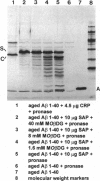
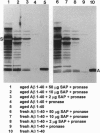
Abstract
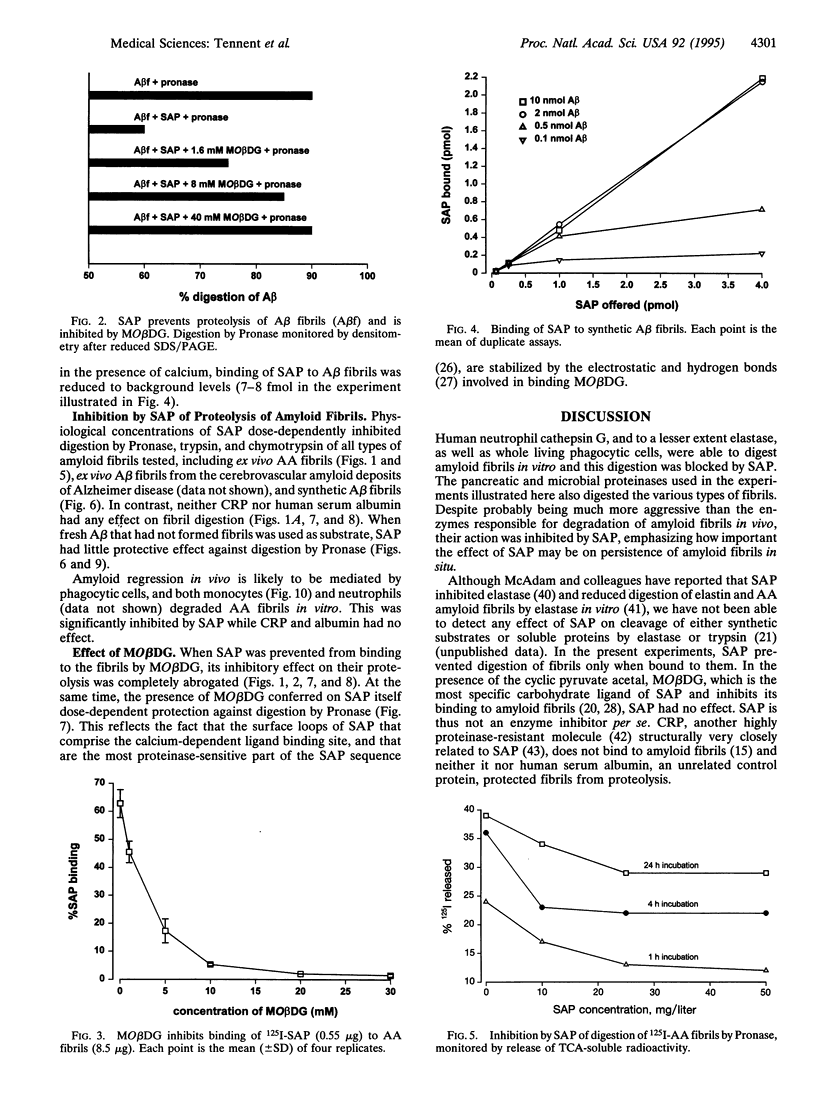
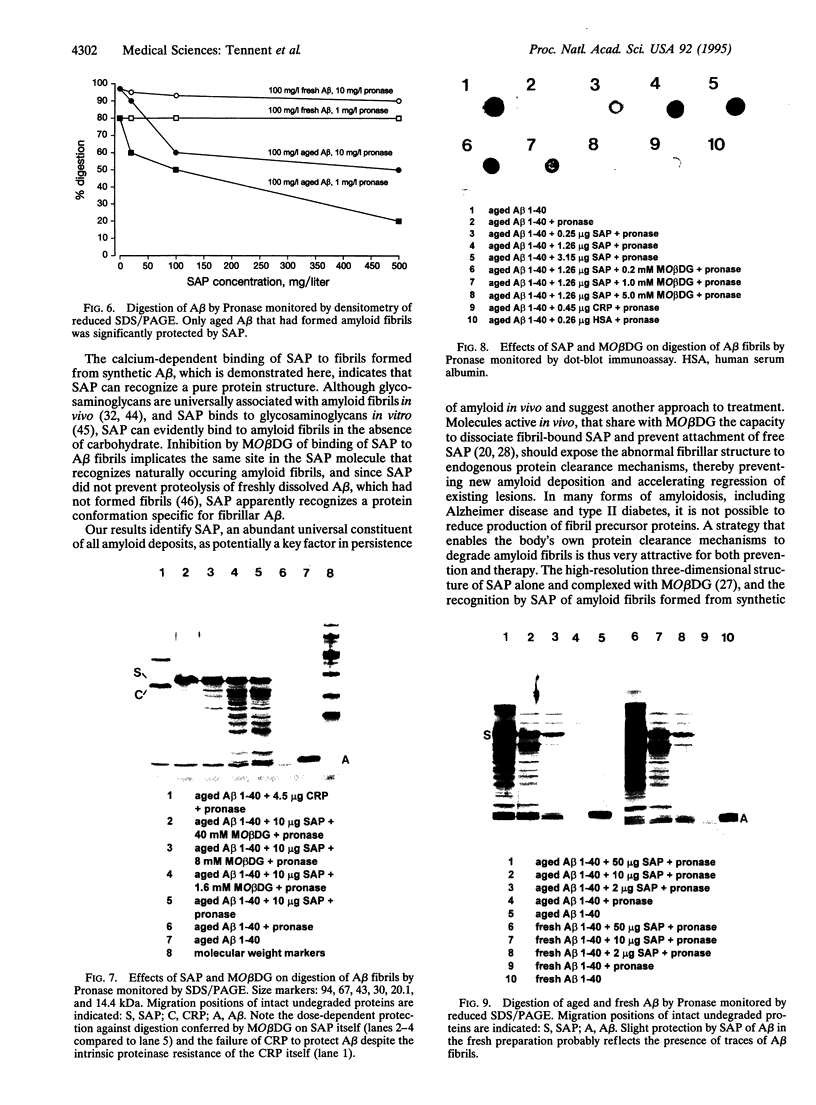
Extracellular deposition of amyloid fibrils is responsible for the pathology in the systemic amyloidoses and probably also in Alzheimer disease [Haass, C. & Selkoe, D. J. (1993) Cell 75, 1039-1042] and type II diabetes mellitus [Lorenzo, A., Razzaboni, B., Weir, G. C. & Yankner, B. A. (1994) Nature (London) 368, 756-760]. The fibrils themselves are relatively resistant to proteolysis in vitro but amyloid deposits do regress in vivo, usually with clinical benefit, if new amyloid fibril formation can be halted. Serum amyloid P component (SAP) binds to all types of amyloid fibrils and is a universal constituent of amyloid deposits, including the plaques, amorphous amyloid beta protein deposits and neurofibrillary tangles of Alzheimer disease [Coria, F., Castano, E., Prelli, F., Larrondo-Lillo, M., van Duinen, S., Shelanski, M. L. & Frangione, B. (1988) Lab. Invest. 58, 454-458; Duong, T., Pommier, E. C. & Scheibel, A. B. (1989) Acta Neuropathol. 78, 429-437]. Here we show that SAP prevents proteolysis of the amyloid fibrils of Alzheimer disease, of systemic amyloid A amyloidosis and of systemic monoclonal light chain amyloidosis and may thereby contribute to their persistence in vivo. SAP is not an enzyme inhibitor and is protective only when bound to the fibrils. Interference with binding of SAP to amyloid fibrils in vivo is thus an attractive therapeutic objective, achievement of which should promote regression of the deposits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Yamada T., Kawamata T., McGeer P. L. Association of amyloid P component with complement proteins in neurologically diseased brain tissue. Brain Res. 1991 May 10;548(1-2):349–352. doi: 10.1016/0006-8993(91)91148-t. [DOI] [PubMed] [Google Scholar]
- Baltz M. L., De Beer F. C., Feinstein A., Pepys M. B. Calcium-dependent aggregation of human serum amyloid P component. Biochim Biophys Acta. 1982 Feb 18;701(2):229–236. doi: 10.1016/0167-4838(82)90118-2. [DOI] [PubMed] [Google Scholar]
