Abstract
Objective
Variations in the intake of folate are capable of modulating colorectal tumorigenesis; however, the outcome appears to be dependent on timing. This study sought to determine the effect of altering folate (and related B vitamin) availability during in-utero development and the suckling period on intestinal tumorigenesis.
Design
Female wildtype mice were fed diets either mildly deficient, replete or supplemented with vitamins B2, B6, B12 and folate for 4 weeks before mating to Apc1638N males. Females remained on their diet throughout pregnancy and until weaning. After weaning, all Apc1638N offspring were maintained on replete diets for 29 weeks.
Results
At 8 months of age tumour incidence was markedly lower among offspring of supplemented mothers (21%) compared with those of replete (59%) and deficient (55%) mothers (p=0.03). Furthermore, tumours in pups born to deficient dams were most likely to be invasive (p=0.03). The expression of Apc, Sfrp1, Wif1 and Wnt5a—all of which are negative regulatory elements of the Wnt signalling cascade—in the normal small intestinal mucosa of pups decreased with decreasing maternal B vitamin intake, and for Sfrp1 this was inversely related to promoter methylation. β-Catenin protein was elevated in offspring of deficient dams.
Conclusions
These changes indicate a de-repression of the Wnt pathway in pups of deficient dams and form a plausible mechanism by which maternal B vitamin intake modulates tumorigenesis in offspring. These data indicate that maternal B vitamin supplementation suppresses, while deficiency promotes, intestinal tumorigenesis in Apc1638N offspring.
Although a few exceptions exist,1 a considerable body of epidemiological evidence accumulated over the past two decades2 3 and scrutinised by recent meta-analyses,4 supports the notion that higher dietary folate intake is associated with a moderately reduced risk of colorectal cancer (CRC). This effect has also been reproduced in rodent CRC models,5 6 establishing true causality. More recently, however, the potential for a paradoxical tumour-promoting effect of folate has become apparent, with our current understanding being that folate insufficiency promotes carcinogenesis by causing DNA breakage and genomic and gene-specific methylation aberrations, while supplemental quantities of the vitamin, when administered in an inappropriate time frame, may paradoxically promote tumorigenesis by providing existing neoplastic lesions with an overly abundant supply of nucleotides to support DNA synthesis during proliferation.7 8
The critical importance of timing in determining the outcome of folate supplementation has prompted us to consider windows of exposure to folic acid other than those in adult life. Because maternal nutrition is becoming increasingly recognised as a determinant of chronic disease in offspring9 and particularly as certain phases of in-utero life are characterised by major transitions in DNA methylation,10 we questioned whether a mother’s folate intake might impact on her offspring’s risk of colorectal carcinogenesis. Epidemiological evidence is not yet available to support (or refute) this idea because suitable databases that attempt to link maternal diet to diseases in the latter decades of the offspring’s adulthood are not yet available; however, data do exist to support a protective role for high maternal folate intake against certain paediatric cancers in offspring, including retinoblastoma,11–14 non-Hodgkin’s lymphoma,15 acute lymphoblastic leukaemia16 and childhood brain tumours.14
It is likely that the above-mentioned pathways by which folate affects genetic and epigenetic stability are also operable in the developing embryo and that the embryo is just as, if not more, vulnerable than adults to nucleotide and methyl donor inadequacies as methylation patterns are rapidly changing10 and cells are dividing rapidly. Evidence that maternal folate intake can impact on the offspring’s epigenome comes from studies with ‘Agouti’ (Avy/a) mice in which maternal supplementation with folate and related dietary methyl donors resulted in a shift in the offspring’s coat colour from yellow to brown in conjunction with de-novo methylation of specific sequences in the agouti gene.17 18
In the current study we sought to determine whether the maternal intake of folate and related B vitamins impacts on intestinal cancer in offspring using the Apc1638N mouse model. Offspring from supplemented mothers had a markedly lower incidence of small intestinal tumours than those from control or deficient mothers. Furthermore, maternal deficiency resulted in a significantly greater proportion of tumours being invasive rather than benign adenomas, compared with tumours in the control group. These data indicate that maternal supplementation with vitamins B2, B6, B12 and folate may significantly lessen the risk of intestinal tumorigenesis in offspring. The relevance of this work is underscored by the fact only approximately one-third of US women report taking vitamins containing folate before conception19 and that mild inadequacies of B2,20 B6 21 and B1222 occur in 10–50% of the US and European populations.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the institutional review board of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University. Two strains of mice were utilised for this study: C57BL6/J (Charles River, Wilmington, Massachusetts, USA) and Apc1638N 23 (NCI Mouse Repository, Frederick, Maryland, USA). Mice were housed in a 12 h light–dark cycle at 23°C and provided with free access to water throughout the experiment.
The Apc1638N mouse model was utilised, which has a targeted modification of exon 15 of one allele of the Apc gene, resulting in a chain-terminating truncation mutation of the Apc protein at codon 1638.23 Mice heterozygous for this particular mutation spontaneously develop between one and five small bowel adenomas or carcinomas.
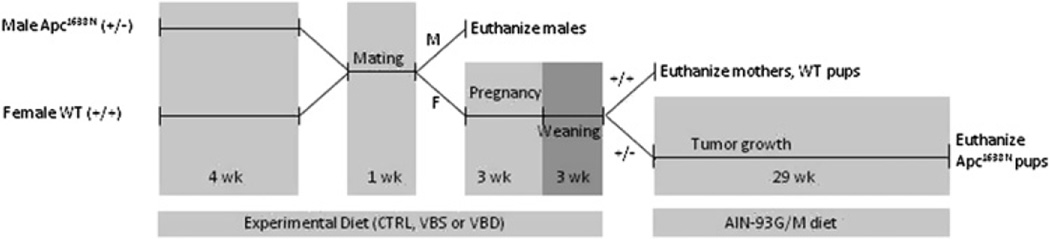
The study design is illustrated in figure 1. Six-week-old female wild-type C57BL6/J mice were housed individually and randomly assigned to be group pair-fed one of three amino acid-defined diets (Dyets, Bethlehem, Pennsylvania, USA) containing either mildly deficient (VBD), replete (CTRL) or supplemental (VBS) quantities of vitamins B2, B6, B12 and folate (see table 1) (n=38, 26, and 22 dams for the VBD, CTRL, and VBS groups, respectively). After 4 weeks, two female mice from the same group and one male Apc1638N were placed in a cage for 1 week to allow mating and continued to be fed their assigned experimental diets. Before this time male mice were all fed standard mouse chow (Harlan Teklad, Madison, Wisconsin, USA) but consumed the experimental diet during the mating period. After mating, females were removed to separate cages and provided free access to the same diets throughout pregnancy and through weaning. Three weeks after birth, pups were genotyped immediately from tail snips. Within 3 days, Apc1638N offspring were removed to separate cages and all fed freely a standard replete diet (AIN-93G; Dyets) regardless of their mother’s diet. At this time mothers and wild-type pups were all killed by carbon dioxide asphyxiation followed by cervical dislocation. Blood was collected by cardiac puncture and stored for later vitamin analyses. The abdomen was then opened and the small intestine removed onto an ice-cold glass plate. The small intestine was flushed with ice-cold saline, opened longitudinally and washed in phosphate-buffered saline (PBS) then PBS plus protease inhibitors (Roche, Indianapolis, Indiana, USA). Approximately 2 cm of the normal proximal small intestine was fixed in formalin (<48 h) before being embedded in paraffin, sectioned and mounted on microscope slides. The remaining normal-appearing small intestine mucosa was scraped with microscope slides, divided into aliquots, frozen in liquid nitrogen and then stored at −80°C. The liver was also removed, frozen and stored (nitrogen, −80°C).
