Abstract
Background:
Long-term regular follow up of ART is an important component of HIV care. Patients who are lost to follow-up (LTFU) while on treatment compromise their own health and the long-term success of ART programs.
Aim:
This study was aimed at determining the incidence and risk factors for LTFU in HIV patients on ART at ART clinic of Mizan-Aman General Hospital, Ethiopia.
Materials and Methods:
A retrospective cohort study of 2133 people living with HIV/AIDS and attending an ART clinic between 2005 and 2013 was undertaken. LTFU was defined as not taking an ART refill for a period of 3 months or longer from the last attendance for refill and not yet classified as ‘dead’ or ‘transferred-out’. The log-rank test was used to measure differences in time to LTFU between groups and Cox proportional hazards modeling was used to measure predictors of LTFU.
Results:
Of 2133 patients, 53.9% were female. The mean (SD) age of the cohort was 31.5 (8.0), 16 (2.2), and 3.8 (3.0) years for adults, adolescents, and children, respectively. Around 574 (26.7%) patients were defined as LTFU. The cumulative incidence of LTFU was 8.8 (95% CIs 8.1-9.6) per 1000 person months. Patients with regimen substitution (HR 5.2; 95% CIs 3.6-7.3), non-isoniazid (INH) prophylaxis (HR 3.7; 95% CIs 2.3-6.2), adolescent (HR 2.1; 95% CIs 1.3-3.4), and had a baseline CD4 count < 200 cells/mm3 (HR 1.7, 95% CIs 1.3-2.2) were at higher risk of LTFU. WHO clinical stage III (HR 0.6; 95% CIs 0.4-0.9) and IV (HR 0.8; 95% CIs 0.6-1.0) patients at entry were less likely to be LTFU than clinical stage I patients. There was no significant difference in risk of LTFU in males and females.
Conclusion:
Overall, these data suggested that LTFU in this study was high. Patients phase of life, drug related factors, and clinical stages were associated with LTFU in this study. Effective control measures in the at-risk population need to be implemented to improve retention.
Keywords: Anti-retroviral therapy, AIDS, CD4, Cohort, Ethiopia, HIV, Lost to follow up, Mizan-Teferi, Predictors
Introduction
The widespread use of antiretroviral therapy (ART) has transformed national AIDS responses and has had a huge positive impact on health.[1] ART has been shown to reduce transmission of HIV and HIV-related morbidity and mortality.[2] In 2012, 9.7 million people received ART in low- and middle-income countries (LMICs)[1] and, as of 2013, ART prevented an estimated 4.2 million deaths in LMICs in 2002-2012.[1] However, while increased access to ART has continued throughout the world, disparities in ART access still exist.
Despite improved and highly successful programmatic coverage with ART, significant numbers of adults and children drop out of care at various points along the treatment pathway and treatment gains fail to reach sufficient numbers of children and adolescents.[1] It is essential to understand how and why people drop out of treatment programs, since retention of people on ART and ensuring adherence to treatment are critical determinants of successful long-term outcomes. Studies in sub-Saharan Africa have shown that about half of people who test HIV-positive are lost between testing and being assessed for eligibility for therapy, and 32% of people considered eligible for ART are then lost between eligibility assessment and initiation of ART.[3] Data from 23 countries indicate that average retention for people on ART decreases over time, from about 86% at 12 months to 72% at 60 months.[1] Loss to follow-up (LTFU) negatively impacts on the immunological benefit of ART and increases AIDS-related morbidity, mortality, and hospitalizations.[4] LTFU in patients receiving ART can result in serious consequences, such as discontinuation of treatment, drug toxicity, treatment failure due to poor adherence, and drug resistance;[5,6,7] this results in an increased risk of death[8,9,10,11,12,13] of up to 40% in studies of patients LTFU in sub-Saharan Africa.[14,15] Poor nutritional status, lower CD4 count, Tuberculosis (TB) co-infection, advanced clinical staging, younger age, adverse drug reactions, gaps in services, and accessibility to services are some of the predictors reported to be associated with LTFU.[3,14,16,17,18,19]
Most studies from sub-Saharan countries have estimated that 20-40% of patients on ART are lost to follow-up due to underlying causative factors.[20,21] Ethiopia has a national HIV prevalence of 1.9%[22] but the magnitude and predictors of LTFU after initiation of ART are not well-investigated. There are ongoing efforts to develop comprehensive strategies and recommendations to improve monitoring and optimize retention in care. This study aimed to determine the prevalence of, and identify potential risk factors for, LTFU in an ART clinic in southwest Ethiopia.
