Abstract
Background:
Drug-eluting stents have been used in daily practice since 2002, with the clear advantages of reducing the risk of target vessel revascularization and an impressive reduction in restenosis rate by 50%-70%. However, the occurrence of a late thrombosis can compromise long-term results, particularly if the risks of this event were sustained. In this context, a registry of clinical cases gains special value.
Objective:
To evaluate the efficacy and safety of drug-eluting stents in the real world.
Methods:
We report on the clinical findings and 8-year follow-up parameters of all patients that underwent percutaneous coronary intervention with a drug-eluting stent from January 2002 to April 2007. Drug-eluting stents were used in accordance with the clinical and interventional cardiologist decision and availability of the stent.
Results:
A total of 611 patients were included, and clinical follow-up of up to 8 years was obtained for 96.2% of the patients. Total mortality was 8.7% and nonfatal infarctions occurred in 4.3% of the cases. Target vessel revascularization occurred in 12.4% of the cases, and target lesion revascularization occurred in 8% of the cases. The rate of stent thrombosis was 2.1%. There were no new episodes of stent thrombosis after the fifth year of follow-up. Comparative subanalysis showed no outcome differences between the different types of stents used, including Cypher®, Taxus®, and Endeavor®.
Conclusion:
These findings indicate that drug-eluting stents remain safe and effective at very long-term follow-up. Patients in the "real world" may benefit from drug-eluting stenting with excellent, long-term results.
Keywords: Drug-Eluting Stents/trends, Treatment Outcome, Effectiveness, Long-Term Effect
Introduction
Drug-eluting stents (DES) have been used in clinical practice since 2002. Multicenter clinical trials have clearly demonstrated the advantages of these stents in reducing major cardiovascular outcomes, particularly target vessel revascularization (TVR), compared with bare metal stents1. Randomized clinical trials have not included DES, despite their increased use; thus, their efficacy and safety in the real world has been questioned. Considerations regarding DES safety have increased since 2006, when preliminary data indicated higher rates of in-stent thrombosis with DES compared with bare metal stents2-5. Despite the widespread use of DES in subsequent years, there is still a lack of long-term studies of patients who have received these devices.
A patient registry was created within this scenario. All the patients from two Brazilian institutions who received DES between 2002 and 2007, often with off-label indications, were clinically followed up for 8 years. Patient outcomes were analyzed based on the current definitions, and the efficacy and safety of this technology were assessed.
Methods
Population
This study included all patients who underwent percutaneous coronary intervention using at least 1 DES (Costar®, Cypher®, Endeavor®, Infinnium®, Janus®, Supralimus®, and Taxus®) from January 2002 to April 2007 at the São Lucas and Mãe de Deus hospitals in Porto Alegre (RS). Every patient that presented with acute coronary syndrome and stable angina, with or without ST-segment elevation, was included. The type of DES used during the procedure was left to the discretion of the interventional cardiologist. Given the predominant use of the Cypher®, Endeavor®, and Taxus® stents, a sub-analysis comparing the performance of these stents was conducted.
Definitions and clinical follow-up
Data regarding the patients' clinical presentations at the time of the procedure were collected through a detailed review of medical records. The patient groups were defined as follows: stable angina, unstable angina, nonST elevation myocardial infarction, ST segment elevation, and recent myocardial infarction (MI) (<3 months before the procedure). Data regarding the procedure and in-hospital outcomes were prospectively collected. Chronic renal failure was defined as a glomerular filtration rate (GFR) of <60 ml/min/1.73 m26.
Clinical outcomes were defined as follows: mortality due to any cause; nonfatal MI; CK-MB increase greater than or equal to three times the upper normal limit, and/or electrocardiographic changes compatible with infarction (i.e., ST-segment elevation or new inactive zone); or in patients who underwent coronary artery bypass graft surgery, CK-MB increase greater than or equal to five times the upper normal limit; target lesion revascularization (TLR) (i.e., percutaneous or surgical revascularization to treat lesions in the segment of the stent or 5 mm proximal or distal to the prior implant); and TVR (i.e., any revascularization of the vessel treated with DES in the index procedure).
