Abstract
Background
End-stage kidney disease patients continue to have markedly increased cardiovascular disease morbidity and mortality. Analysis of genetic factors connected with the renin-angiotensin system that influences the survival of the patients with end-stage kidney disease supports the ongoing search for improved outcomes.
Objective
To assess survival and its association with the polymorphism of renin-angiotensin system genes: angiotensin I-converting enzyme insertion/deletion and angiotensinogen M235T in patients undergoing hemodialysis.
Methods
Our study was designed to examine the role of renin-angiotensin system genes. It was an observational study. We analyzed 473 chronic hemodialysis patients in four dialysis units in the state of Rio de Janeiro. Survival rates were calculated by the Kaplan-Meier method and the differences between the curves were evaluated by Tarone-Ware, Peto-Prentice, and log rank tests. We also used logistic regression analysis and the multinomial model. A p value ≤ 0.05 was considered to be statistically significant. The local medical ethics committee gave their approval to this study.
Results
The mean age of patients was 45.8 years old. The overall survival rate was 48% at 11 years. The major causes of death were cardiovascular diseases (34%) and infections (15%). Logistic regression analysis found statistical significance for the following variables: age (p = 0.000038), TT angiotensinogen (p = 0.08261), and family income greater than five times the minimum wage (p = 0.03089), the latter being a protective factor.
Conclusions
The survival of hemodialysis patients is likely to be influenced by the TT of the angiotensinogen M235T gene.
Keywords: Survival Analysis; Lethality; Renal Dialysis; Polymorphism, Genetic; Peptidyl-Dipeptidase A; Kidney Failure, Chronic
Introduction
The number of patients undergoing renal replacement therapy is on the increase all over the world, including Brazil1-3. Data from the Brazilian Society of Nephrology shows that between 1994 and 2009, the number of patients enrolled in chronic dialysis programs in Brazil more than tripled from 24,000, to 77,5893. Of these, 89.6% were on hemodialysis3.
On July 1st, 2010, the number of Brazilian dialysis patients was 92,091. The annual crude mortality rate for patients with end-stage chronic kidney failure was 17.1% in 20093, and 17.9% in 20104. Despite major advances in recent decades in the treatment of chronic kidney disease, cardiovascular disease is still the main cause of death for these patients, especially among those on dialysis. The cardiovascular mortality of hemodyalized patients is high (40% to 50% of the population of chronic kidney patients), significantly higher than for the general population5,6. The general population trend for cardiovascular mortality is decreasing, but, among hemodialyzed patients, it is increasing4-8.
Traditional cardiovascular risk factors alone are not capable of explaining the high mortality rate for chronic kidney disease, dialysis population. Other factors need to be taken into consideration. Our view is that the key to improving survival is better knowledge of prognostic risk factors, be they traditional or not.
The primary objective of this study was to assess the survival of patients with end-stage chronic kidney failure who received hemodialysis in four centers in the State of Rio de Janeiro. patients were followed up for 11 years and compared to the general population to look at the correlation between mortality for those with cardiovascular risk factors and D/I and M235T polymorphisms of the angiotensin I-converting enzyme and the angiotensinogen genes, respectively. The secondary objective was to analyze cause of death by sex and age group.
Methods
Study Diagram:
The initial study cohort comprised 473 chronic hemodialyzed patients from four hemodialysis centers. Three from the city of Niterói and one from Rio Bonito. This represented all eligible patients in those hemodialysis centers from July 15, 1997 to July 15, 1998.
Inclusion criteria: All adult patients (aged 18 years old or more at the time of selection for this study), regardless of age at the time hemodialysis treatment began, who had been on chronic hemodialysis for at least one year during the period between July 15, 1997 and July 15, 1998.
Exclusion criteria: Exclusion criteria included the presence of Acute Kidney Failure; refusal to participate in this study; and the fact that patients had already been submitted to kidney transplant before selection.
Death records: We used the Mortality Information System. Survival analysis used information from the study population data bases, and death records (1998 to 2008) in the State of Rio de Janeiro, with the objective of identifying individuals who passed away and to determine cause of death. Analysis was conducted with RecLink III software and consisted of six steps:
Standardizing the format of data variables.
Grouping records using identification keys.
Using algorithms to compare chains of characters.
Scoring record pairs on their level of global concordance.
Using threshold definitions to determine whether each pair was real, doubtful, borderline, or non-paired.
Manual revision of the doubtful or borderline pairs, aiming to reclassify as real pairs or non-pairs.
Study duration: Follow-up: 11 years; recruitment: July 15, 1997 to July 15, 1998; conclusion: December 31, 2008.
Variables: The variables studied were defined as follows:
Systolic Blood Pressure before the hemodialysis session: The first measurement recorded in the medical history was used in the recruiting stage of this study.