Figure 1.
Design of animal experiment. M, male. F, female. CTRL, control diet. VBD, vitamin B deficient diet. VBS, vitamin B supplemented diet. Wild-type (WT) (+/+), C57BL6/J mice wild-type for Apc gene. Apc1638N (+/−), mice heterozygous for truncation mutation in the Apc gene.
Table 1.
Vitamin composition of maternal and offspring diets
| Mice | Diet type | Folic acid (mg/kg diet) |
Vitamin B2 (mg/kg diet) |
Vitamin B6 (mg/kg diet) |
Vitamin B12 (µg/kg diet) |
|---|---|---|---|---|---|
| Dams* | Vitamin B deficient (VBD) | 0.5 | 2.0 | 2.0 | 10.0 |
| Control (CTRL) | 2.0 | 6.0 | 7.0 | 50.0 | |
| Vitamin B supplemented (VBS) | 8.0 | 24.0 | 28.0 | 200.0 | |
| Apc1638N offspring † | AIN-93G then M | 2.0 | 6.0 | 7.0 | 25 |
Maternal diet consumption began 4 weeks before mating and lasted through weaning.
Apc1638N offspring diet consumption began at weaning and lasted until animals were killed (29 weeks).
CTRL, control diet; M, maintenance; VBD, vitamin B deficient diet; VBS, vitamin B supplemented diet.
Apc1638N offspring were maintained on replete AIN-93 diet for 29 weeks, ie, until 32 weeks (8 months) of age: the growth formulation for the first 16 weeks and maintenance for the remainder. After this period, Apc1638N mice were killed as described above for the wild-type pups, with the exception that after washing the small intestine, it was systematically examined under a dissecting microscope for the presence of tumours by a blinded and experienced observer. Tumours were photographed, measured with digital calipers then excised and stored in formalin (<48 h) before being embedding in paraffin, sectioned and mounted on microscope slides. Tumour slides were haematoxylin and eosin stained and graded by an expert rodent pathologist (RTB) as either adenoma or invasive carcinoma.
Vitamin analyses
Plasma concentrations of folate and vitamin B12 were quantified using a commercially available chemiluminescence assay (IMMULITE; Siemens Healthcare Diagnostics, Deerfield, Illinois, USA). Hepatic and small intestine folate concentrations were determined by the Lactobacillus casei microbiological assay.24 Plasma vitamin B6 was determined by radioenzymatic assay25 and red cell vitamin B2 concentrations were estimated by the erythrocyte glutathione reductase activation coefficient assay.26
Nucleic acid analyses
DNA was isolated from small intestine mucosal scrapings with phenol:chloroform and quantified with Pico-green reagent (Invitrogen, Carlsbad, California, USA). Genomic DNA methylation was measured by liquid chromatography/mass spectrometry after enzymatic digestion according to the method developed in our laboratory.27 Methylation of three specific regions within the promoter of the Sfrp1 gene was determined by methyl-specific PCR according to the method of Samuel et al.28 Total RNA was extracted from small intestine mucosal scrapings using Trizol reagent (Invitrogen) and 2 µg subjected to reverse transcription using Superscript II enzyme and Oligo dT primers (Invitrogen). The relative expression of a panel of genes was then studied by real-time PCR using SYBR green master mix and an ABI7300 thermocyler (Applied Biosystems, Foster City, CA, USA). Genes selected for analysis included tumour suppressor genes, genes commonly methylated in CRC and/or known to be sensitive to B vitamin status including Apc, Gsk3β, β-catenin, Sfrp1, Wif1, Wnt5a, Tgf-β1, Smad4, Smad7, p53, p21, Cdx2 and MMP9. GAPDH was used as the control gene. Primer sequences specific to each of these genes of interest were retrieved from the qPrimer database (http://mouseprimerdepot.nci.nih.gov).
Apoptosis and proliferation
Apoptosis was assessed in small intestine mucosal scrapings using the CaspACE colorimetric assay according to the manufacturer’s instructions (Promega, Madison, Wisconsin, USA). Results were confirmed by western blotting with rabbit anti-cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA) and goat α-rabbit (Santa Cruz) antibodies. Staining was normalised to GAPDH using mouse primary antibody (Millipore, Billerica, Massachusetts, USA) and goat α-mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cell proliferation was assessed by immunohistochemistry for Ki-67 using rabbit α-Ki-67 primary (Abcam, Cambridge, Massachusetts, USA) followed by detection with the ‘ABC’ peroxidation system (Vector Labs, Burlingame, California, USA). The number of Ki-67-positive cells in the crypt was counted. Villi were scored separately and assigned the number 0–4 based on whether 0, 25, 50, 75 or 100% cells in the lower third of the villus were positively stained. At least 20 crypts and villi were scored per sample.
Statistical analyses
Comparisons of the incidence of tumours in pups between maternal dietary groups were done using a Poisson regression using the mother as the offset variable (PROC GENMOD). Comparisons of tumour grade between maternal dietary groups were performed using χ2 analysis. Comparisons of numerical endpoints between maternal dietary groups (ie, vitamin analyses, gene expression, etc) were made using one-way analysis of variance (ANOVA). Testing for significant trends between diet groups was done using linear regression. Statistical analyses were performed using SAS v9.2 and SYSTAT v11. Significance was accepted when p≤0.05. All data are reported as mean±SEM.
