Abstract
Precise control of mRNA translation is fundamental for eukaryotic cell homeostasis, particularly in response to physiological and pathological stress. Alterations of this program can lead to the growth of damaged cells, a hallmark of cancer development, or to premature cell death such as seen in neurodegenerative diseases. Much of what is known concerning the molecular basis for translational control has been obtained from polysome analysis using a density gradient fractionation system. This technique relies on ultracentrifugation of cytoplasmic extracts on a linear sucrose gradient. Once the spin is completed, the system allows fractionation and quantification of centrifuged zones corresponding to different translating ribosomes populations, thus resulting in a polysome profile. Changes in the polysome profile are indicative of changes or defects in translation initiation that occur in response to various types of stress. This technique also allows to assess the role of specific proteins on translation initiation, and to measure translational activity of specific mRNAs. Here we describe our protocol to perform polysome profiles in order to assess translation initiation of eukaryotic cells and tissues under either normal or stress growth conditions.
Keywords: Cellular Biology, Issue 87, Translation initiation, polysome profile, sucrose gradient, protein and RNA isolation, stress conditions
Introduction
Eukaryotic cells constantly encounter a range of harmful physiological and environmental stress conditions that require a rapid adaptive cell response. Cell stress response involves a precise balance between anti-survival and pro-survival acting factors. Disrupting this balance can have irreversible consequences leading the development of human pathologies such as cancer and neurodegenerative diseases. During the first step of the stress response, cells activate pro-survival pathways that involve the coordinated control of changes in gene expression at the level of mRNA translation.
mRNA translation in eukaryotes is a complex cellular process that involves coordinated interactions between translation initiation factors (eIFs), specific RNA binding proteins (RBDs), and RNA molecules1. mRNA translation is divided into three distinct phases: initiation, elongation, and termination. Although all three phases are subject to regulatory mechanisms, translational control mechanisms target mostly the initiation phase of translation, which thus constitutes the rate-limiting step of protein synthesis2.
Translation initiation is a highly ordered process that begins with the formation of the eIF2a.GTP.Met-tRNAiMet ternary complex and its subsequent binding to the 40S ribosome subunit, leading to the formation of the pre-initiation complex. The next step is the recruitment of the preinitiation complex to mRNA, which involves the activity of translation initiation factors such as eIF4F and eIF3. The 48S preinitiation complex thus formed undergoes specific conformational changes that enable this machinery to start scanning the 5'-untranslated region of the mRNA until it recognizes the initiation codon AUG. Most of the translation initiation factors are then released and 60S subunits are recruited to form an 80S ribosome complex competent for translation, at which point protein synthesis starts (Figure 1). More than one 80S monosome can be translating the same mRNA at a time producing so called polysomes (or polyribosomes). The density of polysomes on a mRNA reflects the initiation, elongation and termination rates and thus is a measure of the translatability of a particular transcript. However, polysome profile is mainly used to assess changes in mRNA translation at the initiation step. Here we have used a proteasome inhibitor as translation initiation inhibitor. Treatment of cancer cells with this drug induces a stress response characterized by activation of the stress kinase named HRI which phosphorylates the translation initiation factor eIF2a3. Phosphorylation of eIF2a is one of the major events leading to the inhibition of translation initiation in mammalian cells4.
Protocol
The protocol follows the guidelinesapproved by Laval's Ethical Review Board.
1. Preparation of Cell Cultures and Brain Manipulation
- Mammalian and Drosophila Cells
- Grow HeLa cervical cancer cells and Schneider Drosophila embryonic cells as recommended by the American Type Culture Collection. Work with cells at a low passage.
- Plate cells in order to reach 80% confluence the day of the experiment. For best results, use ∼12 x 106 of cells for each experimental condition. Before making extracts for polysome analysis, stimulate translation by adding fresh complete medium for at least 90 min.
Brain isolation: Isolate brains from mice aged between 9 and 16 days after birth. Euthanasia involves CO2 inhalation after anesthesia and cervical dislocation. Following sacrifice, isolate the whole brain and either place it at -80 °C or directly transfer it into cold PBS 1X for immediate use.
