal S, Seith A, Sahni P, Ammini AC. Insulinoma: Diagnosis and surgical treatment. Retrospective analysis of 31 cases. Indian J Gastroenterol. 2006;25:244–7. [PubMed] [Google Scholar]
- 11.Kaczirek K, Ba-Ssalamah A, Schima W, Niederle B. The importance of preoperative localisation procedures in organic hyperinsulinism – experience in 67 patients. Wien Klin Wochenschr. 2004;116:373–8. doi: 10.1007/BF03040916. [DOI] [PubMed] [Google Scholar]
- 12.Gritzmann N, Macheiner P, Hollerweger A, Hübner E. CT in the differentiation of pancreatic neoplasms – progress report. Dig Dis. 2004;22:6–17. doi: 10.1159/000078730. [DOI] [PubMed] [Google Scholar]
- 13.Akerström G, Hellman P, Hessman O, Osmak L. Surgical treatment of endocrine pancreatic tumours. Neuroendocrinology. 2004;80(Suppl 1):62–6. doi: 10.1159/000080744. [DOI] [PubMed] [Google Scholar]
- 14.De Herder WW. Insulinoma. Surgical treatment of endocrine pancreatic tumours. Neuroendocrinology. 2004;80(Suppl 1):20–2. doi: 10.1159/000080744. [DOI] [PubMed] [Google Scholar]
- 15.Vázquez Quintana E. The surgical management of insulinoma. Bol Asoc Med P R. 2004;96:33–8. [PubMed] [Google Scholar]
- 16.Lairmore TC, Moley JF. Endocrine pancreatic tumors. Scand J Surg. 2004;93:311–5. doi: 10.1177/145749690409300410. [DOI] [PubMed] [Google Scholar]
- 17.Doherty GM, Doppman JL, Shawker TH, Miller DL, Eastman RC, Gorden P, et al. Results of a prospective strategy to diagnose, localize, and resect insulinomas. Surgery. 1991;110:989–96. [PubMed] [Google Scholar]
- 18.Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved contemporary surgical management of insulinomas: A 25-year experience at the Massachusetts general hospital. Ann Surg. 2008;247:165–72. doi: 10.1097/SLA.0b013e31815792ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grover AC, Skarulis M, Alexander HR, Pingpank JF, Javor ED, Chang R, et al. A prospective evaluation of laparoscopic exploration with intraoperative ultrasound as a technique for localizing sporadic insulinomas. Surgery. 2005;138:1003–8. doi: 10.1016/j.surg.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Brown CK, Bartlett DL, Doppman JL, Gorden P, Libutti SK, Fraker DL, et al. Intraarterial calcium stimulation and intraoperative ultrasonography in the localization and resection of insulinomas. Surgery. 1997;122:1189–93. doi: 10.1016/s0039-6060(97)90226-9. [DOI] [PubMed] [Google Scholar]
- 21.Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: The NIH experience. J Clin Endocrinol Metab. 2009;94:1074–80. doi: 10.1210/jc.2008-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiramoto JS, Feldstein VA, LaBerge JM, Norton JA. Intraoperative ultrasound and preoperative localization detects all occult insulinomas. Arch Surg. 2001;136:1020–5. doi: 10.1001/archsurg.136.9.1020. [DOI] [PubMed] [Google Scholar]
- 23.Virgolini I, Traub-Weidinger T, Decristoforo C. Nuclear medicine in the detection and management of pancreatic islet-cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:213–27. doi: 10.1016/j.beem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC, Schaer JC, Waser B, Mengod G. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54:3455–9. [PubMed] [Google Scholar]
- 25.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–93. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 26.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–47. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]


