Abstract
Background:
Atherosclerotic coronary artery disease (CAD) has long been shown to involve chronic low-grade subclinical inflammation. However, whether there is association between hematological indices assessed by complete blood count (CBC) and coronary atherosclerotic burden has not been well studied.
Materials and Methods:
Consecutive 868 patients without known CAD who presented with acute chest pain to emergency department and underwent coronary artery calcium (CAC) scoring evaluation by multi-detector cardiac computed tomography were included in our study. Clinical characteristics and CBC indices were compared among different CAC groups.
Results:
The cohort comprised 60% male with a mean age of 61 (SD = 14) years. Median Framingham risk of CAD was 4% (range 1-16%). Median CAC score was 0 (IQR 0-43). Higher CAC groups had significantly higher Framingham risk of CAD than lower CAC groups (P < 0.001). Among different CAC categories, there was no statistically significant difference in hemoglobin level (p 0.45), mean corpuscular volume (p 0.43), mean corpuscular hemoglobin (p 0.28), mean corpuscular hemoglobin volume (p 0.36), red cell distribution width (0.42), total white blood cell counts (p 0.291), neutrophil counts (p 0.352), lymphocyte counts (p 0.92), neutrophil to lymphocyte ratio (p 0.68), monocyte count (p 0.48), and platelet counts (p 0.25).
Conclusion:
Our study did not detect significant association between hematological indices assessed with CBC and coronary calcification in symptomatic patients without known CAD.
Keywords: Coronary artery disease, Inflammation, Neutrophil-to-lymphocyte ratio, Red cell distribution width, White blood cell
Introduction
Atherosclerotic coronary artery disease (CAD) has long been shown to involve inflammatory processes. Numerous pathways and markers have been studied in order to detect the presence and evolution of this disease. The role of various biological inflammatory markers as risk factors and prognosticators has been elucidated in different CAD patients ranging from population-based asymptomatic subjects[1] to patients with myocardial infarction undergoing coronary artery bypass graft surgery (CABG).[2] In addition, since the earlier phase of CAD involves asymptomatic coronary artery calcification (CAC), there have also been different studies examining the relationship between multiple classic as well as novel biological markers of inflammation and CAC. However, most of the current studies that investigated associations between inflammatory biomarkers and CAC used novel markers that might not be readily available clinically in general hospital settings and they were conducted in asymptomatic subjects. Data with classic inflammatory markers derived from complete blood count (CBC) such as red blood cell indices, white blood cell indices, and platelet counts is still conflicting and sparse in symptomatic patients. Whether various blood cell counts and ratios can reflect presence and/or extent of CAC in this population with CAD is still uncertain.
The objective of our study is to examine the relationship between hematological indices assessed with CBC and CAC in symptomatic patients without known history of CAD.
Materials and Methods
Study protocol and patient selection
This study is a cross-sectional analysis of a prospective observational cohort study examining usefulness of CAC score in triaging chest pain patients presenting at emergency department in our hospital, which is described elsewhere.[3] Subjects were consecutive patients older than 18 years who were admitted under observational status for further evaluation of acute chest pain of unknown cardiac significance but suggestive of myocardial ischemia within the previous 24 hours. The decision to discharge patients home, admit patients under observational status, or admit patients under full admission status is determined by emergency department physicians or the patient's personal physician. The chest pain observation protocol includes performing serial 12-lead electrocardiograms (EKGs), troponin level, and a stress test. Exclusion criteria included patients with non-cardiac chest pain based on clinical assessment (e.g., pleuritic, musculoskeletal chest pain), history of CAD based on previous coronary angiography or prior coronary revascularization, elevated troponin in initial blood samples, new or presumably new ST-segment elevation or depression (≥1 mm) on baseline electrocardiogram, hemodynamic or clinical instability defined by systolic blood pressure <90 mmHg or clinically significant atrial/ventricular arrhythmia, known or suspected pregnancy and patients who were not to provide informed consent. In this analysis, patients without complete CBC information were also excluded. None of the investigators were involved in clinical management of enrolled patients beyond interpretation of their images. Institutional Review Board approval was obtained for this study.