2. Preparation of the Density Gradient Fractionation System
Wash the tubing system with 0.1% SDS for 5 min.
Wash the tubing system with 70% Ethanol for 5 min, then with RNaseZAP Solution for 5 min before washing it with DEPC water.
Pump air in order to dry the tubing system of the apparatus.
3. Preparation of the Sucrose Gradients
Make 15% and 55% sucrose solutions in 20 mM Tris-HCl pH 7.4, 1.25 mM MgCl2, 150 mM NaCl, and 1 mM DTT.
Generatethe linear 15-55% sucrose gradient using an Isco Model 160 gradient former, as described by the manufacturer's instructions. However, it is important to control well the TRIS peristaltic pump speed in order to avoid creation of bubbles or turbulences in the gradients. Keep the gradients at 4 °C until use.
During the time when the gradient maker program is running, cool the ultracentrifuge to 4 °C.
4. Preparation of Cell and Mouse Brain Extracts
- Preparation of Cell Extracts
- Place plate(s) on ice and wash cells 3x with cold PBS 1X.
- Harvest cells (∼12 x 106) in 1 ml lysis buffer (20 mM Tris-HCl at pH 7.4, 1.25 mM MgCl2, 150 mM NaCl, 1 mM DTT, 1% Nonidet P40, 5 U/ml RNase inhibitor, supplemented with complete mini EDTA-free protease inhibitor cocktail tablets), transfer them to an Eppendorf tube, and mix well by passing them 15x through a 1cc U100 Insulin Syringe 28 G 1/2. Let the cell lysate rest on ice for 15 min.
- Put few drops of cell lysate onto a slide to assess cellular lysis by observing at a phase contrast microscope using a 10X objective. Only nuclei should be visible and no cell membranes should be visible attesting for cell lysis. As a control, observe unlysed cells.
- Clarify the cell lysate by centrifugation at 11,000 x g for 20 min at 4 °C and keep the soluble lysate containing the polyribosomes.
- Preparation of Mouse Brain Extracts
- Homogenize the whole isolated brain (∼500 mg) by 10 strokes in an ice-cold Dounce homogenizer with 2 ml of lysis buffer and clarify the homogenate by centrifugation at 11,000 x g for 15 min at 4 °C.
- Load the resulting supernatant onto a cushion of 50% sucrose and proceed with sedimentation for 2 hr at 200,000 x g using an ultracentrifuge rotor TH-641 at 4 °C.
- Resuspend the translucent pellet containing the polyribosomes in 1 ml of lysis buffer and mix well by pipetting up and down. Let the suspension rest on the ice for 30 min before loading onto gradients.
5. Loading the Extracts onto Sucrose Gradients and Ultracentrifugation
Measure the concentration of RNA present in the cytoplasmic extract using a spectrophotometer. Carefully and slowly load ∼20 OD260 units of the extract onto the 15% to 55% sucrose gradient. Make sure that there is 2-3 mm of available space at the surface of tube to avoid overflowing the tubes during centrifugation.
Centrifuge the gradients using an ultra-centrifuge for 2 hr 30 min at 230,000 x g using an ultracentrifuge rotor TH-641 at 4 °C. When centrifugation ends, remove the tubes and place them on ice carefully not to disturb the gradients.
6. Fractionation of Cytoplasmic Extracts for Polysomes Profiling
Place sucrose gradients on Automated Density Fractionation System and proceed with automated fractionation and collection of fractions (0.5 ml each), as described by the manufacturer.
Collect each fraction (∼500 µl) into individual Eppendorf tubes with continuous monitoring of absorbance at 254 nm. In parallel of the gradient fractionation operation, the polyribosomal profile will be editing on the chart paper. Alternatively, use a data acquisition unit attached to the chart recorder to have an electronic acquisition of the polysome profile.
At the end of each run, transfer collected Eppendorfs on dry ice. At this time, either store collected fractions at -80 °C or directly precipitate protein-RNA complexes as follows.