RESULTS
Vitamin analyses
Blood and tissue vitamin concentrations are reported in table 2 for mothers (wild-type), weanling pups (wild-type) and 8-month-old pups (Apc1638N). Incremental increases in plasma vitamin B6 and B12 concentrations were observed for both mothers and weanling wild-type offspring going from deficient to supplemented maternal diets. Interestingly, these differences were also present, albeit to a lesser degree, in the 8-month-old Apc1638N offspring for plasma B6 and B12.
Table 2.
Blood and tissue B vitamin concentrations in dams consuming different B vitamin intakes and their offspring
| Maternal diet |
|||||
|---|---|---|---|---|---|
| Generation | Vitamin B deficient | CTRL | Vitamin B supplemented | ANOVA p value | |
| Plasma folate (ng/ml) | Mothers | 81.4±7.3 (23) | 84.7±7.8 (7) | 104.4±26.3 (7) | 0.95 |
| 3-Week offspring (wild-type) | 76.1±4.1 (54) | 63.3±6.1 (17) | 107.8±10.3*† (16) | <0.01 | |
| 8-Month offspring (Apc) | 59.3±2.0 (40) | 52.5±3.4 (26) | 50.2±3.2 (21) | 0.12 | |
| Hepatic folate (µg/g) | Mothers | 11.12±0.70 (14) | 13.20±0.88 (9) | 13.29±1.43 (8) | 0.17 |
| 3-Week offspring (wild-type) | 8.38±0.62* (9) | 13.37±1.19 (10) | 14.31±1.45† (10) | <0.01 | |
| 8-Month offspring (Apc) | 12.90±0.65 (9) | 14.14±1.24 (10) | 13.34±1.05 (10) | 0.69 | |
| Small intestine folate (ng/g) | 3-Week offspring (wild-type) | 924.9±89.0 (8) | 1358.2±155.0 (10) | 1295.0±205.3 (9) | 0.16 |
| 8-Month offspring (Apc) | 1264.7±126.1 (10) | 1205.9±136.7 (9) | 1172.4±146.8 (9) | 0.89 | |
| Plasma vitamin B12 (ng/ml) | Mothers | 3.56±0.14* (23) | 10.9±60.72 (7) | 37.59±4.91*† (8) | <0.01 |
| 3-Week offspring (wild-type) | 3.31±0.08* (54) | 8.26±0.80 (17) | 24.48±1.59*† (16) | <0.01 | |
| 8-Month offspring (Apc) | 27.21±0.86 (41) | 24.94±0.84 (26) | 32.87±1.45*† (20) | <0.01 | |
| Plasma vitamin B6 (ng/ml) | Mothers | 151.2±7.2* (7) | 262.9±12.6 (6) | 310.6±29.7† (6) | <0.01 |
| 3-Week offspring (wild-type) | 150.5±13.1* (22) | 281.5±17.3 (13) | 349.7±28.8† (12) | <0.01 | |
| 8-Month offspring (Apc) | 255.9±13.4 (38) | 294.0±18.8 (25) | 319.9±17.2† (20) | 0.02 | |
| Red cell vitamin B2 (activity coeff) | Mothers | 1.43±0.01 (21 | 1.36 (1) | 1.40 (1) | 0.50 |
| 3-Week offspring (wild-type) | 1.27±0.01* (50) | 1.20±0.01 (10) | 1.22±0.03 (3) | <0.01 | |
| 8-Month offspring (Apc) | 1.32±0.01 (41) | 1.28±0.01 (26) | 1.29±0.01 (20) | 0.10 | |
Data=mean±SEM. Sample size in parentheses.
Red cell B2 activity coefficients are inversely related to status, hence a higher value means a lower B2 status.
Significantly different from CTRL.
Significantly different from VBD.
ANOVA, analysis of variance; CTRL, control diet; VBD, vitamin B deficient diet.
No significant differences were detected in plasma and hepatic folate concentrations between mothers of different dietary groups, although significant incremental increases in hepatic folate concentrations were detected among weanling offspring with increasing maternal intake. An effect of maternal supplementation, but not depletion, was also seen on plasma folate in weanling offspring. No differences in folate concentration were detected in small intestinal scrapings of weanling or adult offspring between maternal groups.
No differences in red cell vitamin B2 status could be detected between mothers from different groups. Compared with offspring of control mothers, weanling offspring of deficient mothers did have a significantly reduced B2 status while offspring of supplemented mothers did not display any increase. Although B2 status was slightly reduced in the deficient pups at 8 months, this difference failed to reach statistical significance (p=0.10).
Tumour incidence
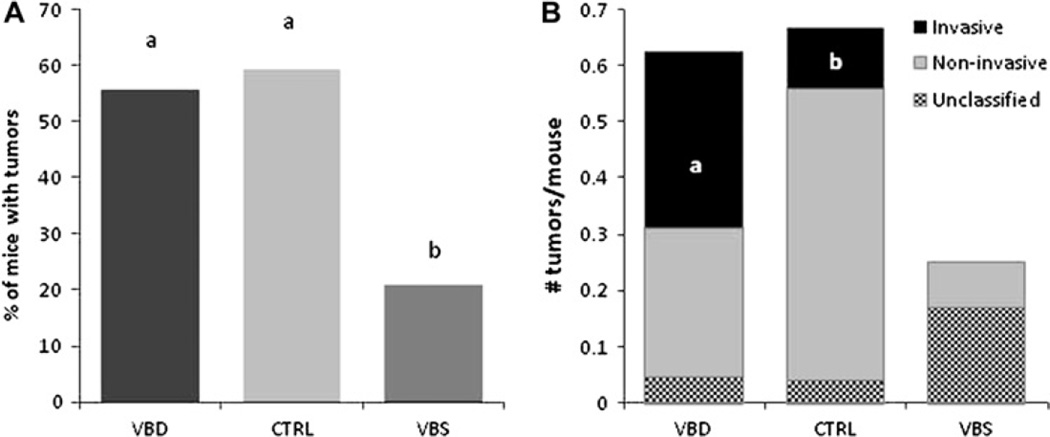
No effects of the maternal diet were observed on mating success, litter size, or genotype distribution in the offspring (additional table 1). No intestinal tumours were detected in wild-type offspring that were killed at weaning. Across all 96 Apc1638N pups, 58 small intestinal tumours were distributed among 46 mice; 33 of these tumours were adenomas, 16 were invasive cancers, while nine could not be classified due to tissue autolysis secondary to premature death. The incidence of small intestinal tumours in 8-month-old Apc1638N offspring was markedly lower among offspring of supplemented mothers compared with those of both control and deficient-fed mothers (p<0.025). Small intestinal tumours were observed in 25 of 45 (55.6%), 16 of 27 (59.3%) and five of 24 (20.8%) offspring from deficient, control and supplemented mothers, respectively (figure 2). The OR for offspring of supplemented dams displaying tumours was 0.18 (95% CI 0.0519 to 0.6308; p=0.009). Among the mice that developed tumours, no difference in tumour multiplicity (tumours/mouse) was observed (p=0.89). Although there was no difference in tumour incidence between offspring of deficient and control mothers, a significant difference in the likelihood of tumours being invasive was observed between these two groups. After excluding tumours that could not be histologically confirmed, tumours in the offspring of deficient dams were significantly more likely to be invasive than those in the offspring of control dams (14 of 26 (53.85%) vs three of 17 (17.65%), respectively; p<0.03). None of the classifiable tumours (n=2) in offspring of supplemented dams was invasive. The average maximum diameter of all tumours from all groups was 3.27±0.17 mm (range 1.82–7.78 mm) with no significant differences between groups (p>0.05).