Materials and Methods
Ethical approval
Ethical approval was obtained from the Institutional Review Board of the Mizan-Tepi University. Written informed consent was not feasible because this was analysis of secondary data retrieved from an electronic database of the Hospital. Data were anonymized and handled confidentially during all phases of research activities.
Design and study setting
A retrospective cohort study was conducted in all patients who initiated ART at the governmental Mizan-Aman General Hospital in the Southern Nations Nationalities People Regional state of Ethiopia, 574 km southwest from Addis Ababa. This ART service was initiated in 2003 and patients who had received ART since 2005 were identified from the program database and selected for study.
Description of loss to follow up
LTFU was defined as not taking an ART refill for a period of 3 months or longer from the last attendance for refill and not yet classified as ‘dead’ or ‘transferred-out’.[3] The time to LTFU was calculated in months according to the time interval between the dates of ART initiation to drop out, as recorded by the ART registration health information data manager. The cohort was stratified into three age-groups: Children (age ≤10 years), adolescents (age 11-19 years), and adults (age ≥20 years) as used by previous studies.[23,24]
Data source and collection
The data for this research was secondary data collected routinely in the hospital for clinical monitoring and evaluation purposes and entered in an ART electronic database during the follow-up time. Further details are fully described elsewhere.[23] The primary outcome variable was LTFU from ART follow-up care after initiation of treatment, confirmed by reviewing medical registration at the hospital, noted by ART adherence supporters. Data recording started from the date that patients started regular HIV care in the clinic to confirmation of a final event. Socio-demographic characteristics such as age and sex, ART drugs received, CD4 counts, clinical staging (I-IV), TB co-morbidity, functional status (working, ambulatory, or bed-ridden), and outcomes were all included for this study.
All data relating to patients with an HIV-positive diagnosis (CD4 count ≤200 cells/mm3 or who met clinical staging (WHO Stage III or IV) according to national ART guidelines effective up to 2012) and who initiated ART prior to 2012 were included. Patients with a CD4 count less than 350 cells/mm3 were eligible to take ART medication, based on 2010 WHO guidelines. Patients of any age who commenced ART at the due date were considered eligible for study. A total of 2655 patients had enrolled to receive ART services and all data from those living with HIV were retrieved for analysis. A proportion of HIV patients (522; 19.7%) were transfer-out who continued their ART medication elsewhere and were therefore excluded from the analysis.