Stent thrombosis was classified according to the definition given by the Academic Research Consortium (ARC) as follows: defined (i.e., acute coronary syndrome with visualization of a thrombus in the segment where the DES was deployed), probable (i.e., unexplained death within 30 days or target vessel infarction), and possible (i.e., any unexplained death after 30 days). Based on the time of occurrence, stent thrombosis was defined as follows: acute (i.e., within the first 24 h), subacute (i.e., within 30 days), late (i.e., after 30 days), and very late (i.e., after 1 year)7.
Total mortality, nonfatal MI, and TVR that occurred during the follow-up period were defined as major adverse cardiac events (MACEs).
Angiographic success was defined as stenosis < 20% and thrombolysis in myocardial infarction (TIMI) flow grade 3 by the end of the procedure. Clinical success was defined as angiographic success and the absence of clinical complications such as death, MI, urgent revascularization, and stroke during the index hospitalization.
The first intervention was considered the index procedure for patients with more than one intervention during the study period.
Clinical follow-up was conducted through medical appointments, phone interviews with the patient, reviews of outpatient and in-hospital medical records, and contact with the attending physician. All the clinical events were adjudicated by analysis of the documentation's sources by a cardiologist who was blinded to the other clinical data. The first clinical follow-up was performed 12 months after the index procedure and a biannual clinical follow-up was performed thereafter.
Quantitative coronary angiographic analysis
An experienced interventional cardiologist analyzed the baseline and post-procedure coronary angiograms. Offline quantitative coronary angiography of the index intervention was performed using a guiding catheter for calibration of the image magnification (CardioNow Websend DICOM Study Sharing Software, HeartLab, Inc., Westerly, Rhode Island). The minimal luminal diameter and the reference vessel diameter were measured, both before and after the intervention, from a single shot showing the smaller luminal diameter. Coronary lesions were classified according to the American Heart Association and the American College of Cardiology (AHA / ACC) guidelines8.
Statistical Analysis
Statistical analysis was performed using the SPSS 19.0 software, assuming a significance level of 5%. Quantitative variables were expressed as a mean ± standard deviation. Categorical variables were presented as absolute and relative frequencies and were compared by the chi-square test or Fisher's exact test, as appropriate. Adjusted residuals greater than 1.96 (α = 0.05) were considered statistically significant, thereby indicating a positive association between the categories. Kaplan-Meier curves were developed for analysis of the clinical outcomes. Cox regression analysis was used to investigate the association between explanatory variables and outcomes. The multivariate analysis initially included all of the variables, for which the p value was < 0.20 in the univariate analysis. Those with the highest p values were removed one by one and only the variables with p values < 0.05 were maintained in the final model.
Results
In total, 611 patients were included in the registry. Clinical follow-up was available for 96.2% of the patients, with an average follow-up of 84 (±12) months and a maximum of 96 months. The demographic characteristics and clinical presentations of the patients are presented in Table 1.
Table 1.
Demographic and clinical characteristics at baseline
| Age | 63.7 (±11) |
|---|---|
| Male gender | 338 (62.4%) |
| GFR < 60 ml/min/1.73 m2 | 165 (32.7%) |
| Diabetes | 204 (34.3%) |
| Insulin dependent | 73 (12.4%) |
| Noninsulin dependent | 166 (28.2%) |
| Hypertension | 468 (76.2%) |
| Dyslipidemia | 453 (76.8%) |
| Previous coronary angioplasty | 174 (28.7%) |
| Prior CABG | 67 (11.1%) |
| Current smoking | 96 (15.7%) |
| Initial clinical presentation | |
| Stable angina | 363 (60.4%) |
| Unstable angina | 151 (25.1%) |
| NSTEMI | 41 (6.8%) |
| STEMI | 24 (4.0%) |
| Recent myocardial infarction | 22 (3.7%) |
GFR: glomerular filtration rate; CABG: coronary artery bypass grafting; NSTEMI: NonST segment elevation myocardial infarction; STEMI: ST segment elevation myocardial infarction.