Length of hemodialysis in months at the time of recruiting. From this variable, it was possible to calculate the date hemodialysis started and the time of survival on hemodialysis. This was calculated by the sum of the time of hemodialysis at the beginning of the treatment, plus the time until the event (death, kidney transplant or conclusion of the study).
Body Mass Index. Calculated using the formula, weight in kg, divided by the squared height in meters. Weight was obtained prior to hemodialysis.
Monthly family income, as provided by the patient when he or she was admitted to the hemodialysis center. This information was registered in the medical record or it was collected from the patient. The variable was measured in number of minimum wages at the time the treatment began in the hemodialysis center. Afterwards, we created the following categories: less than two times the minimum wage; from two to five times the minimum wage; and more than five times the minimum wage.
Age at the beginning of hemodialysis (in years).
Gender.
Basic cause of death: using data from the Mortality Information System of the State Secretariat of Health in the State of Rio de Janeiro, we identified the basic cause of death recorded in the Mortality Information System through selection rules.
Triglycerides: measured by the enzymathic method, at the time of recruiting.
Total cholesterol: measured by the enzymathic method, at the time of recruiting.
High-density lipoprotein: measured by the enzymathic method, at the time of recruiting.
Hematocrit: Percentage occupied by red blood cells in the total blood volume, at the time of recruiting.
Diabetes mellitus, diagnosis registered on the medical record at the time of admission in the hemodialysis center, and collected by us during recruiting.
History of ischemic heart disease, characterized by acute myocardial infarction, angina pectoris, or by some previous invasive coronary procedures. This information was collected from medical records.
History of stroke, collected from medical records.
History of smoking or former smoking, recorded in medical records at the time of admission to the hemodialysis center.
Polymorphism of genes of the angiotensin I-converting enzyme (I/D) and angiotensinogen (M235T). Samples of 5 mL of blood were drawn from patients at the time of recruiting and stored in tubes containing ethylenediaminetetraacetic acid, which were sent to São Paulo. The genetic polymorphism of the angiotensin I-converting enzyme (I/D) was determined by using a standardized essay with three primers. The variable M235T of the angiotensinogen gene was determined by the polymerase chain reaction method, followed by enzymatic digestion. The team who conducted the genotypic assessments did not have access to the clinical information of the study patients.
Patients who had undergone kidney transplant and patients who were alive on December 31, 2008, when the study was concluded, were censored to their respective dates.
The analyzed outcomes were: primary, time of survival; and secondary, basic cause of death.
The Research Ethics Committee in the University Hospital Clementino Fraga Filho, at Universidade Federal do Rio de Janeiro, approved this study.
Statistical Analysis
Each variable was analyzed and its distribution, mean, and standard deviation was calculated. The Chi-Square test was used for categorical variables and the unpaired Student's t-test was used for quantitative variables (quantitative variables had normal distributions, as the sampling number was sufficiently large). A 5% significance level was adopted in both cases. The logistic regression model and the odds ratio calculation were used, as well as the multinomial logistic model, the Cox model, the classification and survival trees. The chosen variables were used as a base allowing a visual analysis of mortality and the variables associated with it.
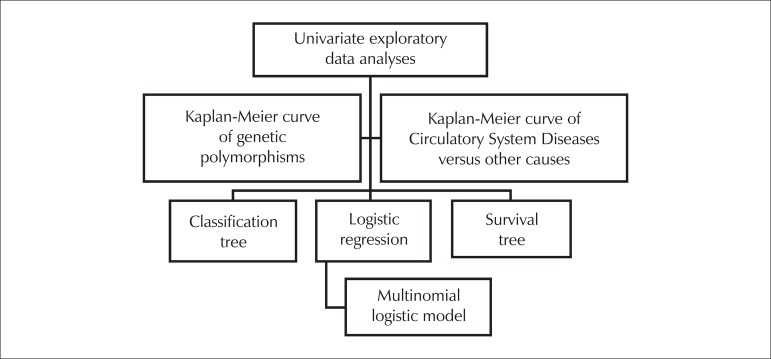
Kaplan-Meier curves were used to visualize the survival of patients according to the chosen variable. The Tarone-Ware, Peto-Prentice and Log-Rank tests were used to assess the difference between curves. The statistical significance level adopted for curves was 5%. Analyses were conducted with R software. Survival curves were measured from the date estimated for the beginning of hemodialysis for each patient. The Hardy-Weinberg equilibrium was calculated. The statistics flowchart, shown in Figure 1 was used, and is described as follows:
Figure 1.
Diagram of the statistical analyses used.
Univariate exploratory data analyses.