7. Protein Extraction and Analysis
To each collected fraction of sucrose gradient, add 2 volumes of 100% cold ethanol and let RNA-protein complexes precipitate at -20 °C overnight.
Centrifuge each RNA-protein precipitate at 11,000 x g for 20 min at 4 °C then wash with 70% ethanol. Dry and resuspend the precipitate in SDS-PAGE sample buffer before proceeding with Western blot using specific antibodies (see Figure 2).
8. RNA Extraction and Analysis
Precipitate RNA-protein complexes as in section 7.1. Resuspend each RNA-protein precipitate in the lysis buffer containing 0.1% SDS. Digest the protein component of the precipitate with 2 mg/ml Proteinase K for 30 min in a 55 °C water bath.
Extract RNA components of the precipitate by adding 1 volume of "phenol: chloroform", 2 volumes of chloroform, and 0.1 volume of 2 M NaOAc pH 4 and centrifuging at 10,000 x g at 4 °C for 20 min. Precipitate RNA from the resulting aqueous phase of each sample overnight at -20 °C by adding 1 volume of isopropanol and 0.2 µg/µl of glycogen. Spin the precipitate for 1 hr at 10,000 x g at 4 °C. Wash the RNA pellet with 70% cold ethanol.
Resuspend the RNA pellet into a small volume of RNAse free water. Assess the quantityand qualityof RNA using the spectrophotometer.
Representative Results
As aforementioned, polysome profile allows the analysis of changes of translation initiation under stress conditions. Figure 1 is a simplified view of translation initiation which as described earlier is a multistep process involving an ordered assembly of translation initiation complexes. Under normal growth conditions, translation initiation complexes are converted into polyribosomes whose detection by polysome profile attest for an active translation initiation (Figure 2; Untreated). Under stress conditions however, translation initiation is blocked resulting in the accumulation of 80S monosomes and a reduction of polysome peaks (Figure 2; proteasome inhibitor).
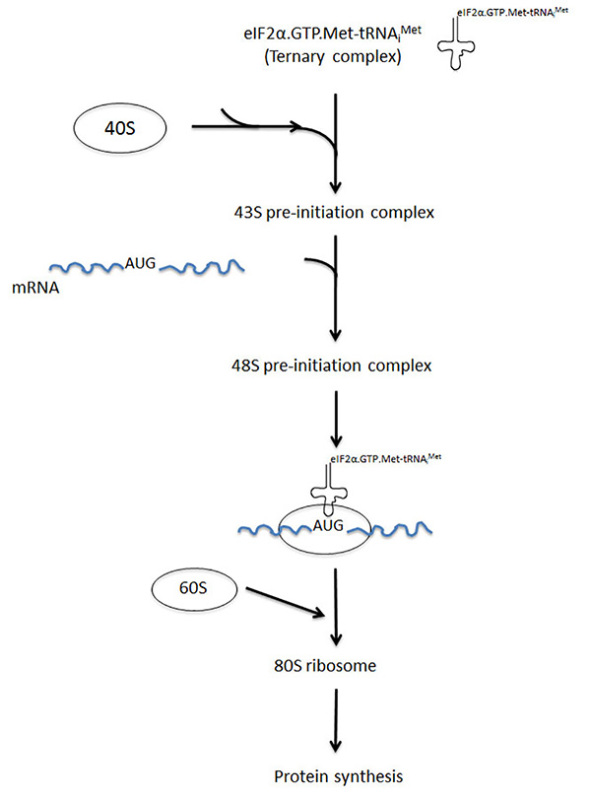 Figure 1. A simplified view of translation initiation. Translation initiation involves several steps: 1) The formation of the eIF2a.GTP.Met-tRNAiMet ternary complex, 2) the association of the ternary complex with 40S ribosomes forming the 43S preinitiation complex, 3) the association of 43S complexes with mRNA forming the 48S preinitiation complex, 4) scanning the 5'-untranslated region of the mRNA by 48S ribosomes until it reaches and identifies the initiation AUG codon, and 5) recruitment of the large ribosomal subunit 60S to 48S forming the 80S complex at which point polypeptides synthesis starts. Each step of translation initiation involves the activity of several translation initiation factors. Click here to view larger image.