The mean average age was 63.7 years, and the male gender was predominant (63%). One-third of the subjects exhibited renal failure or diabetes, more than two-thirds had hypertension and dyslipidemia, and slightly more than one-half were smokers. Stable angina was the most common clinical presentation.
Regarding the angiographic characteristics, most of the lesions were located in the left anterior descending artery (in 348 patients or 56.4%). The majority of the lesions (96.4%) were either type B2 or type C lesions (Table 2).
Table 2.
Qualitative characteristics of the target vessels and lesions
| Patients | 611 |
|---|---|
| Target vessel | |
| Left main coronary arterya | 13 (1.8%) |
| Anterior descending artery | 394 (56.4%) |
| Circumflex artery | 128 (18.0%) |
| Right Coronary artery | 177 (23.0%) |
| Treated lesions | 712 |
| Lesions per patient | 1.17 |
| Type of lesion* | |
| Tipo A | 3 (0.4%) |
| Type B1 | 22 (3.6%) |
| Type B2 | 355 (49.8%) |
| Type C | 332 (46.6%) |
Classification according to the American Heart Association and the American College of Cardiology (AHA / ACC) guidelines.
The mean reference diameter of the target vessel before the procedure was 2.87 mm (± 0.46), with a minimal luminal diameter of 0.92 mm (± 0.51) and a mean lesion length of 15.75 mm (± 8.37). A total of 748 DES and 83 bare metal stents were used in the index procedure. Angiographic success was observed in 98.2% of the cases. Additional angiographic characteristics are presented in Table 3.
Table 3.
Quantitative coronary angiography and procedure data
| Pre-TIMI flow grade 3 | n (%) |
|---|---|
| 0 | 38 (6.20) |
| 1 | 14 (2.40) |
| 2 | 30 (4.90) |
| 3 | 529 (86.50) |
| Média (DP) | |
| Reference diameter (mm) | 2.87 (0.46) |
| Minimal luminal diameter before the procedure (mm) | 0.92 (0.51) |
| Lesion length (mm) | 15.75 (8.37) |
| Maximum pressure of the DES implant (ATM) | 14.88 (2.75) |
| Final minimal luminal diameter in the stent (mm) | 2.82 (0.48) |
| Final minimal luminal diameter in the segment (mm) | 2.41 (0.61) |
| Average number of stents / patient | 1.3 |
| Type of drug-eluting stent | n (%) |
| Costar® | 25 (3.34) |
| Cypher® | 255 (34.2) |
| Endeavor® | 118 (15.7) |
| Infinium® | 9 (1.20) |
| Janus® | 6 (0.80) |
| Supralimus® | 44 (5.80) |
| Taxus® | 291 (38.09) |
| Angiographic success | 600 (98.20) |
| Post-TIMI flow grade 3 | n (%) |
| 0 | 1 (0.16) |
| 1 | 2 (0.32) |
| 2 | 5(0.81) |
| 3 | 603 (98.7) |
| Use of IIb/IIIa inhibitor | 24 (3.90%) |
| Pre-dilation of the lesion | 397 (65.0%) |
The data represent the absolute numbers and percentages or the means and standard deviations.