Statistical analysis
The database was recorded in Microsoft Excel, checked for consistencies and completeness, and then cleaned and edited prior to performing analysis in SPSS 20.0 and STATA 11. The patient characteristics were described in terms of mean/median or percentage, as appropriate. The Kaplan-Meier technique was used to estimate time to LTFU after initiation of ART, with the log-rank test being used to test the significance of observed differences between groups. The Cox proportional hazards regression model was used to determine predictors of LTFU, expressed as estimated hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
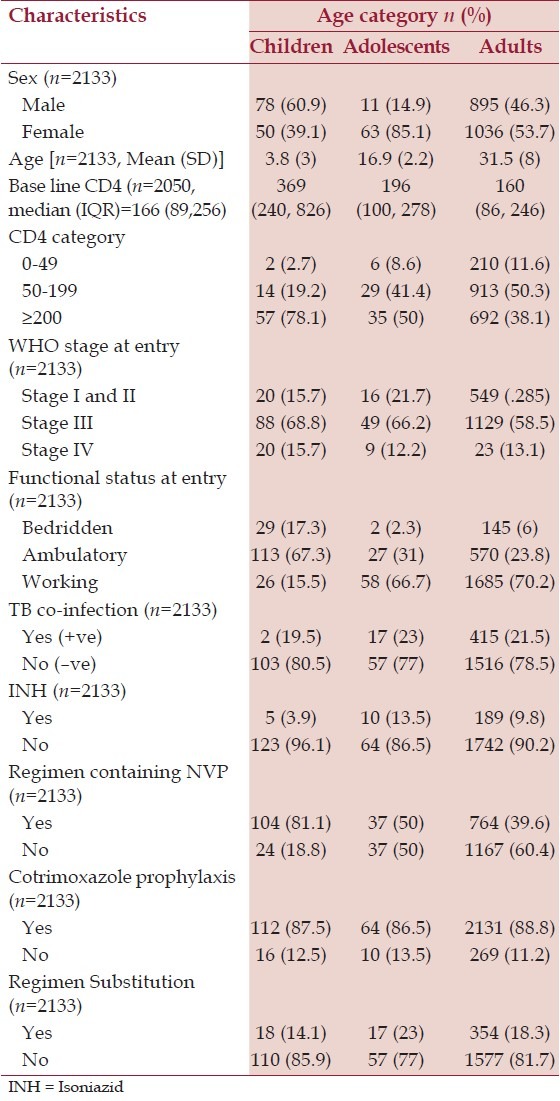
A total of 2133 patients on ART between 2005 and 2013 and followed for 65,022 person-months were included in the statistical analysis. The number of clients who started ART each year is shown in Figure 1. The median (IQR) follow-up was 25 (8-47) months. Of 2133 patients, 128 (6%) were children, 74 (3.5%) were adolescents, and 1931 (90.5%) were adults. The mean (standard deviation) age of the cohort was 31.5 (8.0), 16 (2.2), and 3.8 (3.0) years for adults, adolescents, and children, respectively. 1149 (53.9%) patients were female and there was a higher proportion of females in the adolescent (63, 85.1%) and adult (1036, 53.7%) groups, whereas there were more males in the children group (78, 60.9%), [Table 1]. Only 78%, 50%, and 38% of child, adolescent, and adult participants, respectively, were started on ART with CD4 counts ≥200 cells/mm3. However, there was a trend to a higher baseline CD4 count in adults and adolescents over time with cohort enrolment; those who were enrolled earlier had lower baseline CD4 counts than those who started later. TB co-infection was present in 19.5% of children, 23% of adolescents, and 21.5% of adults throughout the follow-up period.
Figure 1.
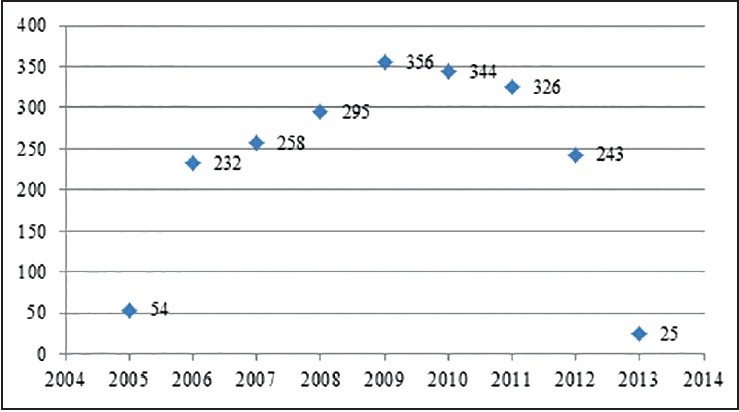
Number of Clients started ART by each year at Mizan-Aman General Hospital, Jan 07, 2005 to May 08, 2013.jpg
Table 1.