Survival Kaplan-Meier curves with the study polymorphisms, comparing deaths caused by the circulatory system (the main cause of death) with other causes. To identify whether the differences found in the Kaplan-Meier curves were significant, we used the Tarone-Ware and the Peto-Prentice tests. We used the log rank test to compare survival curves, as it is the most widely used method. This is also known as the Mantel-Cox test, these tests are only different in the weights used.
Classification tree, with the objective of understanding which variables, or interaction of variables, are responsible for the analyzed phenomenon.
Survival tree, which was used for the same purpose as III.
Logistic regression was employed for all patients, taking death by any cause as the dependent variable and only selecting other variables that were clinically and/or statistically relevant.
Multinomial logistic model for all patients using all of the variables. The dependent variable at this point was death caused by atherothrombotic vascular disease (ischemic heart disease plus stroke), which was the biggest cause of death among diseases of the circulatory system. Multinomial models constitute an extension of logistic models, being used in cases in which the response variable is nominal, with more than two categories, as in this case.
Results
1) Clinical and demographic variables: Only one patient out of the 474 cases that met the selection criteria refused to participate in the study. Out of the 473 analyzed cases, 237 (50.1%) were male. The mean age at the beginning of hemodialysis was 46 years. The mean duration of hemodialysis (HD) at the time of recruiting was 52 months (4.4 years). The mean time of life on hemodialysis was of 144 months. The mean body mass index was > 30 kg/m2. Half of the population consisted of smokers or former smokers. Almost half of the cases (43.4%) had a monthly income of less than twice the minimum wage. The mean systolic blood pressure was 150 mmHg, and most cases (81.6%) were classified as hypertensive patients. Diabetes mellitus was present in 15% of the cases, and generally associated with hypertension. The DI genotype of the angiotensin I-converting enzyme (50%) and the MT genotype of the angiotensinogen (39.3%) were the most prevalent ones. Ischemic heart disease was present in 26% of the cases, and stroke in 7% (Table 1). With respect to biochemical and hematological data, we did not observe major changes from the normal rate for the variables studied, except for the low hematocrit mean (29%) (Table 2) (Figure 2).
Table 1.
Demographic data and clinical and genetic characteristics of the 473 patients at recruitment (July 97/ July 98) or at the beginning of hemodialysis
| Number of patients | 473 | ||
| Gender (M/F) - n | 237/236 | ||
| Age in years at the beginning of hemodialysis mean ± SD | 45.8 ± 16 | ||
| Time of life on HD (months)-mean ± SD | 144 ± 59 | ||
| BMI (Kg/m2)-mean ± SD | 22.6 ± 4.7 | ||
| SBP (mmHg)-mean ± SD | 150 ± 19 | ||
| Smoking or former smoking - n (%) | 237 (50.1%) | ||
| Monthly Family income. n (%): | |||
| < 2 MW | 204 (43.4%) | ||
| ≥ 2 to 5 MW | 139 (29.6%) | ||
| > 5 MW | 127 (27%) | ||
| IGN | 3 (0.6%) | ||
| Genotypes of polymorphisms: | |||
| ACE gene- n (%): | |||
| DD | 156 (33%) | ||
| DI | 239 (50.5%) | ||
| II | 78 (16.5%) | ||
| AGT gene-n (%): | |||
| MM | 139 (29.4%) | ||
| MT | 186 (39.3%) | ||
| TT | 148 (31.3%) | ||
| Combinations of genotypes of polymorphisms of ACE and AGT genes - n (%): | |||
| DD + TT | 46 (9.7%) | ||
| DD + MT | 62 (13.1%) | ||
| DD + MM | 48 (10.2%) | ||
| DI + TT | 79 (16.7%) | ||
| DI + MT | 91 (19.2%) | ||
| DI + MM | 69 (14.6%) | ||
| II + TT | 23 (4.9%) | ||
| II + MT | 33 (6.9%) | ||
| II + MM | 22 (4.7%) | ||
| Comorbidities at recruiting - n (%): | |||
| SAH (total) | 386 (81.6%) | ||
| Diabetes Mellitus (total) | 70 (14.8%) | ||
| SAH with associated diabetes | 59 (12.5%) | ||
| SAH without diabetes | 327 (69%) | ||
| Diabetes mellitus without SAH | 11 (2.3%) | ||
| Circulatory system diseases. n (%): | |||
| Ischemic heart disease | 122 (26%) | ||
| Stroke | 31(7%) | ||
n: number of patients; M: Male gender; F: Female gender; SD: Standard-deviation; Time of life on HD: Time of life on hemodialysis from admission to hemodialysis until the event (death. conclusion of the study or kidney transplant); SBP: Systolic blood pressure; BMI: Body mass index; MW: federal minimum wage at the time of recruiting; IGN: Ignored information; ACE: Angiotensin I-converting enzyme; AGT: angiotensinogen; SAH: Systemic arterial hypertension.