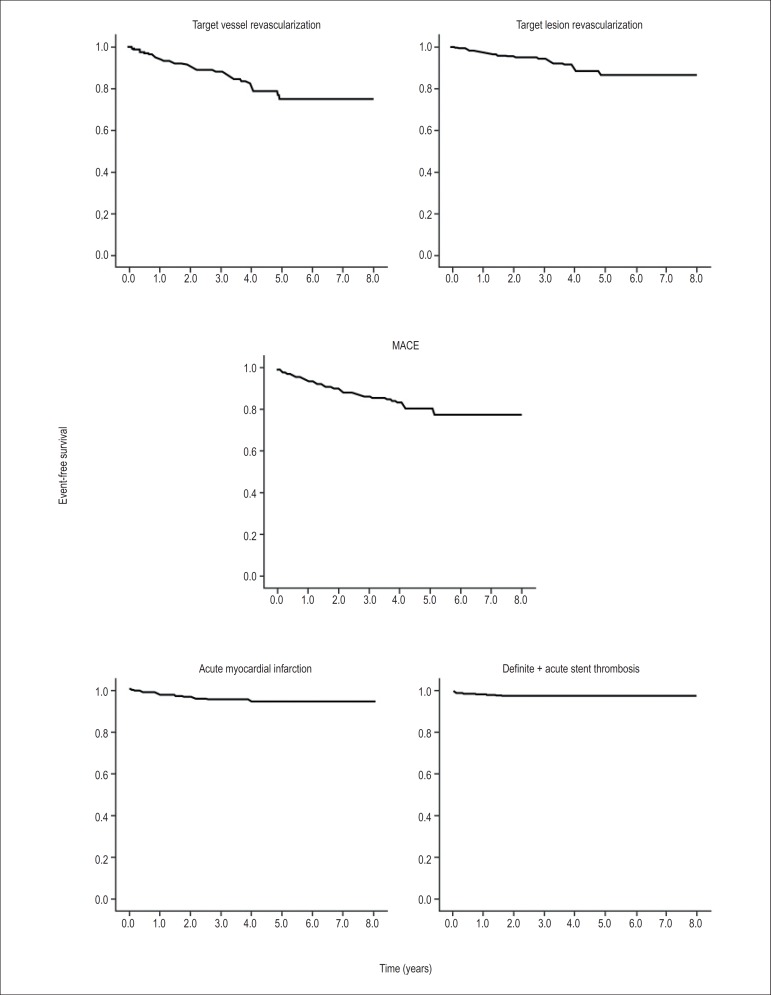
The event-free Kaplan Meier curves are shown in Figure 1. Total mortality was 8.7% at an average follow-up of 84 months (± 12). Only 4.3% of the patients experienced a new nonfatal MI. Eight percent of the patients required new revascularization of the target lesion; 5.4% of the procedures were percutaneous and 2.6% were performed via bypass surgery. The incidence of defined or probable DES thrombosis was 2.1%. Target vessel revascularization and TLR were 12.4% and 8.0%, respectively (Table 4). The occurrence of stent thrombosis during the follow-up period is described in Table 5. Every TVR and TLR was treated by the same operator who performed the index procedure. Of the 13 episodes of thrombosis, 10 were admitted and treated in the same hospital where the index procedures were performed; however the operators were not the same. All of them had a favorable outcome. The three remaining patients were treated in other hospitals by different operators; two of them died in the first 24 h after the rescue procedure.
Figure 1.
Kaplan Meyer curves for the outcomes
Table 4.
Clinical outcomes at final follow-up
| n (%) | |
|---|---|
| Global mortality | 53 (8.7) |
| Nonfatal AMI | 26 (4.3) |
| TVR | 76 (12.4) |
| TLR | 49 (8.0) |
| CABG | 16 (2.6) |
| PTCA | 33 (5.4) |
| Thrombosis | 13 (2.1) |
AMI: acute myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; CABG: coronary artery bypass grafting; PTCA: percutaneous transluminal coronary angioplasty.
Table 5.
Occurrence of thrombosis during follow-up
| Follow-up year | n |
|---|---|
| First | 1 |
| Second | 2 |
| Third | 2 |
| Fourth | 3 |
| Fifth | 5 |
| Sixth | 0 |
| Seventh | 0 |
| Eighth | 0 |
The medical therapy used at the time of the final clinical follow-up is listed in Table 6. At the first year evaluation, 95% of patients were using aspirin and 87% were using clopidogrel. At the end of follow-up, most of the patients were using aspirin (ASA) and statins, and less than one-quarter of the patients were using clopidogrel.
Table 6.