Age-sex distribution and clinical characteristics of ART patients by age category at Mizan-Aman General Hospital, Jan 07, 2005 to May 08, 2013
Regimen substitutions were made in 18% (n = 354) of adults, 14% (n = 18) of children, and 23% (n = 17) of adolescents. Drug toxicities were responsible for 50% of regimen substitutions in adults, 47% in adolescents, and 27% in children. Reasons for regimen substitution included pregnancy 41 (11.6%), new TB infection 24 (6.2%), planning to fall pregnancy 7 (2%), advent of new drug of choice 4 (1%), drug stock-out 2 (0.5%) and others 22 (5.7%). There were no regimen substitutions due to treatment failure in the cohort. The median (IQR) duration of follow-up for patients who were LTFU was 9 months (5-18). At the end of the follow-up period, 65.5% (n = 1398) were actively being followed up and on ART, 7.5% (n = 159) had died and two individuals had reported discontinuing their medications. A total of 26.7% (n = 574) patients were defined as LTFU. The overall proportion of attrition due to both death and LTFU during the study period was 733 (34.4%) and the cumulative incidence (95% CIs) of attrition due to LTFU and death was 11.1 (10.4-11.9) per 1000 person-months. The cumulative incidence of LTFU (95% CIs) was 8.8 (8.1-9.6) per 1000 person-months. After people start ART, the retention rates are initially high and then gradually decline. The probability of retention (95% CIs) on ART at the 6th, 12th, and 24th months after initiation of treatment was 92.0% (90.6-93.0), 82.3% (81.1-84.0) and 75.0% (73.2-77.01), respectively.
Risk factors associated with LTFU after ART initiation
In a multivariate Cox regression model [Table 2], age category, regimen substitution, WHO clinical staging, CD4 cell count, non-isoniazid (INH) prophylaxis, and functional status were independent risk factors for LTFU [Figure 2]. Adolescent aged 11-19 years (HR 2.1; 95% CIs 1.3-3.4) and adults aged >20 yrs were at higher risk of LTFU (HR 1.4; 95% CIs 1.0-2.0) when compared to children aged ≤10 yrs. Men and women were at equal risk of LTFU [Figure 3]. The risk of LTFU in patients with WHO clinical stage III (HR 0.6; 95% CIs 0.44-0.9) and clinical stage IV (HR 0.8; 95% CIs 0.6-1.0) at entry were lower compared to clinical stage I.
Table 2.
Cox regression analysis of factors associated with LTFU HIV infected patients on ART therapy at Mizan-Aman General Hospital, January 2005 to May 2013
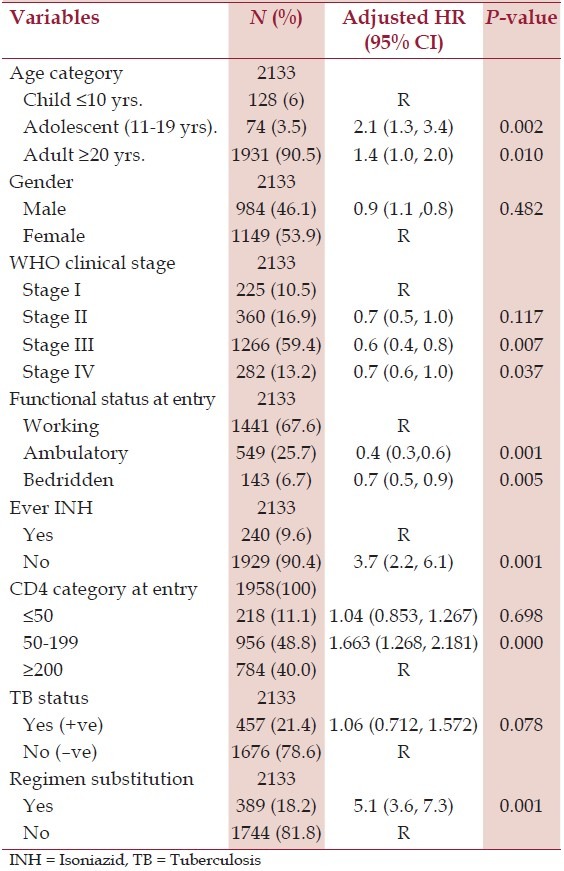
Figure 2.