Figure 2.
Periconceptional maternal B vitamin supplementation suppresses tumour occurrence, while depletion promotes tumour invasiveness in the small intestine of Apc1638N offspring. (A) Tumour incidence (percentage of mice with tumours). (B) Tumour grade (number of tumours per mouse) in 8-month-old Apc1638N mice. Groups with different letters are significantly different (p<0.05). Maternal diets, CTRL, control; VBD, vitamin B deficient; VBS, vitamin B supplemented. N=45, 27 and 24 mice/group respectively (from nine to 15 litters). WT, wild-type.
Molecular analyses of small intestinal mucosa
Genomic DNA methylation was measured in small intestinal scrapings from 3-week-old wild-type and 8-month-old Apc1638N mice. In the former group, maternal diet did not impact on genomic methylation (p=0.28; table 3); however, in Apc1638N offspring there was a trend for offspring of both deficient and supplemented-fed mothers to display a mild, although significant, degree of genomic hypomethylation (p=0.003 and 0.07 for VBD and VBS vs CTRL, respectively). Global methylation was not associated with tumour incidence (p=0.90).
Table 3.
Effect of maternal diet on genomic methylation and proliferation in the small intestine of offspring
| Maternal diet |
|||||
|---|---|---|---|---|---|
| Vitamin B deficient | CTRL | Vitamin B supplemented | ANOVA p value | ||
| Genomic DNA methylation | 3-Week offspring (wild-type) | 4.73±0.02 (49) | 4.74±0.03 (18) | 4.68±0.03 (16) | 0.28 |
| (% cytosine methylated) | 8-Month offspring (Apc) | 4.±0.03* (28) | 4.92±0.05 (16) | 4.79±0.05 (18) | <0.01 |
| Ki-67 staining score | 3-Week offspring (wild-type) | 0.45±0.16 (10) | 0.81±0.21 (10) | 0.87±0.22 (10) | 0.28 |
| (Rating for lower third of villus) (0–5) | 8-Month offspring (Apc) | 1.83±0.18 (17) | 1.78±0.16 (15) | 1.72±0.21 (8) | 0.93 |
Data=mean±SEM. Sample size in parentheses (pups from 7–19 litters/group and 6–12 litters/group for methylation and Ki-67, respectively).
Significantly different from CTRL.
ANOVA, analysis of variance; CTRL, control diet.
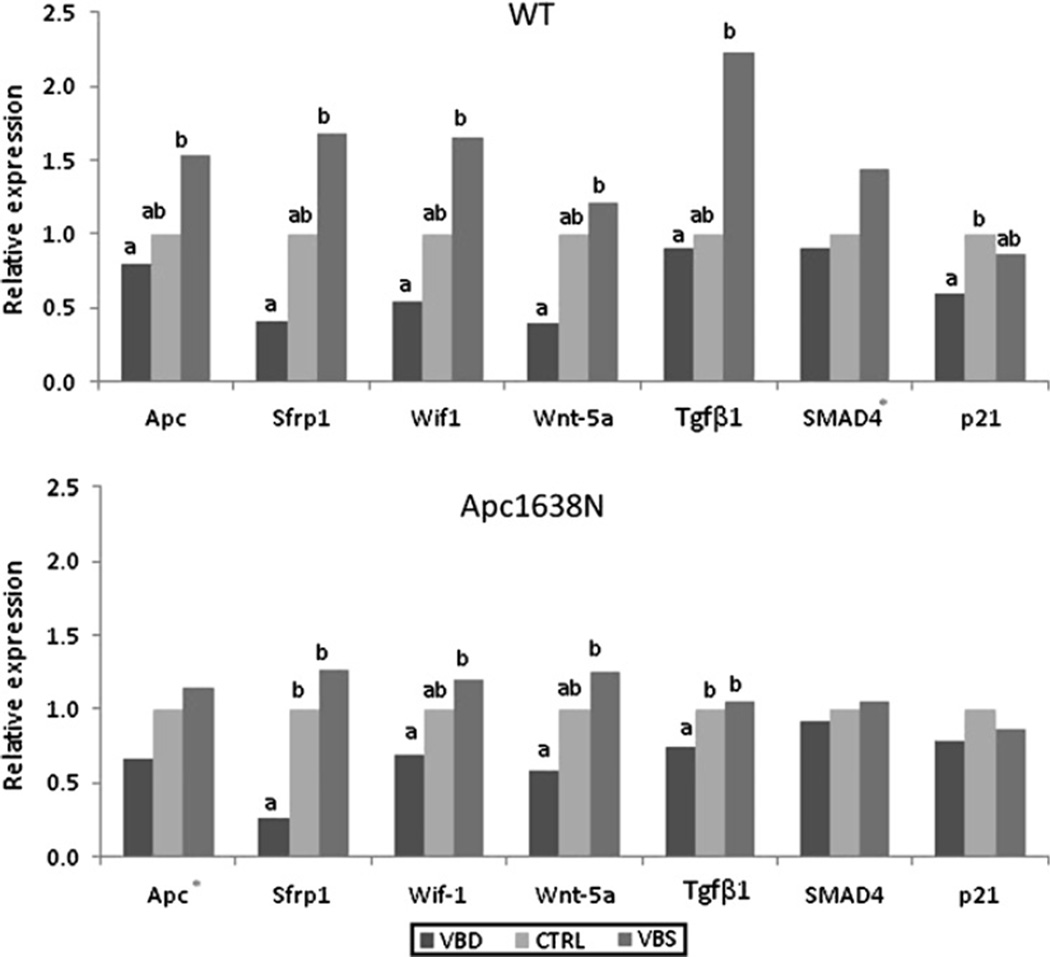
Of the genes for which expression was measured in the normal small intestine mucosa, seven genes were responsive to maternal diet in wild-type and/or Apc1638N offspring, returning an ANOVA p for diet of ≤0.05 or an ANOVA p of ≤0.1 and a ptrend of <0.05 (figure 3). In general, expression changes were of a smaller magnitude in the Apc1638N pups than their wild-type littermates. The genes Apc, Sfrp1, Wif1 and Wnt5a, the products of which negatively regulate the Wnt pathway, displayed significant stepwise reductions in expression in both sets of pups, with reducing maternal vitamin intake. Within the Tgf-β pathway, there was a tendency for the expression for Tgfβ1 and Smad4 to be reduced with decreasing maternal B vitamin intake in either wild-type or Apc1638N offspring. The cell cycle inhibitor p21 was also suppressed in wild-type pups of deficient dams (figure 3). Upon application of the Bonferroni correction for multiple comparisons (p=0.05/13 = 0.003), only differences in Sfrp1 expression in Apc1638N mice remained significant (p=0.002).