Medication in use at the final follow-up
| ASA | 424 (69.6%) |
| Clopidogrel | 147 (24.4%) |
| ASA + clopidogrel | 138 (22.5%) |
| Statin | 420 (71.6%) |
| Beta-blocker | 292 (4.6%) |
| ACE inhibitor | 200 (33.2%) |
| AT2 receptor antagonists | 94 (15.6%) |
| Nitrate | 102 (16.9%) |
ASA: acetylsalicylic acid ; ACE: angiotensin-converting enzyme inhibitors
Multivariate analysis revealed that mortality was correlated with being greater than 60 years old and having a previous MI. TLR was correlated with being greater than 60 years old and the presence of calcium in the lesion. Myocardial infarction events were positively associated with the presence of calcium in the lesion. The only associated predictor for thrombosis was an ECC of < 60 ml/min/1.73 m2 . Previous MI was positively correlated with MACE.
An ECC of < 60 ml/min/1.73 m2 showed a significant positive correlation with all outcomes (Table 7).
Table 7.
Predictors of outcomes by multivariate analysis
| HR (IC 95%) | P | |
|---|---|---|
| Mortality | ||
| Age > 60 years | 3.33 (1.01 - 10.97) | 0.048 |
| Previous myocardial infarction | 5.9 (1.91 - 18.19) | 0.002 |
| GFR < 60 ml/min/1.73 m2 | 6.96 (2.7 - 17.95) | 0 |
| TLR | ||
| Age > 60 years | 0.48 (0.25 - 0.90) | 0.022 |
| GFR < 60 ml/min/1.73 m2 | 2.73 (1.18 - 6.33) | 0.019 |
| Presence of calcium in the lesion | 2.88 (1.23 - 6.72) | 0.015 |
| Infarction | ||
| GFR < 60 ml/min/1.73 m2 | 2.91 (0.96 - 8.83) | 0.06 |
| Presence of calcium in the lesion | 4.43 (1.69 - 11.63) | 0.003 |
| Stent thrombosis | ||
| GFR < 60 ml/min/1.73 m2 | 3.33 (0.88 - 12.63) | 0.077 |
| MACE | ||
| Previous myocardial infarction | 113.74 (48.14 - 268.75) | < 0.0001 |
| GFR < 60 ml/min/1.73 m2 | 2.75 (1.23 - 6.16) | 0.014 |
GFR: glomerular filtration rate; TLR: target lesion revascularization; MACE: major adverse cardiac events.
Comparative sub-analysis between the three most frequently used DES revealed that the Taxus® device trended towards a positive association with the need for TVR in 16.8% of the cases (p = 0.053) compared with the Cypher® and Endeavor® stents, with 9.5% and 10.2%, respectively. This difference was not confirmed when only TLR was evaluated (Table 8). The occurrence of MACE, stent thrombosis (defined + probable), and infarction was not significantly different between the three stents.
Table 8.
Differences between the stents regarding outcomes
| Cypher® | Endeavor® | Taxus® | p value | |
|---|---|---|---|---|
| n | 255 | 118 | 291 | |
| Mortality | 11 (4.3) | 2 (1.7) | 10 (3.4) | 0.47 |
| TVR | 19 (9.5) | 9 (10.2) | 39 (16.8) | 0.05 |
| TLR | 14 (7.0) | 7 (8.0) | 22 (9.5) | 0.63 |
| Infarction | 24 (12.0) | 12 (13.6) | 41 (17.7) | 0.24 |
| Stent thrombosis | 3 (1.5) | 1 (1.1) | 7 (3.0) | 0.42 |
| MACE | 21 (8.20) | 9 (7.6) | 27 (9.2) | 0.88 |
TVR: target vessel revascularization; TLR: target lesion revascularization; MACE: major adverse cardiac event.
Discussion
The results of the registry demonstrate that the use of DES is effective long-term. We further showed that the results of randomized trials can be replicated in clinical practice, despite the inclusion of patients with a wide variety of clinical and angiographic characteristics that are high risk and complex9-17.
In the present study, the low incidence of adverse events, such as new revascularization and stent thrombosis, is similar to recent data that have demonstrated reduced occurrence of these events with DES long-term. The mortality rate observed during the 8 years of follow-up is similar to that demonstrated in most randomized trials with up to 6 years of follow-up9-13.