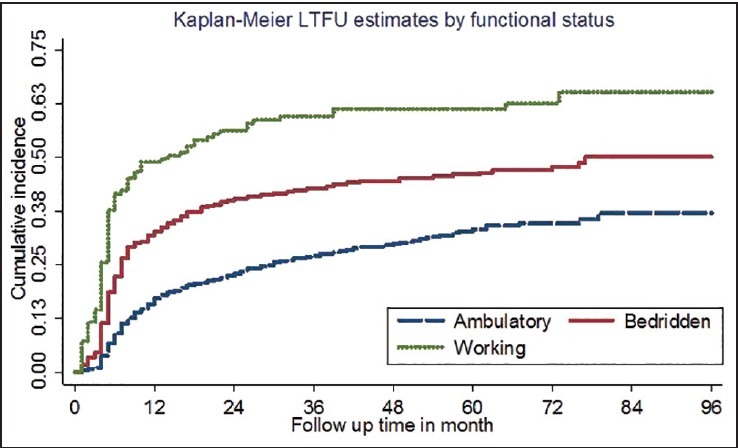
Cumulative incidence of loss to follow up by functional status
Figure 3.
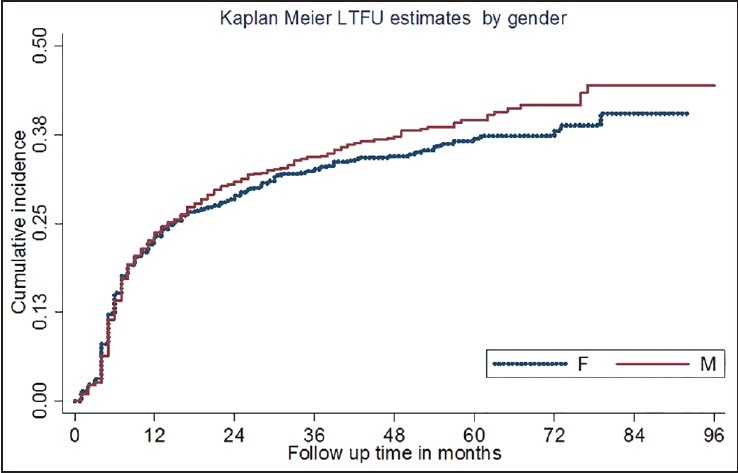
Cumulative incidence of loss to follow up by gender
The risk of LTFU was higher in patients with baseline CD4 cell counts <200 cells/mm3 (HR 1.7; 95% CIs 1.3-2.2) compared to baseline CD4 counts ≥200 cells/mm3. The risk of LTFU in patients who did not take INH prophylaxis was higher than those who did (HR 3.7; 95% CIs 2.3-6.1). Patients who made regimen substitutions during the follow-up period had a higher risk of LTFU (HR 5.2; 95% CIs 3.6-7.3). TB co-infection was not associated with LTFU.
Discussion
Several studies have shown that LTFU poses challenges to the successful implementation of ART programs in LMICs.[1,3] In this study, the incidence rate was estimated to be 8.8 per 1000 person-months. Other studies have shown that patients who discontinued ART developed a rapid increase in viral load and depletion of CD4 T lymphocytes, putting them at risk of opportunistic infections and early death.[25] Therefore, understanding the risk factors for LTFU is necessary to maintain adherence and intervene in groups of patients. In this analysis, it was estimated that the prevalence of LTFU from ART was 26.7%, higher than that reported in other African countries and the Oromia region of Ethiopia.[15,26,27,28] However, ART clinics in the United Kingdom have reported a LTFU of 38.8%; of these, after intensive investigational activities for true outcomes, 51.7% were actually found to be LTFU, either those who were alive but had stopped their ARV or untraceable.[29] LTFU is not only an LMIC problem.
