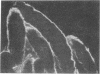
Abstract
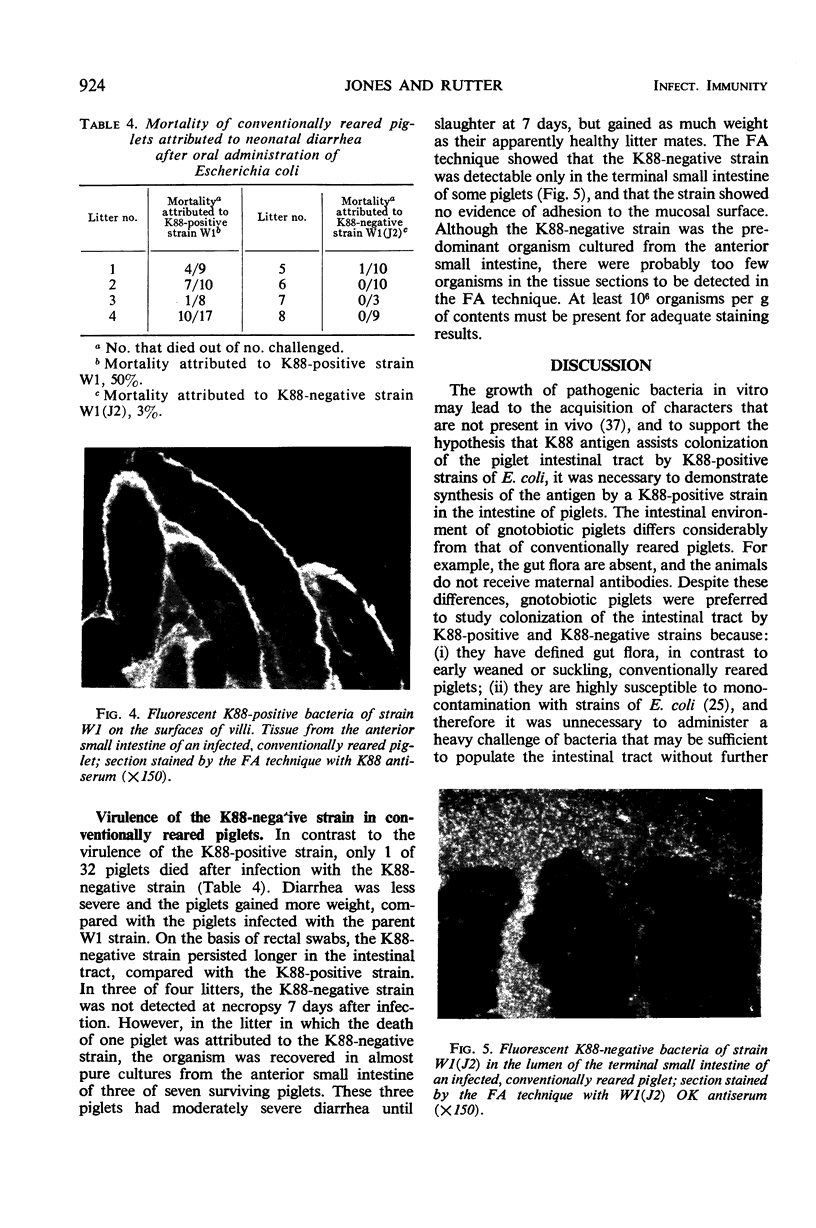
The role of the K88 antigen of Escherichia coli in neonatal diarrhea of piglets was studied by comparing a K88-positive strain with three K88-negative strains derived from the K88-positive strain. K88 antigen was produced by the K88-positive strain in the intestinal tract of gnotobiotic piglets, whereas K88-negative strains did not regain the ability to synthesize K88 antigen. Synthesis of the antigen conferred different colonization characteristics on the four strains; K88-positive bacteria adhered to the mucosa of the small intestine, whereas K88-negative bacteria did not attach and were distributed throughout the lumen. Adhesion of K88-positive bacteria to tissue from the small intestine of gnotobiotic piglets was demonstrated in vitro and was inhibited by antisera that contained K88 antibodies. Attachment did not occur with bacteria grown at 18 C. Adhesion of cell-free K88 antigen was also demonstrated. The K88-positive strain and one of the K88-negative strains were equally virulent in gnotobiotic piglets. In contrast, the K88-positive strain killed 50% of conventionally reared piglets, whereas the K88-negative strain killed only 3%. Adhesion of the K88-positive strain, but not of the K88-negative strain, to the mucosa of the small intestine was demonstrated. Our results show that K88 antigen is responsible for attachment of K88-positive bacteria to the wall of the small intestine, and that adhesion is essential for the virulence of K88-positive bacteria in conventionally reared piglets.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on the resistance of the small intestine to infection. J Bacteriol. 1966 Dec;92(6):1604–1608. doi: 10.1128/jb.92.6.1604-1608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle J. B. Enteropathogenic Escherichia coli on the intestinal mucopolysaccharide layer of pigs. J Pathol. 1971 Jun;104(2):93–98. doi: 10.1002/path.1711040203. [DOI] [PubMed] [Google Scholar]
- Arbuckle J. B. The attachment of Clostridium welchii (Cl. perfringens) type C to intestinal villi of pigs. J Pathol. 1972 Feb;106(2):65–72. doi: 10.1002/path.1711060202. [DOI] [PubMed] [Google Scholar]
- Arbuckle J. B. The location of Escherichia coli in the pig intestine. J Med Microbiol. 1970 May;3(2):333–340. doi: 10.1099/00222615-3-2-333. [DOI] [PubMed] [Google Scholar]
- Beck J. S., Currie A. R. Immunofluorescence localization of growth hormone in the human pituitary gland and of a related antigen in the syncytiotrophoblast. Vitam Horm. 1967;25:89–121. doi: 10.1016/s0083-6729(08)60034-5. [DOI] [PubMed] [Google Scholar]