The long-term follow-up of patients is a key differentiator of this registry. The first report of the efficacy and safety of DES in unselected consecutive patients with complex disease came from the RESEARCH Registry. It demonstrated that the use of the Cypher® stent is associated with significantly lower rates of MACE and TVR compared with bare metal stents during a 6 month follow-up12.
The reduced incidence of new revascularizations at the 8 year follow-up reported in the present investigation also corroborates the data observed in several other clinical studies. They observed a reduced requirement for new revascularization of the target lesion, especially after 1 year9-20. Therefore, the hypothesis that DES would merely delay the phenomenon of restenosis has been rejected.
The incidence of stent thrombosis associated with DES was low in our study. The low number of patients using clopidogrel by the end of the follow-up period was expected because dual antiplatelet therapy has been recommended for only 12 months after DES.
The duration of dual antiplatelet therapy is a point of debate when discussing the risk of stent thrombosis21-24. A higher incidence of thrombosis with DES has been demonstrated when dual antiplatelet therapy is interrupted within the first 6 months after angioplasty24. Nevertheless, the impact of long-term use of dual antiplatelet therapy is debatable. The incidence of stent thrombosis was low in our study, despite the low number of patients using antiplatelet drugs at the end of the follow-up period. The highest rate of stent thrombosis was observed within the first year after the index angioplasty.
Studies comparing the incidence of stent thrombosis between DES and bare metal stents have generated conflicting data. Clinical trials have not included stent thrombosis as a primary outcome due to the low incidence of stent thrombosis (0.5 to 1% per year). In a meta-analysis25,26, the incidence of stent thrombosis was found to be similar for both DES and bare metal stents during the first year. After 1 year, the risk of thrombosis with DES was reported to be higher26. In another study, no difference in stent thrombosis between DES and a bare metal stent was observed during a 15-month follow-up27. In a meta-analysis of trials limited to primary angioplasty for acute MI, the stent thrombosis rate observed with DES and bare metal stents was similar at 1 year of follow-up27.
The frequency of various clinical outcomes of bare metal stents and DES differs between clinical trial data and observational studies. Although clinical trials have demonstrated similar mortality and MI rates for DES and bare metal stents, observational real-world studies indicate a reduction in mortality in the DES group21-25. Recent studies indicate a significant reduction in the occurrence of stent thrombosis in second-generation DES versus bare metal stents9,28.
The effect of different generations of DES on clinical outcomes must also be considered. Few comparisons have been made between the Cypher®, Taxus®, and Endeavor® stents. One study indicated that the Endeavor® stent was not inferior to the Cypher® stent and was superior to the Taxus® stent when mortality from all causes, MI, and TVR were assessed at 12 months29,30. In our registry, the incidence of thrombosis, MI, and TLR was similar among those three stents. We observed a significant difference in the incidence of TVR between the groups, with a higher incidence in the Taxus® group.
Head-to-head comparisons between the Cypher® and Taxus® stents indicate a lower occurrence of stent thrombosis and TLR with the Cypher® stent31. Another head-to-head study comparing the Endeavor® and Taxus® stents with a follow-up period of > 1 year indicated a lower incidence of stent thrombosis with the Endeavor® stent32. Although these studies suggested a higher incidence of thrombosis with the Taxus® stent compared with other first-generation stents, the results were variable and inconclusive when stent thrombosis was assessed as the primary outcome21. Recent studies comparing the second-generation stent Xience V® versus Taxus® have demonstrated a lower incidence of stent thrombosis with Xience V®9,22,23, despite equal patient adherence to dual antiplatelet therapy during the first year of follow-up. A recent meta-analysis of clinical trials revealed a lower incidence of thrombosis with the Xience V®, compared with the Taxus® stent30. Thus, differences in stent thrombosis rates between the types of DES might exist, however, the magnitude of these differences is difficult to measure34-36.