Consistent with our data, report from the British HIV Association 2008[30] has shown that the risk of LTFU increases with decreasing CD4 counts at the entry point of ART, whereas in Switzerland there was an opposite trend in the risk of LTFU; patients with higher CD4 cell counts were more likely to be lost to follow-up.[26] The main reasons in rising incidence of LTFU have been ascribed to poor patient tracing in the low-income setting and due to lack of reporting the risk of death events that can be considered as LTFU.[1] Our study found that adolescents were twice, and adults 1.4 times, more likely to become lost to follow-up than children. A study from Uganda showed that the incidence of mortality was lower in children[31] ; this may suggest that the competing risk of death impacts on this age-group. Children may also be less exposed to stigma and discrimination (two common risks for LTFU) and the caretakers or parents are more likely to look after children that, reducing LTFU. During adolescence, a number of challenges have been identified that may compromise positive outcomes from HIV care. Adolescents may be particularly defiant, may not have caregivers (in contrast to younger children), may show immaturity in analytical thinking, and there may be particular challenges associated with puberty and high LTFU. Previous studies have also shown that adherence is lower in adolescents than adults.[13,32,33]
We detected no gender difference in LTFU, in contrast to several other studies that have shown that men are more likely to become lost to follow-up due to variation in mobility and a high risk of drug abuse in men, that may interfere with adherence.[17] Most men with drug addiction may experience higher toxicity due to interaction with ARV drugs that leads to discontinuation.[34] The detection of a difference in gender was compromised in this study due to cultural influences such as habit of chewing, alcohol consumption, religion, stigmas and lower mobility of the male population for inter-regional trade purpose in this particular study area. Patients with advanced clinical stage (III and IV) at entry were less likely to be lost to follow-up. This is in contrast to other African studies, which have shown the opposite.[35,36] Our study suggested that clinical stage III and IV patients have increased health-seeking behavior, or it may be ascribed to improvements in awareness of community. Outside Africa, a Swiss study showed a statistically non-significant trend,[26] but a French study, similar to ours showed that a history of an AIDS-defining illness was associated with reduced LTFU.[37]
Patients who did not take INH prophylaxis were more likely to be lost to follow-up. Patients generally believe that if they are in the advanced stages of HIV/AIDS or considered immunocompromised, they should strictly maintain follow-up so that they can start prophylaxis and not be deemed unhealthy. Increase in reinforced counseling to patients taking INH prophylaxis might have contributed to better follow-up. TB is a leading cause of morbidity and mortality in people living with HIV, including those on ART. However, we saw no significant association between LTFU status and TB co-infection. Patients who had substitutions in their regimen during the follow-up period were at higher risk of LTFU, similar to an Indian study that reported that substitution of drugs can be risk factor for ART default.[17] The majority of regimen substitution cases in this study were due to adverse drug reactions, so these patients may have become concerned about side effects and the effectiveness of new medication, causing them to seek other treatment options. Skin hypersensitivity reactions are common in patients taking nevirapine (NVP) containing ART regimens. Most patients report that they experience zidovudine (AZT)-induced anemia and stavudine (D4T) induced peripheral neuropathy. All these adverse effects commonly managed through efavirenz (EFZ) in NVP induced rash and tenofovir (TDF) or stavudine for zidovudine-induced anemia.[38] The fear of side-effects is known to be a major cause of default.[25] This subgroup of patients who lose faith in the medication program, irrespective of the reason for substitution, require special attention and frequent counseling in order to preserve retention in the ART program.