Figure 1. A simplified view of translation initiation. Translation initiation involves several steps: 1) The formation of the eIF2a.GTP.Met-tRNAiMet ternary complex, 2) the association of the ternary complex with 40S ribosomes forming the 43S preinitiation complex, 3) the association of 43S complexes with mRNA forming the 48S preinitiation complex, 4) scanning the 5'-untranslated region of the mRNA by 48S ribosomes until it reaches and identifies the initiation AUG codon, and 5) recruitment of the large ribosomal subunit 60S to 48S forming the 80S complex at which point polypeptides synthesis starts. Each step of translation initiation involves the activity of several translation initiation factors. Click here to view larger image.
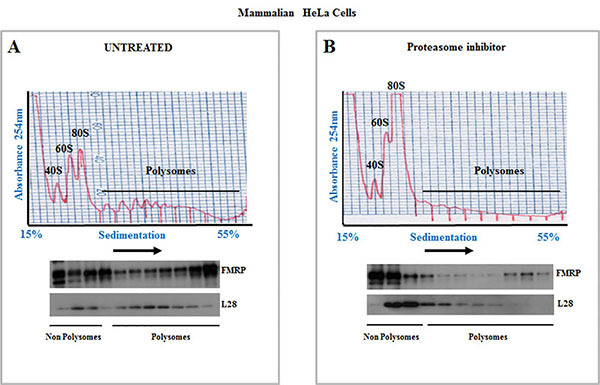 Figure 2. Polysomes profiles and analysis of polysomes associated proteins by western blot in HeLa cells. (A - B) Cytoplasmic extracts were prepared from HeLa cells grown either in normal conditions (A) or upon treatment with the proteasome inhibitor for 4 hr (B) and centrifuged on a 15-55% v/w linear sucrose gradient. Polysomes profiles are indicated in the top panel. The positions of 40S ribosomes, 60S ribosomes, 80S monosomes, and polysomes are indicated in each profile. Fractions from the top (15%) to the bottom (55%) of the gradient are shown from left to right. Fractions were collected and analyzed by western blot (bottom panels) with antibodies against the large ribosomal L28 protein as well as with the polysome-associated protein FMRP that serve as controls for polysome integrity5,6. Note that treatment with the proteasome inhibitor reduces polysomes peaks with a concomitant increases in 80S monosomes indicating an inhibition of translation initiation. Typical profiles obtained from Schneider Drosophila cells or mice brain have been described elsewhere7-10. Click here to view larger image.
Figure 2. Polysomes profiles and analysis of polysomes associated proteins by western blot in HeLa cells. (A - B) Cytoplasmic extracts were prepared from HeLa cells grown either in normal conditions (A) or upon treatment with the proteasome inhibitor for 4 hr (B) and centrifuged on a 15-55% v/w linear sucrose gradient. Polysomes profiles are indicated in the top panel. The positions of 40S ribosomes, 60S ribosomes, 80S monosomes, and polysomes are indicated in each profile. Fractions from the top (15%) to the bottom (55%) of the gradient are shown from left to right. Fractions were collected and analyzed by western blot (bottom panels) with antibodies against the large ribosomal L28 protein as well as with the polysome-associated protein FMRP that serve as controls for polysome integrity5,6. Note that treatment with the proteasome inhibitor reduces polysomes peaks with a concomitant increases in 80S monosomes indicating an inhibition of translation initiation. Typical profiles obtained from Schneider Drosophila cells or mice brain have been described elsewhere7-10. Click here to view larger image.
Discussion
The polysome profile analysis on sucrose gradients allows measurement of translation initiation by analyzing the density of polysomes isolated from cells or tissues9,11-14. This technique is the best (if not the unique) approach to measure translation initiation in vivo. It is used to monitor the translational status of growing cells during cell cycle15, and to assess the effects of various types of stress including viral infections, hypoxia13,16, radiation17, and chemical drugs18 used in therapy, on translation initiation. However, while this technique works well to assess translation initiation of cell cultures and specific tissues such as brain and liver, it remains to be established if it can be used to compare translation initiation of normal versus diseased tissues and tumors.