Table 2.
Laboratory data (biochemical and hematologic) at recruitment (July 97/July 98) of patients on HD
| Laboratory variable | Mean ± SD |
|---|---|
| Total cholesterol (mg/dL) | 189.1 ± 43 |
| HDL-cholesterol (mg/dL) | 37.7 ± 12 |
| Triglycerides (mg/dL) | 184.6 ± 96 |
| Hematocrits (%) | 29.2% ± 4.9 |
HDL-cholesterol: High Density Lipoprotein; SD: Standard-deviation
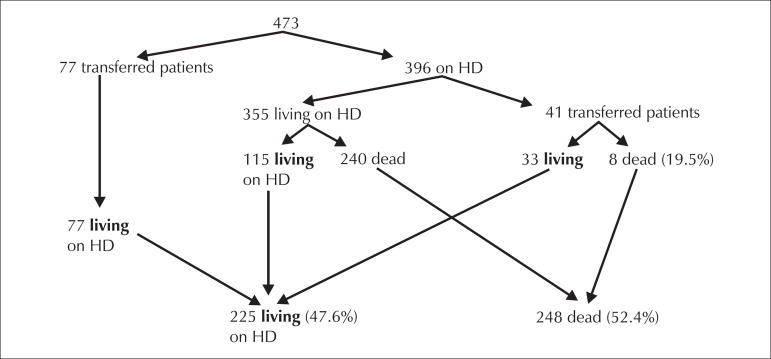
Figure 2.
Patient follow-up flowchart.
2) Survival: The crude death rate at 11 years was 52%. There was a 48% survival rate at 11 years. The mean age at the time of death was 57 years old (± 15).
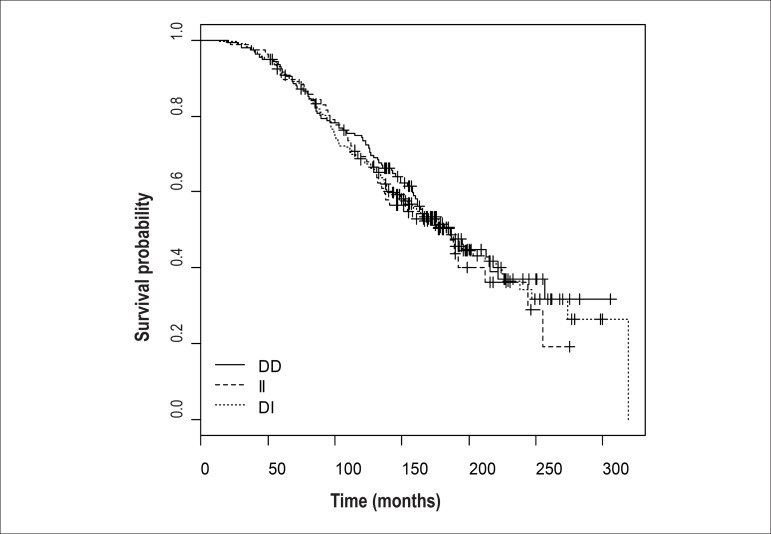
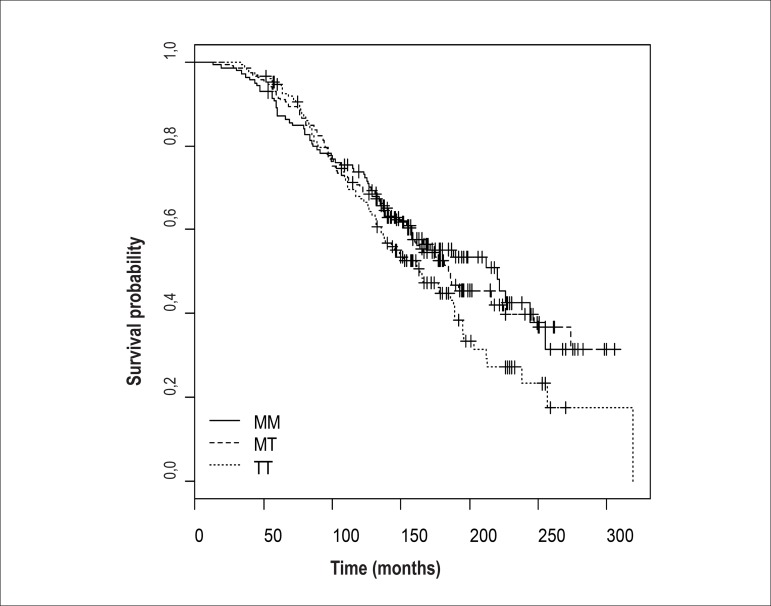
The Kaplan-Meier survival curve showed no differences between genetic polymorphisms of the gene in the converting enzyme (D/I) (Figure 3). We also built a Kaplan-Meier curve for polymorphism of the angiotensinogen. In this, we observed a tendency for the curve for the TT genotype to separate in relation to time of survival, even though the population was smaller (Figure 4). This trend was also confirmed when we separated the TT genotype of the angiotensinogen from the others in the Kaplan-Meier survival curve, with the Tarone-Ware test, and obtained a p value of 0.0976.