Blood cell counts
CBC information used in this analysis was from blood samples drawn on initial emergency department encounter. RBC indices (hemoglobin, Hb; mean corpuscular volume, MCV; mean corpuscular hemoglobin, MCH; mean corpuscular hemoglobin concentration, MCHC and red blood cell distribution width, RDW), WBC counts with differentials (neutrophil; lymphocyte; neutrophil-to-lymphocyte ratio, NLR; and monocytes), and platelet counts data were collected. NLR was calculated by dividing absolute neutrophil count by absolute lymphocyte count.
CAC scoring
CAC scoring was performed within 24 hours after admission from emergency department. CAC score was measured by a 16-slice multi-detector computed tomography (CT) scanner (Philips Precedence, Philips Healthcare, Eindhoven, The Netherlands). Images were acquired during a single breath hold, using prospective EKG gating with imaging triggered at 75% of the R-R interval (collimation 8 × 2.5 mm, voltage 120 keV, current 75 mA). CAC score was calculated, as previously described by Agatston et al.[4] Patients were categorized into four groups based on their CAC extent: Absent CAC (CAC score = 0), mild CAC (CAC score 1-100), moderate CAC (CAC score 101-400), and severe CAC (CAC score > 400).
Data gathering and processing
During the observation period, all clinical information was collected including demographic information, cardiovascular history, and blood samples for lipid profiles, cardiac biomarkers and renal function tests. Cardiovascular history included information on cardiovascular symptoms; history of hypertension, diabetes mellitus, dyslipidemia, smoking, peripheral arterial disease, carotid artery disease, abdominal aortic aneurysm; family history of CAD and current cardiovascular medication profiles. Clinical information was used to calculate Framingham CAD 10-year risk score. 10-year risk of CAD was analyzed from the score. Data were examined for outliers by outlier labeling method.[5] Outliers (less than 5% of all cohorts) were winsorized. All continuous variables were examined for nature of distribution. Logarithmic transformation was performed on all non-normally distributed variables.
Statistical analysis
Patients were divided into four different categories based on their CAC score. Descriptive statistics for studied variables are presented as mean (standard deviation, SD) for normally distributed variables, median (interquartile range, IQR) for non-normally-distributed variables and frequency (percentage) of categorical variables. Spearman correlation was performed between study variables and CAC score. Analysis of variance (ANOVA) and Student's t-test were used to identify differences in means between CAC categories. Kruskal-Wallis H test and Wilcox-Mann-Whitney U-test were used to examine differences in medians between CAC categories. χ2 analysis was used to identify significant heterogeneity in the frequencies. Univariable and multivariable analysis were performed with binary logistic regression and linear regression. Logarithmic transformation of CAC score was used for linear regression test. Each CBC index was adjusted for Framingham CAD risk in multivariable analysis. All statistical tests were performed with IBM SPSS/PASW Statistics 20 (SPSS Inc., Chicago, IL). A two-tailed P value < 0.05 was considered statistically significant.
Results
Study population characteristics
Consecutive 868 patients, who underwent Agatston CAC scoring during observation period and had CBC performed, were included in the final analysis. Baseline and study clinical characteristics are shown in Table 1.
Table 1.