- Caspi D., Baltz M. L., Feinstein A., Munn E. A., Pepys M. B. 'Amyloid degrading activity' of human serum, an in vitro clearing effect which does not involve degradation of amyloid fibrils. Clin Exp Immunol. 1984 Sep;57(3):647–656. [PMC free article] [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Duong T., Doucette T., Zidenberg N. A., Jacobs R. W., Scheibel A. B. Microtubule-associated proteins tau and amyloid P component in Alzheimer's disease. Brain Res. 1993 Feb 12;603(1):74–86. doi: 10.1016/0006-8993(93)91301-8. [DOI] [PubMed] [Google Scholar]
- Duong T., Pommier E. C., Scheibel A. B. Immunodetection of the amyloid P component in Alzheimer's disease. Acta Neuropathol. 1989;78(4):429–437. doi: 10.1007/BF00688180. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P., Hack C. E., Rozemuller J. M., Stam F. C. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56(4):259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- Emsley J., White H. E., O'Hara B. P., Oliva G., Srinivasan N., Tickle I. J., Blundell T. L., Pepys M. B., Wood S. P. Structure of pentameric human serum amyloid P component. Nature. 1994 Jan 27;367(6461):338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Ca2+-mediated association of human serum amyloid P component with heparan sulfate and dermatan sulfate. J Biol Chem. 1987 Feb 5;262(4):1456–1460. [PubMed] [Google Scholar]
- Hawkins P. N., Richardson S., MacSweeney J. E., King A. D., Vigushin D. M., Lavender J. P., Pepys M. B. Scintigraphic quantification and serial monitoring of human visceral amyloid deposits provide evidence for turnover and regression. Q J Med. 1993 Jun;86(6):365–374. [PubMed] [Google Scholar]
- Hawkins P. N., Richardson S., Vigushin D. M., David J., Kelsey C. R., Gray R. E., Hall M. A., Woo P., Lavender J. P., Pepys M. B. Serum amyloid P component scintigraphy and turnover studies for diagnosis and quantitative monitoring of AA amyloidosis in juvenile rheumatoid arthritis. Arthritis Rheum. 1993 Jun;36(6):842–851. doi: 10.1002/art.1780360616. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Tennent G. A., Woo P., Pepys M. B. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis. Clin Exp Immunol. 1991 May;84(2):308–316. doi: 10.1111/j.1365-2249.1991.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. N., Wootton R., Pepys M. B. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J Clin Invest. 1990 Dec;86(6):1862–1869. doi: 10.1172/JCI114917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Caspi D., Baltz M. L., Pepys M. B. Specific chemical dissociation of fibrillar and non-fibrillar components of amyloid deposits. Lancet. 