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim K. A., Taylor J. Soluble antigens of enteropathogenic Escherichia coli. J Med Microbiol. 1970 Nov;3(4):655–667. doi: 10.1099/00222615-3-4-655. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees D. T., Waxler D. L. Enteric colibacillosis in gnotobiotic swine: a fluorescence microscopic study. Am J Vet Res. 1970 Jul;31(7):1147–1157. [PubMed] [Google Scholar]
- Drees D. T., Waxler G. L. Enteric colibacillosis in gnotobiotic swine: an electron microscopic study. Am J Vet Res. 1970 Jul;31(7):1159–1171. [PubMed] [Google Scholar]
- Drucker M. M., Yeivin R., Sacks T. G. Pathogenesis of Escherichia coli enteritis in the ligated rabbit gut. Isr J Med Sci. 1967 May-Jun;3(3):445–452. [PubMed] [Google Scholar]
- FORMAL S. B., ABRAMS G. D., SCHNEIDER H., SPRINZ H. Experimental Shigella infections. VI. Role of the small intestine in an experimental infection in guinea pigs. J Bacteriol. 1963 Jan;85:119–125. doi: 10.1128/jb.85.1.119-125.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossling J., Rhoades H. E. Identification of certain Escherichia coli strains isolated from baby pigs in north central United States. Am J Vet Res. 1967 Sep;28(126):1615–1617. [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. Escherichia coli in ligated segments of pig intestine. J Pathol Bacteriol. 1967 Jul;94(1):189–194. doi: 10.1002/path.1700940124. [DOI] [PubMed] [Google Scholar]
- Hawksworth G., Drasar B. S., Hill M. J. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971 Nov;4(4):451–459. doi: 10.1099/00222615-4-4-451. [DOI] [PubMed] [Google Scholar]
- KENWORTHY R., CRABB W. E. THE INTESTINAL FLORA OF YOUNG PIGS, WITH REFERENCE TO EARLY WEANING, ESCHERICHIA COLI AND SCOURS. J Comp Pathol. 1963 Jul;73:215–228. doi: 10.1016/s0368-1742(63)80025-9. [DOI] [PubMed] [Google Scholar]
- Kohler E. M. Studies of escherichia coli in gnotobiotic pigs. IV. Comparison of enteropathogenic and nonenteropathogenic strains. Can J Comp Med Vet Sci. 1967 Nov;31(11):277–282. [PMC free article] [PubMed] [Google Scholar]
- Kramer T. T., Nderito P. C. Experimental Escherichia coli diarrhea in hysterectomy-derived, one-day-old, fasting pigs. Am J Vet Res. 1967 Jul;28(125):959–964. [PubMed] [Google Scholar]
- Mackenzie A., Wilson A. M. Accumulations of fat in the brains of mice affected with scrapie. Res Vet Sci. 1966 Jan;7(1):45–54. [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., LEACH J. M. Simultaneous occurrence of E. coli B and Lantigens in strains from diseased swine. Influence of cultivation temperature. Two new E. coli Kantigens: K 87 and K 88. Acta Pathol Microbiol Scand. 