Baseline characteristics
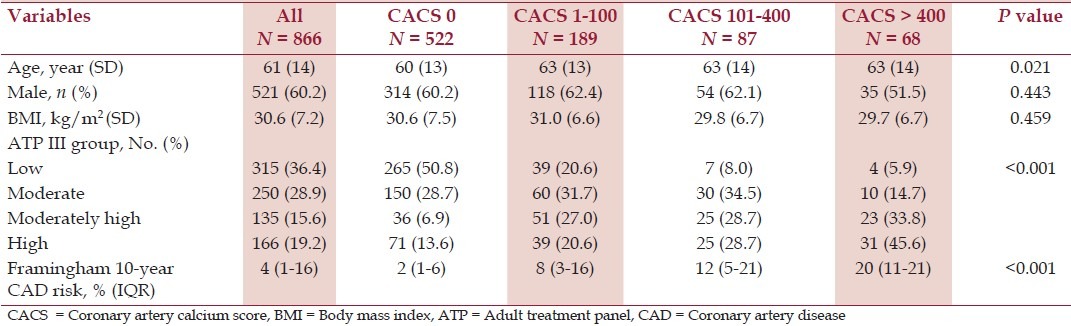
The median CAC score in our study population was 0 (IQR 0-43). Most (522 of 866, 60.3%) had absent CAC, followed by 21.8% (189 of 866) with mild CAC, 10.0% (87 of 866) with moderate CAC, and 7.9% (68 of 866) with severe CAC. Mean age of patients in absent CAC group was lower than patients with CAC (overall P = 0.021). However, only patients with mild CAC were significantly older than patients with absent CAC (P = 0.013), but not patients with moderate CAC (P = 0.074) or severe CAC (P = 0.054). Higher CAC groups had significantly higher 10-year risk for CAD than lower CAC groups predicted by Framingham risk score (overall P < 0.001). Compared to absent CAC group, mild CAC, moderate CAC, and severe CAC all had higher 10-year risk for CAD (P < 0.001 for every group). Moderate CAC and severe CAC had statistically higher CAD risk than mild CAC (P = 0.008 and P < 0.001, respectively). Severe CAC had higher CAD risk than moderate CAC, although it was not statistically significant (P = 0.066). There was no statistically significant difference in gender (overall P = 0.443) or BMI (overall P = 0.459) among CAC groups.
Blood counts and CAC
Overall, all hematological indices were within the normal limit according to our laboratory references as demonstrated in Figures 1 and 2. There was no statistically significant difference in hemoglobin level (p 0.45), mean corpuscular volume (p 0.43), mean corpuscular hemoglobin (p 0.28), mean corpuscular hemoglobin volume (p 0.36), red cell distribution width (0.42), total WBC counts (P = 0.29), relative neutrophil counts (P = 0.88), absolute neutrophil counts (P = 0.35), relative lymphocyte counts (P = 0.85), absolute lymphocyte counts (P = 0.92), NLR (P = 0.68), relative monocyte count (p 0.35), absolute monocyte count (p 0.48) or platelet counts (P = 0.25) among the 4 CAC categories. After reclassifying patients into dichotomous categories: absent CAC (CAC score = 0) and present CAC (CAC score > 0) or absent-to-mild CAC (CAC score ≤ 100) and moderate-to-severe CAC (CAC score > 100), there was still no significant difference between all cell counts or NLR.
Figure 1.
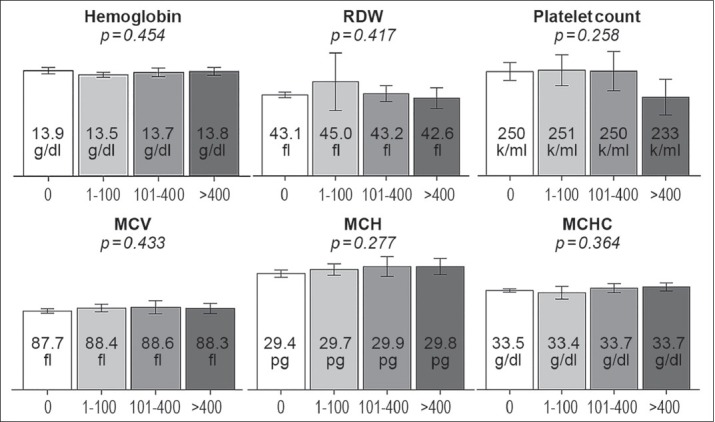
Mean ± 95% confidence interval of red blood cell indices (hemoglobin level, mean corpuscular volume, MCV; mean corpuscular hemoglobin, MCH; mean corpuscular hemoglobin concentration, MCHC; red cell distribution width, RDW) and platelet level among different coronary artery calcium score categories (0, 1– 100, 101– 400, >400)
Figure 2.