Figure 3.
Effect of maternal B vitamin intake on the expression of select genes in the small intestine of weanling wild-type and adult Apc1638N offspring. Gene expression in the small intestinal mucosa of 3-week-old wild-type (upper panel) and 8-month-old Apc1638N (lower panel) offspring. Mothers were fed diets either mildly deficient (VBD), replete (CTRL) or supplemented (VBS) with vitamins B2, B6, B12 and folate. Data expressed as relative expression (2-ΔΔCt). Statistical analyses performed on ΔCt values. Different letters denote significant difference (p<0.05). *indicates ptrend<0.05. N=10–18/group (from six to 12 litters).
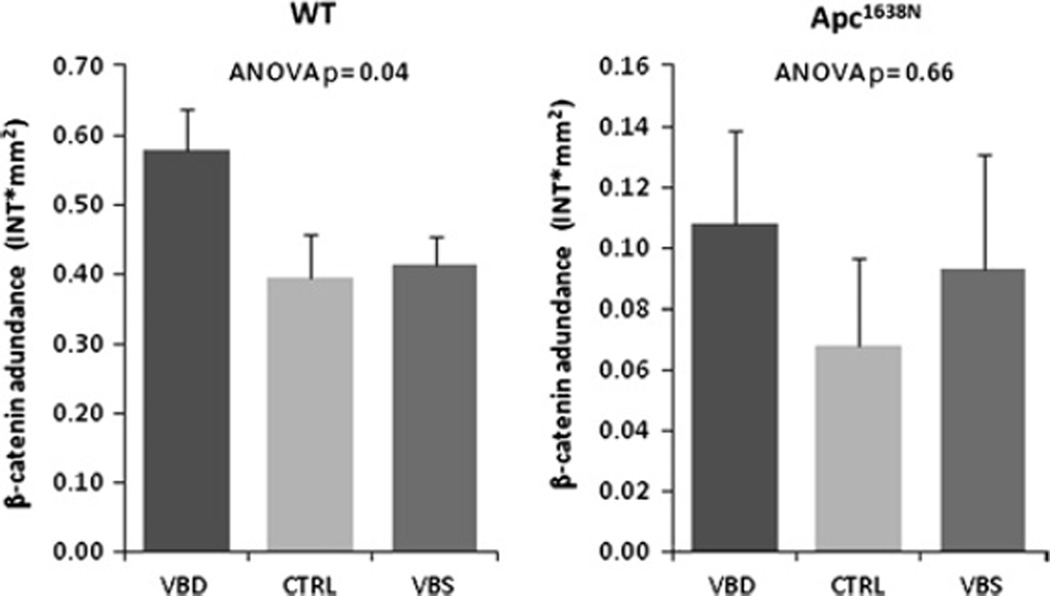
Because we observed a significant repression of Sfrp1 expression with declining maternal B vitamin consumption, we measured the methylation of three specific regions in and around the promoter of this gene in both Apc1638N and wild-type pups. For each of the two strains of animals, two of the three amplicons that were examined were significantly and inversely correlated with expression (table 4), underscoring a potential functional relationship between methylation and expression. In order to determine whether these changes could result in an increased activation of the Wnt pathway, we measured cellular levels of total β-catenin, the effector molecule of this pathway. No differences in nuclear (immunohistochemical) or total β-catenin (western blotting) were detected in the small intestine of the Apc1638N offspring, but this is not unexpected because this animal already has a 40-fold upregulation of the Wnt pathway (Liu et al, in preparation), so modest differences due to diet are difficult to detect on this high background of activation. Therefore, we measured total β-catenin in wild-type offspring and observed an approximately 45% elevation in pups of deficient dams compared with those of replete and supplemented dams (p=0.04; figure 4).
Table 4.
Relationship between Sfrp1 expression and methylation in weanling wild-type and adult Apc1638N offspring
| 3-Week offspring (wild-type) |
8-Month offspring (Apc1638N) |
|||
|---|---|---|---|---|
| p Value | R | p Value | R | |
| Amplicon 1 | NS | − (30) | 0.04 | 0.37 (31) |
| Amplicon 2 | 0.02 | 0.43 (28) | NS | − (30) |
| Amplicon 3 | 0.02 | 0.37 (37) | 0.02 | 0.40 (35) |
Correlation analyses performed on ΔCt values versus methylation (intensity methyl/unmethyl band) of each methyl-specific PCR amplicon. Location of methyl (M) and unmethyl (UM) amplicons in relation to start of gene (NM_013834): amplicon 1 (M: 23–136, UM: 19–139); amplicon 2 (M: −140–5, UM: −140–5); amplicon 3(M: 246–372, UM: 243–375). Note that because ΔCt values are inversely related to gene expression, the positive R values reported here reflect an inverse association. Sample size in parentheses.
NS, not significant.
Figure 4.
Effect of maternal B vitamin intake on the abundance of total β-catenin protein in the small intestine mucosa of offspring. Total β-catenin protein abundance in small intestinal mucosa scrapings of 3-week-old wild-type (WT) (left panel) and 8-month-old Apc 1638N (right panel) offspring of mothers fed diets either mildly deficient (VBD), replete (CTRL) or supplemented (VBS) in vitamins B2, B6, B12 and folate. Protein levels were evaluated by western blotting and band volumes for β-catenin (intensity×mm2) were corrected for those of GAPDH. Data=mean±SEM. N=7–13/group (from six to 12 litters).