Data from studies of this nature cannot be compared with those from clinical trials due to the nonrandomized, all-comers design. However, real world registries allow for the assessment of outcomes in clinical scenarios that are not tested in randomized studies.
The present study revealed adequate safety and efficacy of DES during a long-term clinical follow-up period in the real world. It included a significant proportion of patients at high cardiovascular risk, thereby validating the use of these stents for routine treatment of patients with coronary artery disease.
Footnotes
Author contributions
Conception and design of the research: Gomes VO, Lasevitch R, Caramori P, Pellegrini DO; Acquisition of data: Gomes VO, Lasevitch R, Ledur P, Caramori P, Pellegrini DO; Analysis and interpretation of the data: Smidt L, Azeredo MA, Bodanese R, Sinnott L, Caramori P, Pellegrini DO; Statistical analysis: Ledur P, Caramori P, Pellegrini DO; Writing of the manuscript: Moriguchi E, Caramori P, Pellegrini DO; Critical revision of the manuscript for intellectual content: Moriguchi E, Caramori P.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Denise Oliveira Pellegrini, from Universidade Federal do Rio Grande do Sul.
References
- 1.Daemen J, Serruys PW. Drug-eluting stent update 2007: part II: Unsettled issues. Circulation. 2007;116(8):961–968. doi: 10.1161/CIRCULATIONAHA.107.691451. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR, Jr, Kereiakes DJ, Laskey WK, Colombo A, Ellis SG, Henry TD, et al. Thrombosis and drug-eluting stents: an objective appraisal. J Am Coll Cardiol. 2007;50(2):109–118. doi: 10.1016/j.jacc.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Jensen LO, Maeng M, Kaltoft A, Thayssen P, Hansen HH, Bottcher M, et al. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007;50(5):463–470. doi: 10.1016/j.jacc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 5.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. BASKET-LATE Investigators Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Osorio Gomes V, Blaya P, Lasevitch R, Oliveira D, Hickmann P, Smidt L, et al. Impact of chronic kidney disease on the efficacy of drug-eluting stents: long-term follow-up study. Arq Bras Cardiol. 2011;96(5):346–351. doi: 10.1590/s0066-782x2011005000045. [DOI] [PubMed] [Google Scholar]
- 7.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8, 2006. Circulation. 2007;115(17):2352–2357. doi: 10.1161/CIRCULATIONAHA.107.688416. [DOI] [PubMed] [Google Scholar]
- 8.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, 3rd, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78(2):486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- 9.Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125(23):2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 10.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 2012;345: doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt A, Schoelmerich A, Pollner F, Schlitt M, Raaz U, Maegdefessel L, et al. Comparison of outcome in 1809 patients treated with drug-eluting stents or bare-metal stents in a real-world setting. Vasc Health Risk Manag. 2011;7:693–699. doi: 10.2147/VHRM.S24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemos PA, Hoye A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004;109(11):1366–1370. doi: 10.1161/01.CIR.0000121358.26097.06. [DOI] [PubMed] [Google Scholar]
- 13.Costa JR, Jr, Sousa A, Moreira AC, Costa RA, Cano M, Maldonado G, et al. Incidence and predictors of very late (>or=4 years) major cardiac adverse events in the DESIRE (Drug-Eluting Stents in the Real World)-Late registry. JACC Cardiovasc Interv. 2010;3(1):12–18. doi: 10.1016/j.jcin.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Sousa A, Costa JR, Jr, Moreira AC, Cano M, Maldonado G, Costa RA, et al. Long-term clinical outcomes of the Drug-Eluting Stents in the Real World (DESIRE) Registry. J Interv Cardiol. 2008;21(4):307–314. doi: 10.1111/j.1540-8183.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 15.Saia F, Piovaccari G, Manari A, Guastaroba P, Vignali L, Varani E, et al. Patient selection to enhance the long-term benefit of first generation drug-eluting stents for coronary revascularisation procedures. Insights from a large multicentre registry. EuroIntervention. 2009;5(1):57–66. doi: 10.4244/eijv5i1a10. [DOI] [PubMed] [Google Scholar]