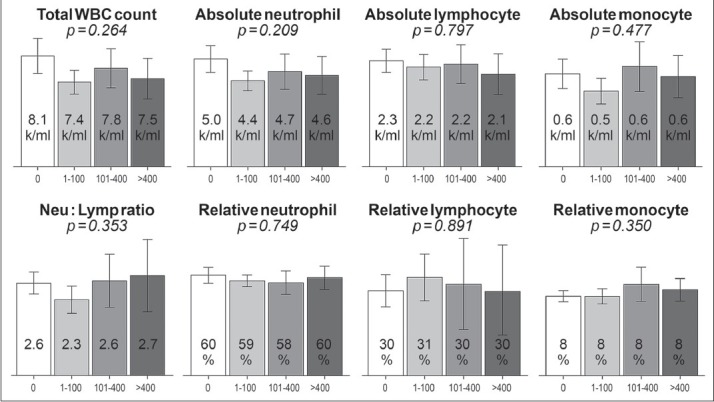
Mean ± 95% confidence interval of white blood cell (WBC) indices including absolute/relative neutrophil counts, absolute/relative lymphocyte counts, absolute/relative monocyte counts, neutrophil-to-lymphocyte ratio (Neu: Lymp ratio) among different coronary artery calcium score categories (0, 1-100, 101-400, >400)
In correlation analysis, MCH had weak but significant association with CAC score (coefficient 0.075, P = 0.03). Examination on other hematological indices did not show statistically significant correlation with CAC score including hemoglobin (P = 0.79), MCV (P = 0.17), MCHC (P = 0.11), RDW (P = 0.64), total WBC counts (P = 0.18), relative neutrophil counts (P = 0.63), absolute neutrophil count (P = 0.17), relative lymphocyte counts (P = 070), absolute lymphocyte count (P = 0.76), NLR (P = 0.77), relative monocyte counts (P = 0.24), absolute monocyte count (P = 0.66), and platelet counts (P = 0.54). With all of the above analysis separately performed for each gender, no statistically significant differences or correlations were shown.
In regression model analyses, higher MCH was found to have significant univariate association with higher absolute CAC score (P = 0.03); however, after adjustment with Framingham risk, the association diminished and did not reach the predefined significant threshold (P = 0.09). Other hematological indices were not associated with either presence of CAC or CAC score as shown in Table 2.
Table 2.
Association of hematological indices with presence of coronary calcification and coronary artery calcium score (CACS)

Discussion
Role of inflammation in the pathogenesis of atherosclerotic CAD has been extensively studied for the past decades. There is evidence of inflammation in every stage of the disease from the very beginning of atherosclerosis when fatty streaks are formed on the vascular wall. This is demonstrated by presence of leukocytes in fatty streaks and their transformation into foam cells. This eventually leads to atherosclerotic plaque formation and to myocardial infarction.[6] As further evidence, medications with anti-inflammatory effect such as aspirin, statins, and steroids have been shown to affect clinical outcomes in various populations of CAD.[7] In addition, in patients with systemic inflammatory disorders such as rheumatoid arthritis (RA), it has been illustrated that prevalence of atherosclerotic CAD is increased and progression of CAC is accelerated compared to those without RA.[8,9]
The main finding of this study is the lack of statistically significant association between CAC detected by cardiac CT and hematological indices using CBC, which is readily available clinically in symptomatic patients without known CAD. This finding is inconsistent with a recently published cardiac CT-based study by Korkmaz et al.[10] which demonstrated a strong relationship between total WBC counts and presence of Agatston CAC score (OR 1.7; 95%CI 1.3-2.1, P < 0.001) as well as extent of CAC (correlation coefficient 0.57, P < 0.001). However, their study was conducted in asymptomatic patients in contrast to our study. WBC count along with its absolute and relative differential counts, mainly neutrophil and lymphocyte, have also been investigated sporadically in other CAD populations for various outcomes. In asymptomatic subjects, Suzuki et al.[11] showed that low relative lymphocyte count was an independent predictor for long-term CAD events in patients with type 2 diabetes mellitus. In symptomatic CAD, Han et al.[12] exhibited that high NLR was associated with worse clinical outcomes including mortality, non-fatal myocardial infarction, and ischemic stroke in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous intervention (PCI). Likewise, Soylu et al.[13] described the role of NLR as an independent predictor for no-reflow development in a similar patient population. In addition, their study also showed a statistically significant inverse correlation between corrected Thrombolysis in Myocardial Infarction (TIMI) frame counts and lymphocyte counts. However, total WBC counts and neutrophil counts did not show significant correlation as such. Correspondingly, Rashidi et al.[2] examined CAD patients undergoing CABG surgery and found that increased total WBC counts preoperatively was an independent predictor for recurrence of ischemic events within 1 year of surgery.