Estimates of apoptosis obtained using a colorimetric assay for caspase-3 activity indicated that in both wild-type and Apc1638N pups, offspring of deficient dams had a significantly higher caspase-3 activity compared with both replete and supplemented dams (figure 5A). Because we expected to see the highest apoptotic activity in the offspring of supplemented dams and the lowest in those of deficient dams we sought to verify these results by western blotting. Protein extracts from Apc1638N pups were analysed in this fashion, and the results concurred with those of the activity assay; caspase-3 expression was significantly higher in offspring of deficient compared with control and supplemented dams (figure 4B). These endpoints were significantly and positively associated with each other (R2=0.21, p=0.006).
Figure 5.
Maternal B vitamin deficiency elevates apoptosis in the small intestine of offspring. Estimation of apoptotic activity in small intestinal mucosal scrapings by: (A) Colorimetric assay for caspase-3 activity in 3-week-old wild-type (WT) and 8-month-old Apc1638N. (B). Western blotting for cleaved caspase-3 protein in 8-month-old Apc1638N mice. Groups with different letters are significantly different. Data for caspase-3 activity and abundance were significantly correlated (R2=0.21, p=0.007). Data=mean±SEM. N=9–18/group (from six to 12 litters). ANOVA, analysis of variance; CTRL, control diet; VBD, vitamin B deficient diet; VBS, vitamin B supplemented diet.
In order to gain an estimate of cellular proliferation we stained for Ki-67 in sections of the small intestine. Because crypts displayed an almost ubiquitous degree of Ki-67 staining we focused on the lower third of the villi in an attempt to detect a possible expansion of the proliferative zone in the high tumour groups. No effect of maternal diet was seen on the abundance of positively stained cells in the lower third of the villi in either wild-type 3-week (p=0.28) or 8-month Apc1638N (p=0.93) offspring (table 3).
DISCUSSION
Our data clearly indicate that periconceptional maternal supplementation with four times the basal requirement of vitamins B2, B6, B12 and folate markedly reduces small intestinal tumour incidence in Apc1638N offspring (OR = 0.18). Furthermore, while a maternal diet mildly deficient in these B vitamins did not elevate tumour incidence above control levels, tumours in the offspring of deficient-fed mothers were significantly more likely to be invasive, rather than benign, compared with tumours in offspring of control-fed mothers (figure 2).
The data from the current study are in good agreement with two other studies investigating the effect of maternal B vitamin intake on intestinal tumorigenesis in offspring. First, similar to the outcome of our depletion arm, McKay et al29 reported that mild maternal folate depletion (0.4 mg/kg), compared with adequacy (2 mg/kg), did not alter the intestinal tumour incidence among replete-fed Apcmin offspring; however, the tumour grade was not reported. Data from Sie et al30 agree with findings from our supplemental arm: periconceptional maternal folate supplementation (5 mg/kg), compared with adequacy (2 mg/kg), significantly reduced azoxymethane-induced colorectal tumour incidence in offspring, irrespective of which diet offspring were fed.
In the current study we were solely concerned with the effect of maternal diet on offspring tumorigenesis, and therefore assigned the offspring of all three maternal groups to consume the same replete AIN-93 diet after weaning. In contrast, Lawrance et al,31 fed mouse dams either folate-deficient, replete or supplemented diets (0.3, 2.0 and 20 mg/kg) periconceptionally and through weaning, but assigned the Apcmin/+ offspring to continue on the same diets their mother received. Significantly fewer intestinal tumours were observed in deplete offspring than in those fed control and supplemented diets. We suggest that these results highlight the dual role of folate in cancer, whereby folate supplementation before the appearance of neoplastic lesions may be protective while supplementation afterwards may fuel the growth of tumours, with the opposite being true for folate depletion. In the Apcmin/+ model, because the tumorigenic phenotype is so severe (15–45 tumours/mouse) and begins so early, the ability of folate to act in its preventive capacity may be overwhelmed, thus biasing the overall results towards the effect folate has in promoting tumour growth and evolution. Although the design of this study precludes dissecting out the effects of maternal versus offspring diet, the findings nevertheless may still have important public health relevance because it is known that a child’s dietary habits are modelled largely upon those of their parents.32
Although the mechanistic importance of genomic DNA methylation in tumorigenesis is unclear, a graded decrease in whole-genome methylation going from normal to malignant tissue has been repeatedly observed in CRC.33 Because folate status34 and variants in folate-metabolising enzymes35 have been reported to impact on genomic methylation we also studied this endpoint. No significant differences in genomic methylation were observed between weanling wild-type pups of different maternal dietary groups; however, a small but significant reduction in genomic methylation was seen among adult Apc1638N pups of VBD dams (ie, those possessing the highest likelihood of developing invasive cancers, compared with CTRL dams) (table 3). We speculate that maternal deficiency may initiate a specific metabolic programme, which, over time, results in genomic hypomethylation that might subsequently promote tumorigenesis. Mechanistically, genomic DNA hypomethylation may cause chromosomal instability36 and loss,37 both recognised risk factors for cancer.38 39
Our previous work showing that multiple B vitamin depletion can cause aberrant activation of the Wnt pathway40 prompted us again to consider a role for this pathway in the current study. Taken together, the reduced expression of Wnt pathway negative regulators (figure 3) with the elevated level of total β-catenin protein observed in wild-type VBD pups (figure 4) points towards a de-repression of this pathway in offspring with decreasing maternal B vitamin intake. In the case of Sfrp1, we observed a significant inverse relationship between expression and methylation (table 4), which is consistent with observations of previous investigators who have demonstrated a functional relationship between these two phenomena in human colorectal carcinogenesis.41 42 This is of particular relevance as previous work indicates that loss of Sfrp1 function is an early event in human colorectal tumorigenesis that results in constitutive Wnt signalling.41 Furthermore, expression of exogenous Sfrp1 inhibits the growth of established tumours by promoting apoptosis and suppressing vascularisation,43 and this latter observation may have relevance to the increased risk of invasiveness that was observed with depletion in our study.
In an effort to explain the kinetic mechanisms underlying observed differences in tumour incidence we evaluated apoptosis and proliferation in the small intestine of offspring. We observed a significantly elevated caspase-3 activity in VBD pups compared with pups of both CTRL and VBS dams, a finding subsequently confirmed by western blotting in the Apc1638N pups (figure 5). Since apoptosis is highest in the group with an elevated tumour incidence and the highest proportion of invasive tumours, apoptosis is unlikely to be a key factor explaining the differences in tumour burden seen here. Rather, we suggest that the elevated apoptosis among the pups of deficient dams may be explained by an elevated frequency of cells harbouring deleterious genetic and epigenetic aberrations and is an effort to remove these cells for the benefit of the organism.