Changes in polysome profiles can also occur under conditions that alter translation elongation or termination. In these cases, the inhibition of elongation rates of translation will lead to an increase of polysomes peaks while an inhibition of termination should result in larger polysomes relative to the control. In contrast, inhibitors of translation initiation prevent reformation of polysomes because formation of active translation initiation complexes is blocked. This is reflected in polysomes profiles by a reduction of polysomes peaks with a concomitant increase of 80S monosomes. Because translation inhibitors stall translation initiation complexes, it is expected that both 40S and 60S peaks will also increase. However, very often an increase in only 80S monosomes is observed upon inhibition of translation initiation. The reason why an increase in the other ribosomal complexes is not observed is currently unknown. One possible explanation could be that fortuitous association between stalled ribosomal complexes may occur during extraction leading to the observed accumulation of only 80S monosomes.
Beside the analysis of translation initiation, polysome profile technique can help to confirm the identity of translation initiation factors (such as eIF4E, eIF4A and eIF4G) and to identify novel translation regulators. As translation initiation factors are released from ribosomes at the last translation initiation step, they should not be detected over translating ribosomes fractions. The association of proteins such as FMRP14, SMN19,20, TDRD321 with both polysomes and non-polysomes fractions indicates that the regulatory role of these proteins in translation is not restricted to the initiation step. Monitoring the association of proteins with polysomes also constitutes a powerful means to assess how stress conditions could affect the translational activity of specific proteins. However, the association of specific proteins with polysomes by itself is not sufficient to conclude a translational role, which then needs to be confirmed by functional studies. Finally, the polysome profile technique allows determination of the translational activity of specific mRNAs under various conditions, and for examination of specific mechanisms that regulate translation such as miRNA mediated translational repression22 . The general use of polysome analysis can be further extended using a variety of downstream applications including genome-wide microarray analysis and more recently the transcriptome-scale ribosome profiling23-26 allowing for robust prediction of protein expression and more importantly providing a new powerful experimental tool for analysis of translational control23-27.
As with any methodology, precautions need to be taken while performing polysome profiling. In this respect, multiple parameters such as cell passage, cell lysis, amount of cell extract to be loaded onto the gradient, and RNA integrity should be well controlled. Optimization of ultracentrifugation conditions is also required in order to obtain the best separation of ribosomal population through the sucrose gradients. Components that constitute the gradients and lysis buffer such as sucrose and detergents, may give absorbance in the 254-nm wavelength. It is thus important to include a control gradient containing only lysis buffer. Finally, the gradients themselves should be handled with caution as minimal perturbation of gradients can cause huge effects on polysomes profiles.
Disclosures
The authors have nothing to disclose.
Acknowledgments
P. A. is a recipient of a scholarship "Pierre Durand" from the Faculty of medicine of Laval University. This work was supported by the Natural Sciences and Engineering Research Council of Canada (MOP-CG095386) to R. M. The polysome fractionator was acquired through a Canadian Foundation for innovation grant (MOP-GF091050) to R. M. R. M holds a new CIHR investigator salary award.
We are grateful to Drs. E. Khandjian, I. Gallouzi, S. Di-Marco and A. Cammas for helpful advice.
