Abstract
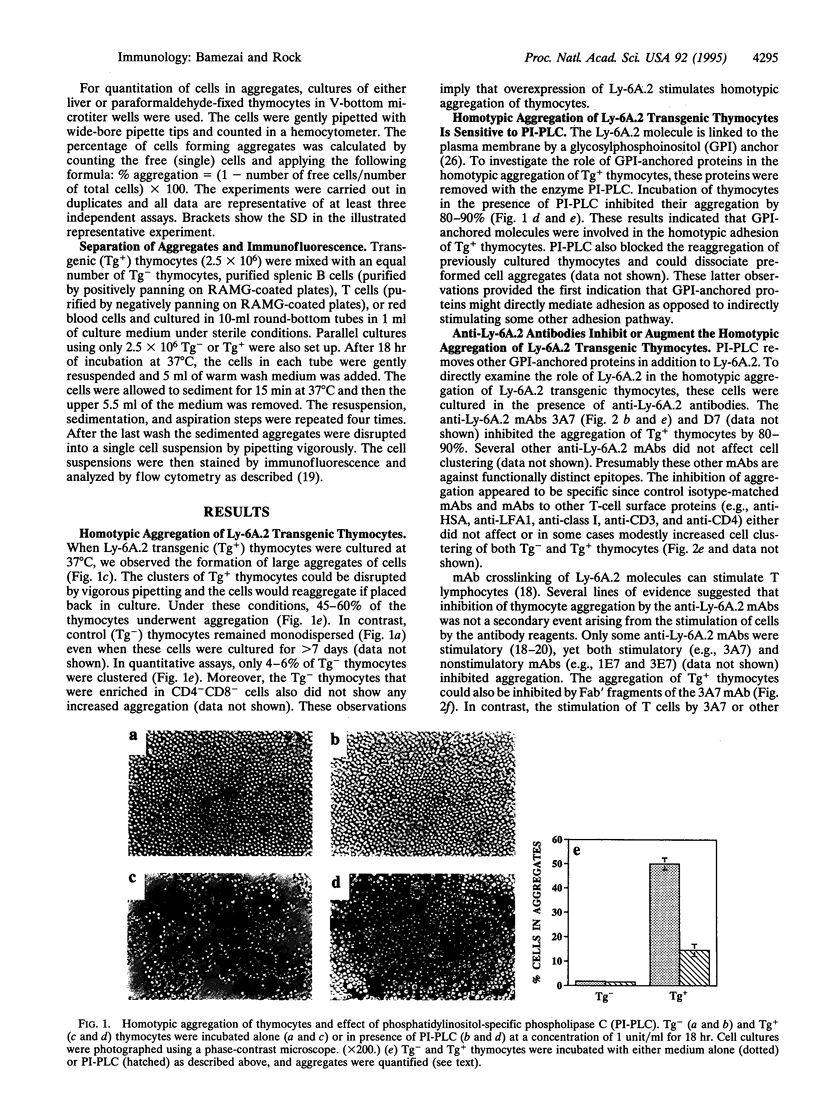
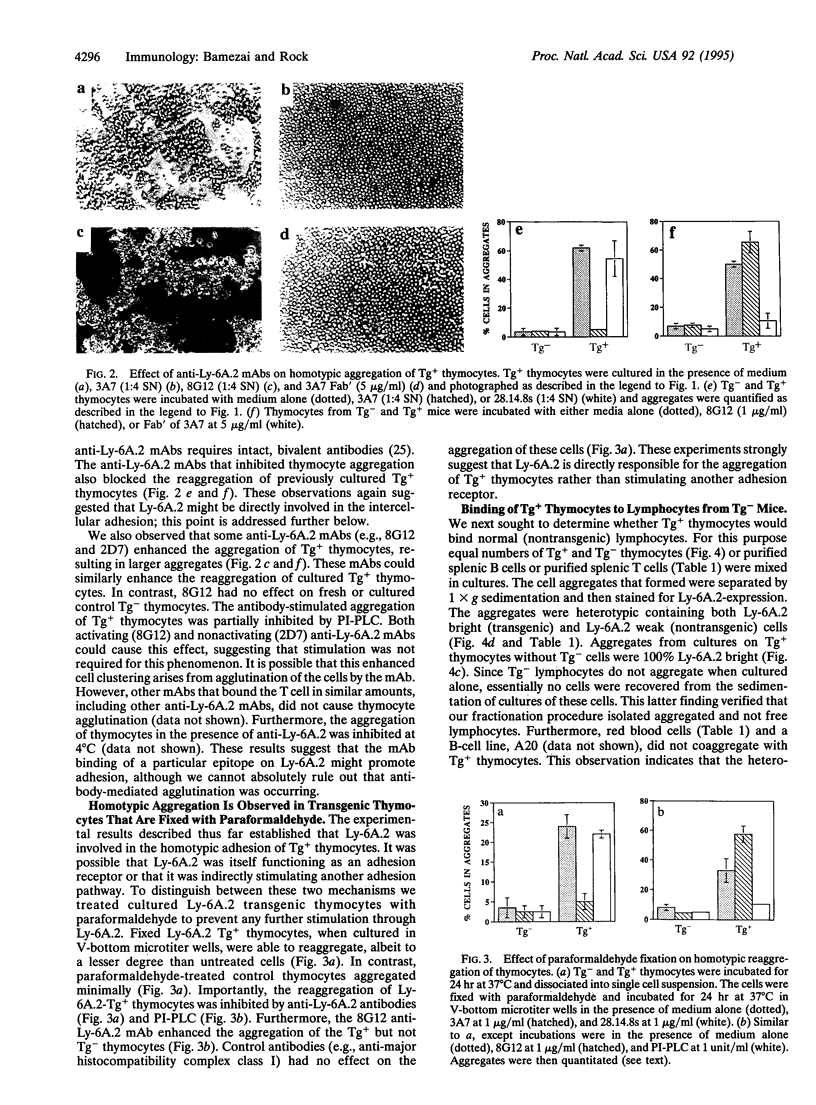
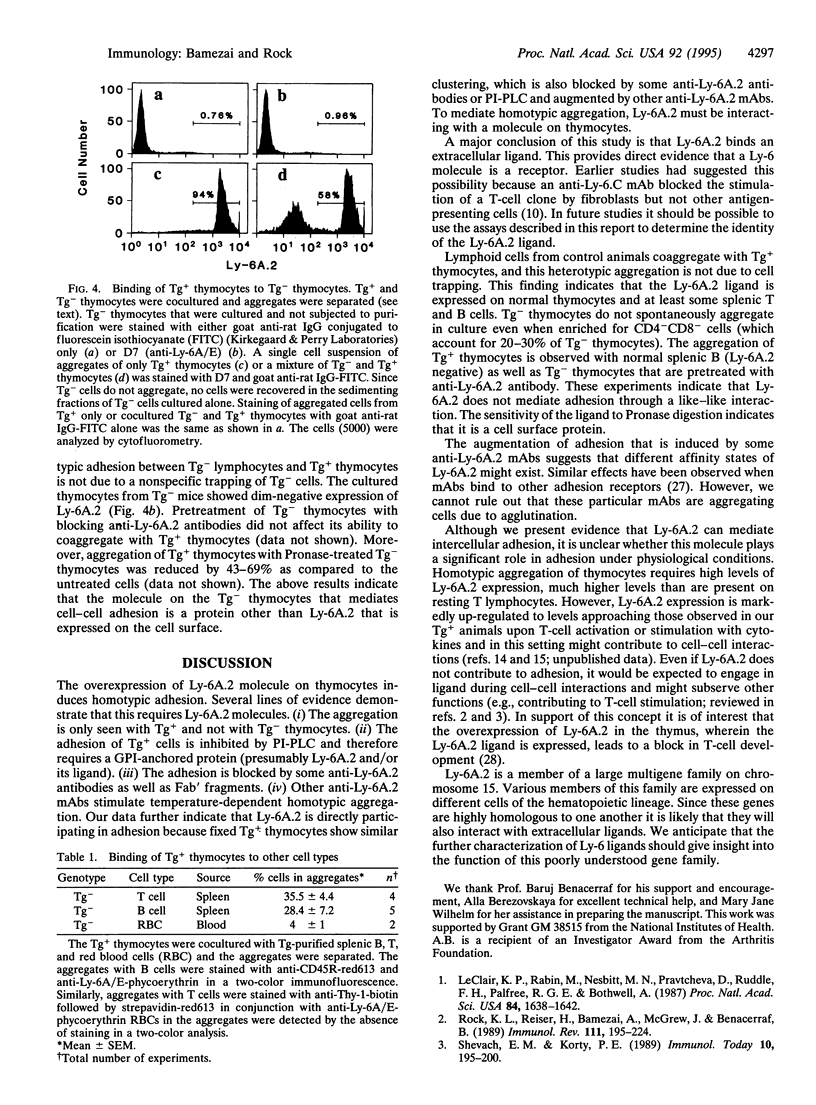
The Ly-6 locus encodes several cell surface proteins whose functions are unknown. Although it is hypothesized that these proteins may be receptors, there is no direct evidence that they bind a ligand. Herein we present evidence that Ly-6A.2, a Ly-6 protein expressed on T lymphocytes, binds a ligand expressed on normal thymocytes and splenic B and T cells. We find that transgenic thymocytes that overexpress Ly-6A.2 spontaneously aggregate in culture. This homotypic adhesion requires the overexpression of Ly-6A.2 because it is not observed in cultures of nontransgenic thymocytes. The aggregation of Ly-6A.2 transgenic thymocytes is inhibited by phosphatidylinositol-specific phospholipase C (which removes Ly-6A.2 and other glycosylphosphatidylinositol-anchored proteins from the membrane). Some anti-Ly-6A.2 monoclonal antibodies, including nonactivating ones and Fab' fragments, inhibit this aggregation. In contrast, other anti-Ly-6A.2 monoclonal antibodies increase the aggregation of transgenic but not nontransgenic thymocytes. To further examine whether Ly-6A.2 mediates adhesion (versus inducing another adhesion pathway) reaggregation assays were performed with paraformaldehyde-fixed Tg+ thymocytes. Paraformaldehyde-fixed Tg+ thymocytes reaggregate in culture and this aggregation is also blocked by phosphatidyl-inositol-specific phospholipase C and anti-Ly-6A.2 monoclonal antibodies. These results indicate that the homotypic adhesion of cultured Ly-6A.2 transgenic thymocytes is directly mediated by Ly-6A.2 and, more importantly, strongly suggests that Ly-6A.2 binds a ligand that is expressed on thymocytes. Tg+ thymocytes also bind to nontransgenic thymocytes, B cells, and T cells, indicating that normal cells naturally express the Ly-6A.2 ligand.