Figure 3.
Kaplan–Meier curve for D/I polymorphism of the ACE gene.
Figure 4.
Kaplan–Meier curve for the M235T polymorphism of the angiotensinogen.
3) Probability of death and its association with the variables used in statistical models: The logistic regression model, using death by any cause as a dependent variable, found the following p values and odds ratios (OR) with 95% confidence intervals. Age (p value = 0.000038, OR = 1.027), angiotensinogen TT (in relation to MM) (p value = 0.08261, OR = 1.534) and monthly income greater than five times the minimum wage (a protective factor) (p value = 0.03089, OR = 0.600).
Using a multinomial logistic model for all patients and using all variables, with death caused by atherothrombotic vascular disease (ischemic heart disease plus stroke, the main cause of death in this cohort), as the dependent variable, we found the following variables to be statistically significant. Age (p = 0.019802), and TT polymorphism of the angiotensinogen (p = 0.001394). TT was significant for both for men (p = 0.039402) and women (p = 0.01518).
Because the study population at the time of recruiting had different periods of hemodialysis, and because it was not in Hardy-Weinberg equilibrium with regards to polymorphism of the angiotensinogen (p value < 0.0001), we decided to study those patients who had been on hemodialysis for up to one year at recruitment separately and compare the results of this subgroup with those of the total population. This cohort was more homogeneous with respect to length of disease, and comprised 82 patients. This cohort will be described, analyzed, and compared with the total population in another study.
Discussion
In our study, there were very slightly more males (50.1%) than females, which is in accordance with 2008 Census from the Brazilian Society of Nephrology. It found that men make up 57% of the population with end-stage chronic kidney disease1. A study in Bahia found a 56.6% of patients to be male9. A cross-sectional analysis conducted in six hemodialysis clinics in the State of Rio de Janeiro also found a higher proportion of men (56%)10.
The mean age of the study population was 46 years, which is lower than that found in a study in the State of Rio de Janeiro10 (54 years old), and also lower than another cross-sectional analysis conducted in a single dialysis center in the State of Rio de Janeiro, where the mean age was 52 years11. However, it was similar to the Bahia study9, which found a mean age of 49 years. In the 2010 Census from the Brazilian Society of Nephrology, 30.7% of the patients were aged 65 years or older4.
The prevalence of arterial hypertension in our study (82%) was higher than the Bahia study (71%)9, and higher than the analysis of six hemodialysis clinics in the State of Rio de Janeiro (61%)10; it was also higher than the single center study conducted in the State of Rio de Janeiro (55%)11.
Diabetes mellitus prevalence in this study (15%) was much lower than that found in the 2010 Census by the Brazilian Society of Nephrology (28%)4, lower than the Bahia study (21%)9, and lower than the single center study in the State of Rio de Janeiro (20%)11. However, it was similar to the analysis involving six hemodialysis clinics in the State of Rio de Janeiro (17%)10.
The overall survival rate found in this study was 48% at 11 years. This is higher than that found in studies with large patient cohorts, such as the European Dialysis and Transplant Association study, which recorded a 50% survival rate over five years12. It is also higher than the rate found in the United States, which was only 36% over five years13. Our survival results were also higher than those found in diabetic hemodialyzed patients in Japan14 (28% over 10 years). These differences can be explained by the fact that our population was younger, as well as the lower prevalence of diabetes in our study population.
With regards to the demographics of our patient cohort, it is worth mentioning that the mean age of 46 years represents a significant social impact, since this is the most economically active age group. Life expectancy in the general Brazilian population was 73.48 years (73 years, 5 months and 24 days) in 2010, (77.32 years for females and 69.73 years for males) according to the research Tábuas Completas de Mortalidade, published on 01/12/2011 by the Brazilian Institute of Geography and Statistics15. At the age of 60, life expectancy in the Brazilian population in 2009, according to the same institute15, for both sexes was + 21.27 years (+ 19.55 for men, + 22.83 for women). This suggests that even though there was a good survival rate in our cohort, it is still lower than that of the same age group in the general Brazilian population.