1961;53:404–422. [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Episome-carried surface antigen K88 of Escherichia coli. I. Transmission of the determinant of the K88 antigen and influence on the transfer of chromosomal markers. J Bacteriol. 1966 Jan;91(1):69–75. doi: 10.1128/jb.91.1.69-75.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Wittig W., Sweeney E. J. A new E. coli serotype O149:K9 (B), K88ac (L): H10 isolated from diseased swine. Acta Pathol Microbiol Scand. 1969;75(3):491–498. [PubMed] [Google Scholar]
- ROSS C. A. Mucinase activity of intestinal organisms. J Pathol Bacteriol. 1959 Apr;77(2):642–644. doi: 10.1002/path.1700770237. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Anderson J. C. Experimental neonatal diarrhoea caused by an enteropathogenic strain of Escherichia coli in piglets: a study of the disease and the effect of vaccinating the dam. J Med Microbiol. 1972 May;5(2):197–210. doi: 10.1099/00222615-5-2-197. [DOI] [PubMed] [Google Scholar]
- SMITH H. W., JONES J. E. OBSERVATIONS ON THE ALIMENTARY TRACT AND ITS BACTERIAL FLORA IN HEALTHY AND DISEASED PIGS. J Pathol Bacteriol. 1963 Oct;86:387–412. [PubMed] [Google Scholar]
- Scherer R. K. Colonial morphology of Escherichia coli on Tergitol-7 medium. Appl Microbiol. 1966 Mar;14(2):152–155. doi: 10.1128/am.14.2.152-155.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Observations by the ligated intestinal segment and oral inoculation methods on Escherichia coli infections in pigs, calves, lambs and rabbits. J Pathol Bacteriol. 1967 Apr;93(2):499–529. doi: 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The production of oedema disease and diarrhoea in weaned pigs by the oral administration of Escherichia coli: factors that influence the course of the experimental disease. J Med Microbiol. 1968 Aug;1(1):45–59. doi: 10.1099/00222615-1-1-45. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Jones E. W., Corley L. D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969 Sep;56(3):371–392. [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J., MALTBY M. P., PAYNE J. M. Factors influencing the response of ligated rabbit-gut segments to injected Escherichia coli. J Pathol Bacteriol. 1958 Oct;76(2):491–499. doi: 10.1002/path.1700760218. [DOI] [PubMed] [Google Scholar]
- Tavernor W. D., Trexler P. C., Vaughan L. C., Jones D. G. The production of gnotobiotic piglets and calves by hysterotomy under general anaesthesia. Vet Rec. 1971 Jan 2;88(1):10–14. doi: 10.1136/vr.88.1.10. [DOI] [PubMed] [Google Scholar]
- Trexler P. C. Microbiological isolation of large animals. Vet Rec. 1971 Jan 2;88(1):15–20. doi: 10.1136/vr.88.1.15. [DOI] [PubMed] [Google Scholar]