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamezai A., Goldmacher V., Reiser H., Rock K. L. Internalization of phosphatidylinositol-anchored lymphocyte proteins. I. Documentation and potential significance for T cell stimulation. J Immunol. 1989 Nov 15;143(10):3107–3116. [PubMed] [Google Scholar]
- Bamezai A., Reiser H., Rock K. L. T cell receptor/CD3 negative variants are unresponsive to stimulation through the Ly-6 encoded molecule, TAP. J Immunol. 1988 Sep 1;141(5):1423–1428. [PubMed] [Google Scholar]
- Dumont F. J., Boltz R. C. The augmentation of surface Ly-6A/E molecules in activated T cells is mediated by endogenous interferon-gamma. J Immunol. 1987 Dec 15;139(12):4088–4095. [PubMed] [Google Scholar]
- Dumont F. J., Dijkmans R., Palfree R. G., Boltz R. D., Coker L. Selective up-regulation by interferon-gamma of surface molecules of the Ly-6 complex in resting T cells: the Ly-6A/E and TAP antigens are preferentially enhanced. Eur J Immunol. 1987 Aug;17(8):1183–1191. doi: 10.1002/eji.1830170816. [DOI] [PubMed] [Google Scholar]
- Eckhardt L. A., Herzenberg L. A. Monoclonal antibodies to ThB detect close linkage of Ly-6 and a gene regulating ThB expression. Immunogenetics. 1980;11(3):275–291. doi: 10.1007/BF01567794. [DOI] [PubMed] [Google Scholar]
- Engel P., Nojima Y., Rothstein D., Zhou L. J., Wilson G. L., Kehrl J. H., Tedder T. F. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993 Jun 1;150(11):4719–4732. [PubMed] [Google Scholar]