A selection of survival rates from other studies are as follows. The French Tassin group found a 73% survival rate at five years16 with prolonged slow hemodialysis. A Brazilian study (3,082 hemodialysis patients, in seven Brazilian States) found a 58.2% survival rate at five years17. A national study of chronic hemodialysis survival in a cohort of 1,009 patients over 25 years, at three dialysis units in Santa Maria, Rio Grande do Sul, found a global survival rate of 64%, at five years, and 41%, at 10 years18.
Our research showed similar mortality rates for male and female patients, which is different than the findings of the cohort study across seven Brazilians States17 which found higher mortality among males.
In our study, diabetic patients had statistically higher mortality than non-diabetic ones (p = 0.0432). Lower survival among diabetics on hemodialysis has also been found in other analyses18,19.
Body mass index was not associated with mortality in our study. A similar result was found by De Matos et al17. However, this finding is different from that published by Kalantar-Zadch et al20.
Our findings showed that the highest cause of death was circulatory system diseases (34%), followed by infectious diseases (15%). Of the circulatory system conditions, the main cause was atherothrombotic vascular diseases (ischemic heart disease plus stroke), with 48% of cases. Infectious causes may be related to vascular access and with the immunosuppressive state associated with uremia. It is interesting to note that while the number of cardiovascular deaths is declining in the general population, the same is not true for patients on dialysis21. This difference is partly due to the demographic conditions of these individuals, when they started on dialysis. There were multiple comorbidities in our study, 15% of the cohort were diabetic, 92% were hypertensive, 26% already had ischemic heart disease, and 7% had had a stroke. A study9 conducted in Salvador concerning overall cardiovascular mortality and risk factors of patients on hemodialysis also showed high cardiovascular mortality of 41.7%.
We also analyzed the genetic polymorphisms of the angiotensin I-converting enzyme and of the angiotensinogen with mortality on hemodialysis. The results of the study by Inácio et al22, in Niterói and Rio Bonito, of patients who presented as "healthy", collected in practically the same period as our study, are different from ours for the D/I homozygotic polymorphisms in the gene of the angiotensin I-converting enzyme studied by these authors.
The literature on mortality in hemodialysis and its association with polymorphisms in the gene of the angiotensin I-converting enzyme, or of the angiotensinogen, is slim. Most studies in this area are of patients with particular diseases, diabetes being the most common.
Our study looked for a prognostic association between some polymorphisms of the renin-angiotensin system in chronic hemodialyzed patients in two important cities of the State of Rio de Janeiro. We found lower survival rates only the TT polymorphism of the angiotensinogen, and no difference in the survival rate for the D/I polymorphism of the angiotensin I-converting enzyme. Such results, showing a worse prognosis for the TT genotype, are similar to those found in analyses by Buraczynska et al23, Lovati et al24 and Miguel et al27. The differences found by the last two studies mentioned may have been due to the cohort being composed entirely of diabetics.
The study by Bzome et al25 was designed to assess arterial hypertension complications. There was no association found between D/I polymorphisms of the angiotensin I-converting enzyme and poorer outcomes, unlike the findings in studies by Sakka et al28, Losite et al29, van der Sman-de Beer et al30, Yoshida et al26, Pérez-Oller et al31; Lovati et al24. These studies found decreasing survival rates for the DD genotype of the angiotensin I-converting enzyme, and many of them had short follow-up periods for a population of diabetics. Other studies32-34 did not find any association for polymorphisms of the angiotensin I-converting enzyme or of the angiotensinogen. These studies also had short follow-ups and were conducted in a specific population with diabetic nephropathy.
Study limitations
The analysis of only two genetic polymorphisms in a highly complex system, such as the renin-angiotensin system is a limiting factor. The absence of other important variables to analyze the survival among hemodialyzed patients, such as hemoglobin, calcium, vitamin D, parathormone, erythropoietin, phosphorus, C-reactive protein, and serum albumin can also be limiting, as was the lack of information about the associations and dosages of medicines used by these patients.
Conclusions
The survival rate in our population was considered low when compared to the general population of the same age group. However, the survival in this cohort was high when compared to other studies involving Brazilian and North-American chronic hemodialyzed patients, as well as in comparison to most European studies. The main cause of death was circulatory system diseases, for both sexes, especially in older age groups, followed by infectious diseases and diabetes mellitus. In this study, it was not possible to demonstrate an association between polymorphisms in the gene that codifies the angiotensin I-converting enzyme (D/I) and mortality in hemodialyzed patients. The TT polymorphism of the M235 T angiotensinogen, age, and monthly family income were the variables that were associated with mortality in this cohort.