The probability of attrition from care was directly associated with the length of engagement with ART care. A higher proportion of LTFU was recorded in the first 6 months after ART initiation. Generally, the likelihood of dropping out from care gradually increases with longer retention period. The high risk factors for LTFU after initiation of ART were thought to be due to gaps in counseling services while refilling, assessment of ART outcomes by physicians, and tracing service by cell phones only. The main drawback of this service in this particular community assumed to be limited coverage of network and higher rate of cell phone non-users. This finding is consistent with many other studies, which reported that a large proportion of patients dropped-out from care within the first year of ART, the peak period being the first 6 months of ART. This has been suggested to be due to less awareness at these early time-points about treatment outcomes, whether adverse or beneficial.
Our study had some limitations that resulted from poor tracing of patients in the ART program's monitoring and evaluation system, patients who were LTFU may have died or self-referred to other facilities. Thus, the findings addressed crudely LTFU who might be dead, untraceable, self-transfer outs, and defaulters. The concerted influences of these factors may affect the accurate record keeping procedures in this clinic. Decreasing LTFU of patients through provision of tracking system is crucial for minimizing early mortality, complications of HIV, reducing viral transmission, and ensuring success of ART programs.
Conclusion
We report a comparatively high rate of LTFU from an Ethiopian ART clinic. Low CD4 counts, adolescence, being in good health, regimen substitution, and advanced clinical stage were found to be risk factors for LTFU. Improving comprehensive counseling services, follow-up for adverse reactions and introducing an ART outcome evaluation program may help reduce LTFU to an acceptable level. Our results highlight the need to better understand the health-seeking behaviors of patients with ART and to implement strategies in HIV clinics for better tracking services and minimizing LTFU from HIV care. Change in way of tracing services like community education, scaling up of health extension services community wide, and increasing awareness by media may reduce LTFU. The lost to follow-up is of particular importance to ART programs because they potentially endanger not only their own life, but also contribute to increased HIV drug resistance due to ART default.[11,12] Further studies that address the profiles of LTFU patients and the contributing factors are required for clarity.
Abbreviations
AIDS: acquired immunodeficiency syndrome; ART: Antiretroviral therapy; CIs: confidence intervals; HIV: human immunodeficiency virus; HR: hazard ratios; INH: Isoniazid; IQR: interquartile range; LMICs: Low- and middle-income countries; LTFU: loss to follow up; SD: standard deviations; TB: Tuberculosis; WHO: World Health Organization
Acknowledgment
We acknowledge the kind assistance given by all the staff in the ART clinics. The authors also gratefully acknowledge editorial assistance from the Nextgenediting Global Initiative.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.WHO. Global update on HIV treatment: Results, impact and opportunities. 2013. [Accessed March 14, 2014]. at: http://www.who.int/about/licensing/copyright_form/en/index.html .
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individualson combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: Experience from western Kenya. AIDS. 2006;20:41–8. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 4.Hogg RS, Heath K, Bangsberg D, Yipa B, Press N, O’Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–8. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 6.Low-Beer S, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–1. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 7.Taiwo B. Understanding transmitted HIV resistance through the experience in the USA. Int J Infect Dis. 2009;13:552–9. doi: 10.1016/j.ijid.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–7. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 9.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: A retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13:49. doi: 10.1186/1758-2652-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bygrave H, Kranzer K, Hilderbrand K, Whittall J, Jouquet G, Goemaere E, et al. Trends in loss to follow-up among migrant workers on antiretroviral therapy in a community cohort in Lesotho. PLoS One. 2010;5:e13198. doi: 10.1371/journal.pone.0013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam BD, Maticka-Tyndale E, Cohen JJ. Adherence practices among people living with HIV. AIDS Care. 2003;15:263–74. doi: 10.1080/0954012031000068407. [DOI] [PubMed] [Google Scholar]
- 12.Malcolm SE, Ng JJ, Rosen RK, Stone VE. An examination of HIV⁄AIDS patients who have excellent adherence to HAART. AIDS Care. 2003;15:251–61. doi: 10.1080/0954012031000068399. [DOI] [PubMed] [Google Scholar]