Proliferation was also measured in the small intestine of offspring. Among both wild-type and Apc1638N pups there were no differences in Ki-67 staining between pups of different dietary groups (table 3). When comparing proliferation scores between the two offspring genotypes it was clear that Apc1638N mice had a greater expansion of the proliferative cells into the villi than wild-type mice (p<0.0001). Although caution should be exercised when comparing these two genotypes, others have previously shown that small intestine proliferation is not different between wild-type and Apc1638N mice of the same age.44 Therefore we speculate that, although we did not observe differences in proliferation between groups at either time point, it is plausible that pups of deficient and CTRL dams attained a hyperproliferative state significantly earlier than pups of supplemented dams. In this case we may have missed the critical window of time when differences in proliferation were apparent. Sampling of Apc1638N offspring at various ages would have been required to detect such a difference.
It is well recognised that all non-human models of CRC have limitations, and this includes the Apc1638N mouse. Virtually all genetic models of colorectal carcinogenesis in the rodent (including the widely used Apcmin mouse) have a strong predilection for forming small intestinal, rather than colonic, neoplasms and we have found this to be true with the Apc1638N mouse model as well. Although the original characterisation of this model23,45 indicated that it also develops colorectal tumours, we have found this to be a relatively rare event. Nevertheless, genetically induced models of colorectal carcinogenesis have been used with considerable effectiveness to study the effects of diet on cancer development because the small intestinal tumorigenesis generally responds in a fashion that mimics the effects of diet on the colon, and this has certainly been true of the Apc1638N mouse.46,47 Although tumour location differs from the human situation, in which the vast majority of tumours appear in the colorectum as opposed to the small intestine, there are aspects of tumorigenesis in Apc1638N mice that closely adhere to molecular carcinogenesis in the human. For instance, Apc protein is reported to be absent in 71% of human colorectal adenocarcinomas (and only 31% of small intestine adenocarcinomas).48 Similarly, inactivation of Apc is critical in Apc1638N tumorigenesis in which 81% of tumours display a loss of the wild-type Apc allele.45
In conclusion, our data clearly demonstrate that maternal B vitamin intake can modulate both the incidence of small intestinal neoplasms in mouse offspring as well as the likelihood of progressing to an invasive phenotype. Our data are supportive of a relative de-repression of the Wnt pathway in pups of CTRL, but especially of deficient dams. Quite aside from the well-documented benefits in the prevention of birth defects, our observations indicate that, at least within the framework of this animal model, mothers who initiate B vitamin supplementation before conception may also be protecting their offspring against CRC in adulthood.
Significance of this study.
What is already known about this subject?
-
▶
Epidemiological data and controlled animal studies support a protective role for dietary folate and related B vitamins against colorectal cancer.
-
▶
Maternal diet and environmental exposure are becoming increasingly recognised as important determinants of the risk for chronic disease in offspring.
-
▶
In addition to its established role in preventing birth defects, maternal folate supplementation appears to be protective against several paediatric cancers.
What are the new findings?
-
▶
Maternal supplementation with vitamins B2, B6, B12 and folate markedly suppresses intestinal tumorigenesis in mouse offspring (OR 0.18; 95% CI 0.0519 to 0.6308; p=0.009).
-
▶
Exceedingly mild maternal B vitamin inadequacy increases the likelihood of tumours in offspring acquiring an invasive phenotype.
-
▶
A de-repression of the Wnt pathway characterised by the hypermethylation and suppression of Sfrp1 and accumulation of β-catenin was observed with declining maternal B vitamin intake.
How might it impact on clinical practice in the foreseeable future?
-
▶
Mild deficiencies of vitamins B2, B6 and B12 persist in 10–50% of the population of industrialised nations such as the USA and UK. In addition, although maternal folic acid supplementation is widespread, it is frequently not initiated until after conception. These data indicate that maternal B vitamin supplementation may not only protect offspring against birth defects but also against colorectal cancer in adulthood.
Acknowledgements
The authors would like to thank the staff of the Nutrition Evaluation Laboratory at the HNRCA for their kind measurement of blood vitamin concentrations, as well as Dr Donald Smith and Andrea Pinilla for their assistance with animal care and maintenance. Thanks also to Drs Larry Feig and Xiang-Dong Wang for their input.
Funding This work was supported by grants from the NIH; 2-T32-DK062032-18 (EDC) and 5-K05- CA100048-05 (JBM) and Prevent Cancer Foundation (ZL). This material is based upon work supported by the US Department of Agriculture, under agreement no 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
Footnotes
An additional table is published online only. To view this file please visit the journal online (http://gut.bmj.com).