- 16.Ortolani P, Balducelli M, Marzaroli P, Piovaccari G, Menozzi A, Guiducci V, et al. Two-year clinical outcomes with drug-eluting stents for diabetic patients with de novo coronary lesions: results from a real-world multicenter registry. Circulation. 2008;117(7):923–930. doi: 10.1161/CIRCULATIONAHA.107.730416. [DOI] [PubMed] [Google Scholar]
- 17.Marzocchi A, Saia F, Piovaccari G, Manari A, Aurier E, Benassi A, et al. Long-term safety and efficacy of drug-eluting stents: two-year results of the REAL (REgistro AngiopLastiche dell'Emilia Romagna) multicenter registry. Circulation. 2007 Jun 26;115(25):3181–3188. doi: 10.1161/CIRCULATIONAHA.106.667592. [DOI] [PubMed] [Google Scholar]
- 18.Marroquin OC, Selzer F, Mulukutla SR, Williams DO, Vlachos HA, Wilensky RL, et al. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358(4):342–352. doi: 10.1056/NEJMoa0706258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 20.Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112(21):3306–3313. doi: 10.1161/CIRCULATIONAHA.105.552190. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. TAXUS V Investigators Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294(10):1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 23.Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Baldus S, et al. Long-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II study. EuroIntervention. 2010;6(5):562–567. doi: 10.4244/EIJV6I5A95. [DOI] [PubMed] [Google Scholar]
- 24.Venkitachalam L, Lei Y, Stolker JM, Mahoney EM, Amin AP, Lindsey JB, et al. Clinical and economic outcomes of liberal versus selective drug-eluting stent use: insights from temporal analysis of the multicenter Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Circulation. 2011;124(9):1028–1037. doi: 10.1161/CIRCULATIONAHA.110.978593. [DOI] [PubMed] [Google Scholar]
- 25.Holmes DR Jr, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent thrombosis. J Am Coll Cardiol. 2010;56(17):1357–1365. doi: 10.1016/j.jacc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2011;125(3):505–513. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 27.Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 28.Ko DT, Chiu M, Guo H, Austin PC, Goeree R, Cohen E, et al. Safety and effectiveness of drug-eluting and bare-metal stents for patients with off- and on-label indications. J Am Coll Cardiol. 2009;53(19):1773–1782. doi: 10.1016/j.jacc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 29.Roukoz H, Bavry AA, Sarkees ML, Mood GR, Kumbhani DJ, Rabbat MG, et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am J Med. 2009;122(6):581.e1–581.10. doi: 10.1016/j.amjmed.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs AT, Kuehnl A, Pelisek J, Rolland PH, Mekkaoui C, Netz H, et al. Meta-analysis shows similar risk of thrombosis after drug-eluting stent, bare-metal stent, or angioplasty. Endothelium. 2008;15(1):93–100. doi: 10.1080/10623320802092534. [DOI] [PubMed] [Google Scholar]
- 31.Brodie B, Pokharel Y, Fleishman N, Bensimhon A, Kissling G, Hansen C, et al. Very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction: a 15-year single-center experience. JACC Cardiovasc Interv. 2011;4(1):30–38. doi: 10.1016/j.jcin.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 33.Jaffery Z, Prasad A, Lee JH, White CJ. Drug-eluting coronary stents - focus on improved patient outcomes. Patient Relat Outcome Meas. 2011;2:161–174. doi: 10.2147/PROM.S24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park DW, Kim YH, Yun SC, Kang SJ, Lee SW, Lee CW, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010;56(15):1187–1195. doi: 10.1016/j.jacc.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 35.Schomig A, Dibra A, Windecker S, Mehilli J, Suarez de Lezo J, Kaiser C, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. 2007;50(14):1373–1380. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 36.Leon MB, Nikolsky E, Cutlip DE, Mauri L, Liberman H, Wilson H, et al. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2010;3(10):1043–1050. doi: 10.1016/j.jcin.2010.07.008. [DOI] [PubMed] [Google Scholar]