- 13.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR. Adolescent Medicine HIV/AIDS Research Network. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157:249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 14.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: Systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61:e50–8. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 16.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, et al. Mortality and loss to- follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: Data from an HIV cohort study in India. Glob Health Action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanoy E, Mary-Krause M, Tattevin P, Dray-Spira R, Duvivier C, Fischer P, et al. Clinical Epidemiology Group of French Hospital Database on HIV Infection. Predictors identified for losses to follow-up among HIV-sero positive patients. J Clin Epidemiol. 2006;59:829–35. doi: 10.1016/j.jclinepi.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Bagchi S. Telemedicine in rural India. PLoS Med. 2006;3:e82. doi: 10.1371/journal.pmed.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: A systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12:687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 22.Central Statistical Agency [Ethiopia] and ICF International: Ethiopia Demographic and Health Survey. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International; 2012. [Accessed February 25, 2014]. at http://www.usaid.gov/sites/default/files/documents/1860/Demographic%20Health%20Survey%202011%20Ethiopia%20Final%20Report.pdf . [Google Scholar]
- 23.Moshago T, Haile DB, Enqusilasie F. Survival analysis of HIV infected people on antiretroviral therapy at Mizan-Aman General Hospital, Southwest Ethiopia. Int J Sci Res. 2014;3:1462–9. [Google Scholar]
- 24.Bakand C, Birungi J, Mwesigwa R, Nachega J, Chan K, Palmer A, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: Findings from a nationally representative cohort in Uganda. PLoS One. 2011;6:e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deribe K, Hailekiros F, Biadgilign S, Amberbir A, Beyene BK. Defaulters from antiretroviral treatment in Jimma University Specialized Hospital, Southwest Ethiopia. Trop Med Int Health. 2008;13:328–33. doi: 10.1111/j.1365-3156.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 26.Schoni-Affolter F, Keiser O, Mwango A, Stringer J, Ledergerber B, Mulenga L, et al. Swiss HIV Cohort Study, IeDEA Southern Africa. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: The effect of the competing risk of death in Zambia and Switzerland. PLoS One. 2011;6:e27919. doi: 10.1371/journal.pone.0027919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–4. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alemu AW, San Sebastian M. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3 doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerver SM, Chadborn TR, Ibrahim F, Vatsa B, Delpech VC, Easterbrook PJ. High rate of loss to clinical follow up among African HIV-infected patients attending a London clinic: A retrospective analysis of a clinical cohort. J Int AIDS Soc. 2010;13:29. doi: 10.1186/1758-2652-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mocroft A, Kirk O, Aldins P, Chies A, Blaxhult A, Chentsova N, et al. EuroSIDA study group. Loss to follow-up in an international, multicentre observational study. HIV Med. 2008;9:261–9. doi: 10.1111/j.1468-1293.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 31.Weidle PJ, Malamba S, Mwebaze R, Sozi C, Rukundo G, Downing R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: Patients’ response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CM, Wright PF, Safrit JT, Rudy B. Epidemiology of HIV infection and risk in adolescents and youth. J Acquir Immune Defic Syndr. 2010;54:S5–6. doi: 10.1097/QAI.0b013e3181e243a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M. Adolescent medicine HIV/AIDS research network. Antiretroviral medication adherence among REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 34.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Socio demographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 35.Brinkhof MW, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Messou E, et al. Adjusting mortality for loss to follow-up: Analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5:e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15:63–9. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebouche B, Yazdanpanah Y, Gerard Y, Sissoko D, Ajana F, Alcaraz I, et al. Incidence rate and risk factors for loss to follow-up in a French clinical cohort of HIV-infected patients from January 1985 to January 1998. HIV Med. 2006;7:140–5. doi: 10.1111/j.1468-1293.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 38.Ministry of Health (MOH) of Ethiopia: Guideline for implementation of antiretroviral therapy. Federal HIV/AIDS Prevention and Control Office. Federal Ministry of Health. 2010. [Accessed March 25, 2014]. at http://www.etharc.,org/.../SPM%20II%20Final%20version%20sept%2026.pdf .