Ethics approval All animal procedures were approved by the institutional review board of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Eussen SJ, Vollset SE, Igland J, et al. Plasma folate, related genetic variants, and colorectal cancer risk in EPIC. Cancer Epidemiol Biomarkers Prev. 2010;19:1328–1340. doi: 10.1158/1055-9965.EPI-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannucci E, Rimm EB, Ascherio A, et al. Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA, Sandler RS, Haile RW, et al. Folate intake, alcohol consumption, cigarette smoking, and risk of colorectal adenomas. J Natl Cancer Inst. 1998;90:57–62. doi: 10.1093/jnci/90.1.57. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Smith-Warner SA, Spiegelman D, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010;21:1919–1930. doi: 10.1007/s10552-010-9620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YI, Salomon RN, Graeme-Cook F, et al. Dietary folate protects against the development of macroscopic colonic neoplasia in a dose responsive manner in rats. Gut. 1996;39:732–740. doi: 10.1136/gut.39.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravo ML, Mason JB, Dayal Y, et al. Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine-treated rats. Cancer Res. 1992;52:5002–5006. [PubMed] [Google Scholar]
- 7.Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev. 2009;67:206–212. doi: 10.1111/j.1753-4887.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arasaradnam RP, Commane DM, Bradburn D, et al. A review of dietary factors and its influence on DNA methylation in colorectal carcinogenesis. Epigenetics. 2008;3:193–198. doi: 10.4161/epi.3.4.6508. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(Suppl 6):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 10.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 11.French AE, Grant R, Weitzman S, et al. Folic acid food fortification is associated with a decline in neuroblastoma. Clin Pharmacol Ther. 2003;74:288–294. doi: 10.1016/S0009-9236(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 12.Orjuela MA, Titievsky L, Liu X, et al. Fruit and vegetable intake during pregnancy and risk for development of sporadic retinoblastoma. Cancer Epidemiol Biomarkers Prev. 2005;14:1433–1440. doi: 10.1158/1055-9965.EPI-04-0427. [DOI] [PubMed] [Google Scholar]
- 13.Baethmann M, Wendel U, Hoffmann GF, et al. Hydrocephalus internus in two patients with 5,10-methylenetetrahydrofolate reductase deficiency. Neuropediatrics. 2000;31:314–317. doi: 10.1055/s-2000-12947. [DOI] [PubMed] [Google Scholar]
- 14.Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685–691. doi: 10.1038/sj.clpt.6100100. [DOI] [PubMed] [Google Scholar]
- 15.Schüz J, Weihkopf T, Kaatsch P. Medication use during pregnancy and the risk of childhood cancer in the offspring. Eur J Pediatr. 2007;166:433–441. doi: 10.1007/s00431-006-0401-z. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JR, Gerald PF, Willoughby ML, et al. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case—control study. Lancet. 2001;358:1935–1940. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 17.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(Suppl 8):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 18.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.March-of-Dimes. Improving preconception health: women’s knowledge and use of folic acid. White Plains, NY: March of Dimes Foundation; 2008. [Google Scholar]
- 20.Henderson L, Gregory J. [accessed 27th May 2011];National diet and nutrition survey: adults aged 19–64. 2004 4 http://www.food.gov.uk/multimedia/pdfs/ndnsfour.pdf. [Google Scholar]
- 21.Planells E, Sanchez C, Montellano MA, et al. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur J Clin Nutr. 2003;57:777–785. doi: 10.1038/sj.ejcn.1601610. [DOI] [PubMed] [Google Scholar]
- 22.Lindenbaum J, Rosenberg IH, Wilson PW, et al. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 23.Fodde R, Edelmann W, Yang K, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–347. doi: 10.1136/jcp.45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camp VM, Chipponi J, Faraj BA. Radioenzymatic assay for direct measurement of plasma pyridoxal 59-phosphate. Clin Chem. 1983;29:642–644. [PubMed] [Google Scholar]
- 26.Nichoalds GE. Assessment of status riboflavin nutriture by assay of erythrocyte glutathione reductase activity. Clin Chem. 1974;20:624–628. [PubMed] [Google Scholar]
- 27.Friso S, Choi SW, Dolnikowski GG, et al. A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal Chem. 2002;74:4526–4531. doi: 10.1021/ac020050h. [DOI] [PubMed] [Google Scholar]
- 28.Samuel MS, Suzuki H, Buchert M, et al. Elevated Dnmt3a activity promotes polyposis in Apc(Min) mice by relaxing extracellular restraints on Wnt signaling. Gastroenterology. 2009;137:902–913. 913, e1–e11. doi: 10.1053/j.gastro.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 29.McKay JA, Williams EA, Mathers JC. Gender-specific modulation of tumorigenesis by folic acid supply in the Apc mouse during early neonatal life. Br J Nutr. 2008;99:550–558. doi: 10.1017/S0007114507819131. [DOI] [PubMed] [Google Scholar]
- 30.Sie KKY, Medline A, van Weel J, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut. 2011;60:1687–1694. doi: 10.1136/gut.2011.238782. [DOI] [PubMed] [Google Scholar]
- 31.Lawrance AK, Deng L, Rozen R. Methylenetetrahydrofolate reductase deficiency and low dietary folate reduce tumorigenesis in Apc min/+ mice. Gut. 2009;58:805–811. doi: 10.1136/gut.2007.143107. [DOI] [PubMed] [Google Scholar]
- 32.Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes (Lond) 2010;34:1116–1124. doi: 10.1038/ijo.2010.43. [DOI] [PubMed] [Google Scholar]
- 33.Cravo M, Fidalgo P, Pereira AD, et al. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. Eur J Cancer Prev. 1994;3:473–479. doi: 10.1097/00008469-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Jacob RA, Gretz DM, Taylor PC, et al. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr. 1998;128:1204–1212. doi: 10.1093/jn/128.7.1204. [DOI] [PubMed] [Google Scholar]
- 35.Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez J, Frigola J, Vendrell E, et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res. 2006;66:8462–9468. doi: 10.1158/0008-5472.CAN-06-0293. [DOI] [PubMed] [Google Scholar]
- 37.Guttenbach M, Schmid M. Exclusion of specific human chromosomes into micronuclei by 5-azacytidine treatment of lymphocyte cultures. Exp Cell Res. 1994;211:127–132. doi: 10.1006/excr.1994.1068. [DOI] [PubMed] [Google Scholar]
- 38.Duesberg P, Rasnick D, Li R, et al. How aneuploidy may cause cancer and genetic instability. Anticancer Res. 1999;19:4887–4906. [PubMed] [Google Scholar]
- 39.Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059–2072. doi: 10.1053/j.gastro.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Choi SW, Crott JW, et al. Mild depletion of dietary folate combined with other B vitamins alters multiple components of the Wnt pathway in mouse colon. J Nutr. 2007;137:2701–2708. doi: 10.1093/jn/137.12.2701. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 42.Caldwell GM, Jones CE, Ashley AM, et al. Wnt signalling in adenomas of familial adenomatous polyposis patients. Br J Cancer. 2010;103:910–917. doi: 10.1038/sj.bjc.6605790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu J, Dong A, Fernandez-Ruiz V, et al. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 44.Mahmoud NN, Bilinski RT, Churchill MR, et al. Genotype-phenotype correlation in murine Apc mutation: differences in enterocyte migration and response to sulindac. Cancer Res. 1999;59:353–359. [PubMed] [Google Scholar]
- 45.Smits R, Kartheuser A, Jagmohan-Changur S, et al. Loss of Apc and the entire chromosome 18 but absence of mutations at the Ras and Tp53 genes in intestinal tumors from Apc1638N, a mouse model for Apc-driven carcinogenesis. Carcinogenesis. 1997;18:321–327. doi: 10.1093/carcin/18.2.321. [DOI] [PubMed] [Google Scholar]
- 46.Yang WC, Mathew J, Velcich A, et al. Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosa. Cancer Res. 2001;61:565–569. [PubMed] [Google Scholar]
- 47.Yang K, Edelmann W, Fan K, et al. Dietary modulation of carcinoma development in a mouse model for human familial adenomatous polyposis. Cancer Res. 1998;58:5713–5717. [PubMed] [Google Scholar]
- 48.Zhang MQ, Chen ZM, Wang HL. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma: a comparison with colorectal adenocarcinoma. Mod Pathol. 2006;19:573–580. doi: 10.1038/modpathol.3800566. [DOI] [PubMed] [Google Scholar]