1984 Aug 18;2(8399):376–378. doi: 10.1016/s0140-6736(84)90544-0. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren G., Ericzon B. G., Groth C. G., Steen L., Suhr O., Andersen O., Wallin B. G., Seymour A., Richardson S., Hawkins P. N. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993 May 1;341(8853):1113–1116. doi: 10.1016/0140-6736(93)93127-m. [DOI] [PubMed] [Google Scholar]
- Husby G. Nomenclature and classification of amyloid and amyloidoses. J Intern Med. 1992 Dec;232(6):511–512. doi: 10.1111/j.1365-2796.1992.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson W. L., Noble G. E., Hawkins P. N., Pepys M. B. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. J Clin Invest. 1994 Oct;94(4):1390–1396. doi: 10.1172/JCI117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. T., Marzloff K., Arriagada P. V. The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993 Nov;52(6):594–600. doi: 10.1097/00005072-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Iseki E., Amano N., Matsuishi T., Yokoi S., Arai N., Yagishita S. A case of familial, atypical Alzheimer's disease: immunohistochemical study of amyloid P-component. Neuropathol Appl Neurobiol. 1988 Mar-Apr;14(2):169–174. doi: 10.1111/j.1365-2990.1988.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Galloway P. G., Perry G. Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol Appl Neurobiol. 1991 Jun;17(3):189–201. doi: 10.1111/j.1365-2990.1991.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Golde T. E., Cohen M. L., Younkin S. G. Serum amyloid P in Alzheimer's disease. Implications for dysfunction of the blood-brain barrier. Ann N Y Acad Sci. 1991;640:145–148. doi: 10.1111/j.1749-6632.1991.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Grahovac I. Serum amyloid P immunoreactivity in hippocampal tangles, plaques and vessels: implications for leakage across the blood-brain barrier in Alzheimer's disease. Brain Res. 1990 May 21;516(2):349–353. doi: 10.1016/0006-8993(90)90941-4. [DOI] [PubMed] [Google Scholar]
- Kinoshita C. M., Gewurz A. T., Siegel J. N., Ying S. C., Hugli T. E., Coe J. E., Gupta R. K., Huckman R., Gewurz H. A protease-sensitive site in the proposed Ca(2+)-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1992 Jun;1(6):700–709. doi: 10.1002/pro.5560010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C. M., Ying S. C., Hugli T. E., Siegel J. N., Potempa L. A., Jiang H., Houghten R. A., Gewurz H. Elucidation of a protease-sensitive site involved in the binding of calcium to C-reactive protein. Biochemistry. 1989 Dec 12;28(25):9840–9848. doi: 10.1021/bi00451a044. [DOI] [PubMed] [Google Scholar]