Footnotes
Author contributions
Conception and design of the research: Alves M, Silva NAS, Salis LHA; Acquisition of data: Alves M, Oliveira JMF; Analysis and interpretation of the data: Alves M, Silva NAS, Salis LHA, Godoy PH; Statistical analysis: Pereira BB, Nascimento EM; Writing of the manuscript: Alves M; Critical revision of the manuscript for intellectual content: Alves M, Silva NAS, Salis LHA, Oliveira JMF.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of Doctoral submitted by Mauro Alves, from Universidade Federal do Rio de Janeiro.
References
- 1.Sesso R, Lopes AA, Thomé FS, Bevilacqua JL, Romão Junior JE, Lugon J. Relatório do censo brasileiro de diálise, 2008. J Bras Nefrol. 2008;30(4):233–238. [Google Scholar]
- 2.National Institutes of Health (NIH) National Institutes of Diabetes and Digestive and Kidney Disease. U.S. Renal Data System . USRDS Annual Data Report. Bethesda: 2009. [Accessed on 2010 Feb 10]. Available from: htpp://www.usrds.org. [Google Scholar]
- 3.Sesso RC, Lopes AA, Thomé FS, Lugon JR, Burdmann EA. Brazilian dialysis census, 2009. J Bras Nefrol. 2010;32(4):374–378. [PubMed] [Google Scholar]
- 4.Sociedade Brasileira de Nefrologia [Acesso 2011 dez 20];Censo 2010. Disponível em: htpp://www.sbn.org.br/censo.asp.
- 5.Silva Júnior AC, Lopes H, Lotaif LD, Amodeo C, Piegas LS. Novos fatores de risco cardiovascular. Rev Soc Cardiol Estado de São Paulo. 2007;17(1):50–59. [Google Scholar]
- 6.Canziane ME. Doenças cardiovasculares na doença renal crônica. J Bras Nefrol. 2005;26(supl. 1):20–21. [Google Scholar]
- 7.Romão Junior JE. Doença renal crônica: definição, epidemiologia e classificação. J Bras Nefrol. 2004;26(3) supl.1:1–5. [Google Scholar]
- 8.Sesso R, Gordon P. Dados disponíveis sobre a doença renal crônica no Brasil. J Bras Nefrol. 2007;29(1) supl. 1:9–12. [Google Scholar]
- 9.Almeida FA, Machado FC, Moura JA Jr, Guimarães AC. Mortalidade global e cardiovascular e fatores de risco de pacientes em hemodiálise. Arq Bras Cardiol. 2010;94(2):187-92, 201-6, 190-5. doi: 10.1590/s0066-782x2010005000003. [DOI] [PubMed] [Google Scholar]
- 10.Miguel JB, Strogoff de Matos JP, Ruzany F, Miguel CS, Miguel SJ, Naveiro LT, et al. Associação do índice tornozelo-braço com inflamação e alterações minerais ósseas em pacientes em hemodiálise. Arq Bras Cardiol. 2011;96(5):405–409. doi: 10.1590/s0066-782x2011005000031. [DOI] [PubMed] [Google Scholar]
- 11.Miguel SB, Miguel JB, Velarde LG, Sampaio Ede A, Matos JP, Lugon JR. Prevalência e correlatos de doença vascular no exame de ultrassom em pacientes em hemodiálise. Arq Bras Cardiol. 2011;96(4):260–265. doi: 10.1590/s0066-782x2011005000027. [DOI] [PubMed] [Google Scholar]
- 12.European Renal Association. European Dialysis and Transplant Association . ERA-EDTA Registry: ERA-EDTA Registry 2006 Annual Report. Academic Medical Center, Department of Medical Informatics; Amsterdam: 2008. [Google Scholar]
- 13.United States Renal Data System (US RDS) U.S. Renal Data System, USRDS . Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health. (HIH); 2008. 2008. [Google Scholar]
- 14.Imai E, Yamagata K, Iseki K, Iso H, Horio M, Mkino H, Hishida A, et al. Kidney disease screening program in Japan: history, outcome and perspectives. Clin J Am Soc Nephrol. 2007;2(6):1360–1366. doi: 10.2215/CJN.00980207. [DOI] [PubMed] [Google Scholar]
- 15.Instituto Brasileiro de Geografia e Estatística. (IBGE) [Acesso em 2012 jan 6];Tábuas Completas de Mortalidade. Disponível: http://www.ibge.gov.br.