Relationship between CAC and other inflammatory markers has also been extensively studied. Most investigations demonstrated positive but weak association between these biological markers and presence or extent of CAC. After adjustment with baseline characteristics such as body mass index, the associations were lost. C-reactive protein (CRP) is one of the most studied markers. It has been shown that, in asymptomatic subjects without apparent CAD, high CRP was associated with the presence of CAC (CAC score > 0) and CAC score > 10.[1,14] In addition, value of CRP was also shown to correlate with value of CAC score.[14] In contrast, Redberg et al.[15] demonstrated an inverse relationship between high CRP and CAC detected by electron beam CT (EBCT) in postmenopausal women. However, their population size was modest and comprised high proportion of patients without CAC (44%). Other markers that have been described in association with CAC in recent literature include fibrinogen,[1,14] monocyte chemotactic protein-1 (MCP-1),[14] resistin,[14] lipoprotein-associated phospholipase A2 (Lp-PLA2),[14] tumor necrosis factor alpha (TNF-α),[14] beta fibroblast growth factor (β-FGF),[14] and interleukin-6 (IL-6).[16] In addition, relationships between clinical events or other surrogates and these inflammatory biomarkers have also been examined. Those markers are not only limited to the aforementioned markers but also include interleukin-18,[17] gelatinase-associated lipocalin,[18] vaspin,[19] macrophage migration inhibiting factor (MMIF),[20] and fetuin-A.[21]
In summary, we have demonstrated the lack of significant association between CBC indices and CAC in symptomatic patients suspected for CAD. These findings are in contrary to most of the other current studies in literature, regarding inflammatory markers and CAD. This discrepancy might possibly be from robustness of CBC indices in detecting chronic low level of inflammation in atherosclerotic CAD, heterogeneity between previous studies and our studies in the studied population, definition of each CAD group as well as imaging modalities to detect CAC. Nevertheless, our findings suggest that none of CBC indices can be used reliably as a marker of CAC in clinical setting.
The limitation of this study is the single-center cross-sectional nature of examining association between the hematological indices and CAC score. Causal relationship cannot be established from this study design. Also some patients did not have CBC performed, so we excluded those patients from the study. This might create a bias in our series.
Conclusion
Our study did not detect significant association between Agatston coronary artery calcification score and hematological indices assessed with CBCs including hemoglobin level, MCV, MCH, MCHC, RDW, total WBC counts, absolute/relative neutrophil counts, absolute/relative lymphocyte counts, neutrophil-to-lymphocyte ratio, absolute/relative monocyte counts, and platelet counts in symptomatic patients without history of CAD. The findings were in contrast with the previously reported data and potentially suggested that CBC indices could not be used reliably as a marker of CAD in clinical setting.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, et al. Associations of inflammatory markers with coronary artery calcification: Results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–9. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rashidi F, Jamshidi P, Kheiri M, Ashrafizadeh S, Ashrafizadeh A, Abdolalian F, et al. Is leukocytosis a predictor for recurrence of ischemic events after coronary artery bypass graft surgery. A cohort study? ISRN Cardiol. 2012;2012:824730. doi: 10.5402/2012/824730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabi F, Chang SM, Pratt CM, Paranilam J, Peterson LE, Frias ME, et al. Coronary artery calcium scoring in the emergency department: Identifying which patients with chest pain can be safely discharged home. Ann Emerg Med. 2010;56:220–9. doi: 10.1016/j.annemergmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 5.Hoaglin DC, Iglewicz B. Fine tuning some resistant rules for outlier labeling. J Am Stat Assoc. 1987;82:1147–9. [Google Scholar]