- Li J. J., McAdam K. P. Human amyloid P component: an elastase inhibitor. Scand J Immunol. 1984 Sep;20(3):219–226. doi: 10.1111/j.1365-3083.1984.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Maggio J. E., Stimson E. R., Ghilardi J. R., Allen C. J., Dahl C. E., Whitcomb D. C., Vigna S. R., Vinters H. V., Labenski M. E., Mantyh P. W. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. R., Lyon M., Gallagher J. T., Johnson E. A., Pepys M. B. Isolation and characterization of the integral glycosaminoglycan constituents of human amyloid A and monoclonal light-chain amyloid fibrils. Biochem J. 1991 Apr 1;275(Pt 1):67–73. doi: 10.1042/bj2750067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Amyloidosis: some recent developments. Q J Med. 1988 Apr;67(252):283–298. [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Rademacher T. W., Amatayakul-Chantler S., Williams P., Noble G. E., Hutchinson W. L., Hawkins P. N., Nelson S. R., Gallimore J. R., Herbert J. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest. 1987 Jan;56(1):120–123. [PubMed] [Google Scholar]
- Soutar A. K., Hawkins P. N., Vigushin D. M., Tennent G. A., Booth S. E., Hutton T., Nguyen O., Totty N. F., Feest T. G., Hsuan J. J. Apolipoprotein AI mutation Arg-60 causes autosomal dominant amyloidosis. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7389–7393. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan N., White H. E., Emsley J., Wood S. P., Pepys M. B., Blundell T. L. Comparative analyses of pentraxins: implications for protomer assembly and ligand binding. Structure. 1994 Nov 15;2(11):1017–1027. doi: 10.1016/S0969-2126(94)00105-7. [DOI] [PubMed] [Google Scholar]
- Vachino G., Heck L. W., Gelfand J. A., Kaplan M. M., Burke J. F., Berninger R. W., McAdam K. P. Inhibition of human neutrophil and Pseudomonas elastases by the amyloid P-component: a constituent of elastic fibers and amyloid deposits. J Leukoc Biol. 1988 Dec;44(6):529–534. doi: 10.1002/jlb.44.6.529. [DOI] [PubMed] [Google Scholar]
- Vigushin D. M., Pepys M. B., Hawkins P. N. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993 Apr;91(4):1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]