- 16.Innes A, Charra B, Burden RP, Morgan AG, Laurent G. The effect of long, slow haemodialysis on patient survival. Nephrol Dial Transplant. 1999;14(4):919–922. doi: 10.1093/ndt/14.4.919. [DOI] [PubMed] [Google Scholar]
- 17.De Matos JP, Almeida JR, Guinsburg A, Marelli C, Barra AB, Vasconcellos MS, et al. Avaliação da sobrevida de cinco anos em hemodiálise no Brasil: uma coorte de 3.082 pacientes incidentes. J Bras Nefrol. 2011;33(4):436–441. [PubMed] [Google Scholar]
- 18.Da Silva LA, Mezzomo NF, Pansard HM, Arantes LC, Rempel W, Argenta LC, et al. Sobrevida em hemodiálise crônica: estudo de uma coorte de 1.009 pacientes em 25 anos. J Bras Nefrol. 2009;31(3):190–197. [Google Scholar]
- 19.Peres LA, Matsuo T, Delfino VD, Peres CP, Almeida Netto JH, Ann HK, et al. Increase in prevalence of diabetes mellitus as a cause of dialytic end-stage renal disease: analysis of 20 years in the west regional of Paraná. Arq Bras Endocrinol Metabol. 2007;51(1):111–115. doi: 10.1590/s0004-27302007000100018. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadch K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailloux LU, Henrich WL. [Up To Date, 2005]; [Access in 2011 Mar 20];Patient survival and maintenance dialysis. 2005 Available from: http://www.uptodate.com/subscribers/tutorial/index.asp.
- 22.Inácio J, Goulart Filho LR, Vieira GS. Frequência genotípica e alélicas do gene do polimorfismo da ECA I/D na população brasileira. Biosci J. 2004;20(1):47–51. [Google Scholar]
- 23.Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A. Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant. 2006;21(4):979–983. doi: 10.1093/ndt/gfk012. [DOI] [PubMed] [Google Scholar]
- 24.Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001;60(1):46–54. doi: 10.1046/j.1523-1755.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 25.Bzoma B, Debska-Slizien A, Dudziak M, Raczynska K, Slizien W, Brylowska A, et al. Genetic predisposition to systemic complications of arterial hypertension in maintenance haemodialysis patients. Pol Merkur Lekarski. 2008;25(147):209–216. [PubMed] [Google Scholar]
- 26.Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A, et al. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996;50(2):657–664. doi: 10.1038/ki.1996.362. [DOI] [PubMed] [Google Scholar]
- 27.Padró-Miguel A, Alía-Ramos P, González-Alvarez MT, Navarro-Moreno MA. Survival in type 2 diabetic patient in dialysis and the number of risk alleles in polymorphisms of the renin-angiotensin system genes. Clin Biochem. 2009;42(1-2):5–11. doi: 10.1016/j.clinbiochem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Sakka Y, Babazono T, Sato A, Ujihara N, Iwamoto Y. ACE gene polymorphism, left ventricular geometry, and mortality in diabetic patients with end-stage renal disease. Diabetes Res Clin Pract. 2004;64(1):41–49. doi: 10.1016/j.diabres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Losito A, Parente B, Cao PG, Jeffery S, Afzal AR. ACE gene polymorphism and survival in atherosclerotic renovascular disease. Am J Kidney Dis. 2000;35(2):211–215. doi: 10.1016/s0272-6386(00)70328-3. [DOI] [PubMed] [Google Scholar]
- 30.van der Sman-de Beer F, Verhagen C, Rombach SM, Boorsma P, van Manen JG, Korevaar JC, et al. NECOSAD Study Group ACE I/D is associated with mortality in a cohort study of patients starting with dialysis. Kidney Int. 2005;68(5):2237–2243. doi: 10.1111/j.1523-1755.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Oller L, Torra R, Badenas C, Milà M, Darnell A. Influence of the ACE gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1999;34(2):273–278. doi: 10.1016/s0272-6386(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 32.Aucella F, Margaglione M, Vigilante M, Gatta G, Grandone E, Forcella M, et al. PAI-1 4G/5G and ACE I/D gene polymorphisms and the occurrence of myocardial infarction in patients on intermittent dialysis. Nephrol Dial Transplant. 2003;18(6):1142–1146. doi: 10.1093/ndt/gfg118. [DOI] [PubMed] [Google Scholar]
- 33.Wetmore JB, Johansen KL, Sen S, Hung AM, Lovett DH. An angiotensin converting enzyme haplotype predicts survival in patients with end stage renal disease. Hum Genet. 2006;120(2):201–210. doi: 10.1007/s00439-006-0191-4. [DOI] [PubMed] [Google Scholar]
- 34.Higashiuesato Y, Tana T, Tozawa M, Iseki C, Iseki K, Fukiyama K, et al. Angiotensin-converting enzyme (ACE) insertion/deletion polymorphism and survival in a cohort of chronic hemodialysis patients. Clin Nephrol. 2002;58(5):370–375. doi: 10.5414/cnp58370. [DOI] [PubMed] [Google Scholar]