References
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nature Reviews. Molecular Cell Biology. 2004;5(10):827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CUT, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Reviews. Molecular Cell Biology. 2010;11(2):113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M-J, Gareau C, Mazroui R. The chemotherapeutic agent bortezomib induces the formation of stress granules. Cancer Cell International. 2010;10(12) doi: 10.1186/1475-2867-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nature Reviews. Molecular Cell Biology. 2005;6(4):318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- Mazroui R, Huot M-E, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Human Molecular Genetics. 2002;11(24):3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- Mazroui R, Huot M-E, Tremblay S, Boilard N, Labelle Y, Khandjian EW. Fragile X Mental Retardation protein determinants required for its association with polyribosomal mRNPs. Human Molecular Genetics. 2003;12(23):3087–3096. doi: 10.1093/hmg/ddg335. [DOI] [PubMed] [Google Scholar]
- Farny NG, Kedersha NL, Silver P. RNA. 10. Vol. 15. New York, N.Y: 2009. a Metazoan stress granule assembly is mediated by P-eIF2alpha-dependent and -independent mechanisms; pp. 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau C, Houssin E, et al. Characterization of fragile x mental retardation protein recruitment and dynamics in Drosophila stress granules. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett DM, Qing F, Amieux PS, Sellers DL, Horner PJ, Morris DR. FMR1 transcript isoforms: association with polyribosomes; regional and developmental expression in mouse brain. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian EW, Huot M-E, Tremblay S, Davidovic L, Mazroui R, Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13357–13362. doi: 10.1073/pnas.0405398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson A, Winblad B, Wallace W. Translational control of gene expression in the human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(3-4):469–479. doi: 10.1016/0278-5846(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Stephens SB, Nicchitta C. V In vitro and tissue culture methods for analysis of translation initiation on the endoplasmic reticulum. Methods in Enzymology. 2007;431:47–60. doi: 10.1016/S0076-6879(07)31004-5. [DOI] [PubMed] [Google Scholar]
- Koritzinsky M, Wouters BG. Hypoxia and regulation of messenger RNA translation. Methods in Enzymology. 2007;435:247–273. doi: 10.1016/S0076-6879(07)35013-1. [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Corbin F, Woerly S, Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nature Genetics. 1996;12:91–93. doi: 10.1038/ng0196-91. [DOI] [PubMed] [Google Scholar]
- Sivan G, Kedersha N, Elroy-Stein O. Ribosomal slowdown mediates translational arrest during cellular division. Molecular and Cellular Biology. 2007;27(19):6639–6646. doi: 10.1128/MCB.00798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Johannes GJ. Identification of mRNAs that continue to associate with polysomes during hypoxia. RNA. 2007;13:1116–1131. doi: 10.1261/rna.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraswamy S, Chinnaiyan P, Shankavaram UT, Lü X, Camphausen K, Tofilon PJ. Radiation-induced gene translation profiles reveal tumor type and cancer-specific components. Cancer Research. 2008;68(10):3819–3826. doi: 10.1158/0008-5472.CAN-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M-J, Coudert L, et al. Inactivation of the mTORC1-eIF4E Pathway alters Stress Granules Formation. Molecular and Cellular Biology. 2013;33(11):2285–2301. doi: 10.1128/MCB.01517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez G, Dury AY, et al. A novel function for the survival motoneuron protein as a translational regulator. Human Molecular Genetics. 2013;22(4):668–684. doi: 10.1093/hmg/dds474. [DOI] [PubMed] [Google Scholar]
- Béchade C, Rostaing P, et al. Subcellular distribution of survival motor neuron (SMN) protein: possible involvement in nucleocytoplasmic and dendritic transport. The European Journal of Neuroscience. 1999;11(1):293–304. doi: 10.1046/j.1460-9568.1999.00428.x. [DOI] [PubMed] [Google Scholar]
- Goulet I, Boisvenue S, Mokas S, Mazroui R, Côté J. TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules. Human Molecular Genetics. 2008;17(19):3055–3074. doi: 10.1093/hmg/ddn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nature Structural & Molecular Biology. 2006;13(12):1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- Genolet R, Araud T, Maillard L, Jaquier-Gubler P, Curran J. An approach to analyse the specific impact of rapamycin on mRNA-ribosome association. BMC Medical Genomics. 2008;1(33) doi: 10.1186/1755-8794-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete MJ, Vernal R, Dolznig H, Müllner EW, Garcia-Sanz J. RNA. 3. Vol. 13. New York, N.Y: 2007. a Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments; pp. 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Brar GA, Rouskin S, Mcgeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nature Protocols. 2012;7(8):1534–1550. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DR. Ribosomal footprints on a transcriptome landscape. Genome Biology. 2009;10(4) doi: 10.1186/gb-2009-10-4-215. [DOI] [PMC free article] [PubMed] [Google Scholar]