- Fleming T. J., Fleming M. L., Malek T. R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993 Sep 1;151(5):2399–2408. [PubMed] [Google Scholar]
- Flood P. M., Dougherty J. P., Ron Y. Inhibition of Ly-6A antigen expression prevents T cell activation. J Exp Med. 1990 Jul 1;172(1):115–120. doi: 10.1084/jem.172.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Lancki D. W., Fitch F. W. Accessory molecules involved in antigen-mediated cytolysis and lymphokine production by cytotoxic T lymphocyte subsets. I. Identification of functions for the T cell surface molecules Ly-6C and Thy-1. J Immunol. 1993 Sep 15;151(6):2986–2999. [PubMed] [Google Scholar]
- Kimura S., Tada N., Liu-Lam Y., Hämmerling U. Studies of the mouse Ly-6 alloantigen system. II. Complexities of the Ly-6 region. Immunogenetics. 1984;20(1):47–56. doi: 10.1007/BF00373446. [DOI] [PubMed] [Google Scholar]
- Lancki D. W., Ma D. I., Havran W. L., Fitch F. W. Cell surface structures involved in T cell activation. Immunol Rev. 1984 Oct;81:65–94. doi: 10.1111/j.1600-065x.1984.tb01105.x. [DOI] [PubMed] [Google Scholar]
- LeClair K. P., Rabin M., Nesbitt M. N., Pravtcheva D., Ruddle F. H., Palfree R. G., Bothwell A. Murine Ly-6 multigene family is located on chromosome 15. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1638–1642. doi: 10.1073/pnas.84.6.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Segal D. M., Shevach E., Bluestone J. A. Activation of murine T lymphocytes with monoclonal antibodies: detection on Lyt-2+ cells of an antigen not associated with the T cell receptor complex but involved in T cell activation. J Immunol. 