- 6.Pamukcu B, Lip GY, Devitt A, Griffiths H, Shantsila E. The role of monocytes in atherosclerotic coronary artery disease. Ann Med. 2010;42:394–403. doi: 10.3109/07853890.2010.497767. [DOI] [PubMed] [Google Scholar]
- 7.Namdari M, Ghafarzadeh M, Nikoo MA. Efficacy of intramuscular methyl prednisolone in preventing restenosis after coronary artery stenting with bare-metal stainless steel stent: A double-blind, randomised, controlled clinical trial. Cardiovasc J Afr. 2011;22:67–9. doi: 10.5830/CVJA-2010-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deo SS, Chogle AR, Mistry KJ, Shetty RR, Nadkar UL. Increased prevalence of subclinical atherosclerosis in rheumatoid arthritis patients of Indian descent. Exp Clin Cardiol. 2012;17:20–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 10.Korkmaz L, Kul S, Korkmaz AA, Akyüz AR, Aðaç MT, Erkan H, et al. Increased leucocyte count could predict coronary artery calcification in patients free of clinically apparent cardiovascular disease. Turk Kardiyol Dern Ars. 2012;40:223–8. doi: 10.5543/tkda.2012.37801. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Futami-Suda S, Igari Y, Watanabe K, Ouchi M, Suzuki K, et al. Low-molecular-weight lipoprotein (a) and low relative lymphocyte concentration are significant and independent risk factors for coronary heart disease in patients with type 2 diabetes mellitus: Lp(a) phenotype, lymphocyte, and coronary heart disease. Lipids Health Dis. 2013;12:31. doi: 10.1186/1476-511X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han YC, Yang TH, Kim DI, Jin HY, Chung SR, Seo JS, et al. Neutrophil to lymphocyte ratio predicts long-term clinical outcomes in patients with st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013;43:3–9. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soylu K, Yuksel S, Gulel O, Erbay AR, Meric M, Zengin H, et al. The relationship of coronary flow to neutrophil/lymphocyte ratio in patients undergoing primary percutaneous coronary intervention. J Thorac Dis. 2013;5:258–64. doi: 10.3978/j.issn.2072-1439.2013.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: A systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Redberg RF, Rifai N, Gee L, Ridker PM. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: Implications for coronary artery disease screening. J Am Coll Cardiol. 2000;36:39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 16.Saremi A, Anderson RJ, Luo P, Moritz TE, Schwenke DC, Allison M, et al. VADT. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT) Atherosclerosis. 2009;203:610–4. doi: 10.1016/j.atherosclerosis.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferis BJ, Papacosta O, Owen CG, Wannamethee SG, Humphries SE, Woodward M, et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis. 2011;217:227–33. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kafkas N, Demponeras C, Zoubouloglou F, Spanou L, Babalis D, Makris K. Serum levels of gelatinase associated lipocalin as indicator of the inflammatory status in coronary artery disease. Int J Inflam. 2012;2012:189797. doi: 10.1155/2012/189797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobat MA, Celik A, Balin M, Altas Y, Baydas A, Bulut M, et al. The investigation of serum vaspin level in atherosclerotic coronary artery disease. J Clin Med Res. 2012;4:110–3. doi: 10.4021/jocmr841w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller II, Müller KA, Schönleber H, Karathanos A, Schneider M, Jorbenadze R, et al. Macrophage migration inhibitory factor is enhanced in acute coronary syndromes and is associated with the inflammatory response. PLoS One. 2012;7:e38376. doi: 10.1371/journal.pone.0038376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkmen K, Gorgulu N, Uysal M, Ozkok A, Sakaci T, Unsal A, et al. Fetuin-A, inflammation, and coronary artery calcification in hemodialysis patients. Indian J Nephrol. 2011;21:90–4. doi: 10.4103/0971-4065.82128. [DOI] [PMC free article] [PubMed] [Google Scholar]