1987 Aug 15;139(4):1214–1222. [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Reiser H., Bamezai A., McGrew J., Benacerraf B. The LY-6 locus: a multigene family encoding phosphatidylinositol-anchored membrane proteins concerned with T-cell activation. Immunol Rev. 1989 Oct;111:195–224. doi: 10.1111/j.1600-065x.1989.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Korty P. E. Ly-6: a multigene family in search of a function. Immunol Today. 1989 Jun;10(6):195–200. doi: 10.1016/0167-5699(89)90324-1. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Springer T. A., Mescher M. F. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J Immunol. 1981 Sep;127(3):923–930. [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Bamezai A., Rock K. L. TAP transcription and phosphatidylinositol linkage mutants are defective in activation through the T cell receptor. Cell. 1988 Mar 11;52(5):665–674. doi: 10.1016/0092-8674(88)90404-7. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Benacerraf B., Rock K. L. The expression, function, and ontogeny of a novel T cell-activating protein, TAP, in the thymus. J Immunol. 1986 Aug 15;137(4):1232–1238. [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Benacerraf B., Rock K. L. The expression, function, and ontogeny of a novel T cell-activating protein, TAP, in the thymus. J Immunol. 1986 Aug 15;137(4):1232–1238. [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Daley J., Rock K. L. Stimulation of T cells via the TAP molecule, a member in a family of activating proteins encoded in the Ly-6 locus. J Immunol. 1987 Jan 1;138(1):91–97. [PubMed] [Google Scholar]





