Abstract
In vitro differentiation of mouse and human stem cells into early T cells has been successfully demonstrated using artificial Notch signaling systems. However, generation of mature, antigen-specific, functional T cells, directly from human stem cells has remained elusive, except when using stromal co-culture of stem cells retrovirally transfected with antigen-specific T cell receptors (TCRs). Here we show that human umbilical cord blood (UCB)-derived CD34+CD38−/low hematopoietic stem cells (HSCs) can be successfully differentiated into functional, antigen-specific cytotoxic CD8+ T cells without direct stromal co-culture or retroviral TCR transfection. Surface-immobilized Notch ligands (DLL1) and stromal cell conditioned medium successfully induced the development of CD1a+CD7+ and CD4+CD8+ early T cells. These cells, upon continued culture with cytomegalovirus (CMV) or Influenza-A virus epitope-loaded HLA-A*0201 tetramers, resulted in the generation of a polyclonal population of CMV-specific or Influenza-specific CD8+ T cells respectively. Upon further activation with antigen-loaded target cells, these antigen-specific, stem cell-derived T cells exhibited cytolytic functionality, specifically CD107a surface mobilization, IFNγ production, and Granzyme B secretion. Such scalable, in vitro generation of functional, antigen-specific human T cells from human stem cells could eventually provide a readily available cell source for adoptive transfer immunotherapies and also allow better understanding of human T cell development.
Keywords: CD34+ cells, adult hematopoietic stem cells, human cord blood, Cytotoxic T cells
Introduction
T cells, unlike other lymphocyte subsets that mature in the bone marrow, develop exclusively in the thymus. It is well established that in addition to soluble cytokines, T lineage commitment and maturation of lymphoid precursors is critically dependent on two temporally- and spatially-controlled signals provided by direct contact with thymic stromal cells: (i) Notch receptor - Delta-Like Ligand signaling and (ii) Major histocompatibility complex (MHC)/HLA - TCR signaling. Notch signaling is required for T lineage commitment and development of double positive CD4+CD8+ (DP), early T cells.1,2 DP cells undergo positive selection by interacting with antigen-loaded MHC/HLA molecules on thymic cortical epithelial cells. Specifically, cells with developing TCRs that are capable of interacting with either Class I or Class II MHC/HLA complexes escape apoptosis and further differentiate into single positive (SP) CD8+CD4− or CD4+CD8− T cells, respectively. Subsequent negative selection in the thymic medulla and cortex eliminates SP T cells with TCRs that bind with high affinity to self-antigen-loaded MHC/HLA complexes, resulting in a diverse repertoire of mature, functional T cells.1,2
Fully mature T cells are characterized by the surface expression of a TCR heterodimer (α and β chain) which recognizes foreign antigen-MHC/HLA complexes on target cells. In particular, mature CD8+ T cells, also known as Cytotoxic T Lymphocytes (CTLs), are responsible for eliminating pathogen-infected cells and tumor cells, thereby playing an essential role in immune function. This unique ability of antigen-specific CD8+ T cells provides much promise for the field of cellular immunotherapy. The use of T cells for autologous adoptive transfer has shown promise in treating many cancers including melanoma, renal cancer, leukemia, multiple myeloma, prostate cancer, Hodgkin’s disease, and nasopharyngeal cancer, as well as post-transplant lymphoproliferative diseases.3–9 Currently, T cells for such therapies are isolated from a patient’s peripheral blood, expanded in vitro for several weeks, and selected for antigen-specificity before being transplanted back into the patient.4 Thus, despite its immense clinical promise, adoptive T cell transfer is severely constrained by the difficulty and inefficiency of patient cell isolation, problems with expansion of primary cells in vitro, a limited availability of HLA-matched donor cells, and the time required to process patient-isolated cells. Thus, scalable technologies for providing efficient, high throughput and readily available sources for antigen-specific, therapeutic T cells are needed.
In vitro T cell generation from stem cells has been explored extensively using co-culture with stromal cells known to support hematopoiesis. Retrovirally-transfected, mouse bone marrow-derived stromal cells (OP9) that stably express the Notch ligands, DLL1 (OP9-DL1) or DLL4 (OP9-DL4), are capable of supporting the differentiation of mouse hematopoietic, embryonic, and induced pluripotent stem cells, as well as human hematopoietic stem cells into early T cells and CD8+ SP T cells.10–13 Recent studies have also shown that plate-bound Notch ligands and a defined combination of soluble cytokines induce early T cell development from mouse Lin-c-kit+Sca-1+ or human CD34+ HSCs.14–17 Our group has previously shown that culturing mouse Lin-c-kit+Sca-1+ HSCs with DLL4-functionalized microbeads in an insert co-culture system using OP9-DL1 cells can induce early T lineage commitment and differentiation without direct stromal cell contact.18 However, generation of mature, functional SP cells from these in vitro culture systems has not been reported extensively. Recently, a bulk population of OP9-DL1-derived mouse T cells were successfully expanded into antigen-specific, functional CD8+ T cells using bone marrow-derived dendritic cells (DCs) induced to express various antigen epitopes.19 Our group also demonstrated the ability of antigen-loaded MHC Class I tetramers to generate, from mouse DP cells or mouse embryonic stem cells, a population of CD8+ T cells specific for that particular antigen and capable of in vitro cytotoxic killing of target cells.20 However, to date, direct in vitro generation of antigen-specific, functional human T cells from any stem cell population has not been achieved, except through stromal cell co-culture with HSCs retrovirally transduced with specific TCRs.21,22
We hypothesized that the thymic HLA-TCR interaction can be recreated in vitro using foreign antigen-loaded HLA tetramers, thereby differentiating Notch-directed, human stem cell-derived early T cells into functional SP T cells specific for the same antigen. Here, we report that by culturing human umbilical cord blood (UCB)-derived CD34+CD38−/low HSCs with plate-immobilized DLL1, human HSCs can be directed into CD1a+CD7+ and CD4+CD8+ early T cells. Further culture with CMV or GIL epitope-loaded HLA-A*0201 tetramers resulted in the generation of CMV-specific or GIL-specific CD8+ T cells, respectively. These cells exhibited activation and in vitro cytolytic functionality against peptide-loaded target cells as demonstrated by surface presentation of the degranulation marker CD107a, production of IFNγ, and Granzyme B secretion.
Materials and Methods
Human HSC Expansion
5 × 105 CD34+ human cord blood mononuclear cells (CB-MN) (StemCell Technologies) were expanded in T25 tissue-culture treated flasks (Corning) using StemSpan® Serum Free Expansion Medium (StemCell Technologies) supplemented with the following human recombinant cytokines from Peprotech: Flt3L (100 ng/mL), SCF (100 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), G-CSF (20 ng/mL), TPO (50 ng/mL), and Human LDL (40 µg/mL) (StemCell Technologies). Cells were grown at 37°C and 5% CO2. After 3 days, cells were transferred to T150 tissue-culture treated flasks (Corning) and fresh media and cytokines were added to the cultures. Cells were expanded for a total of 7 days.
CD34+CD38− Cell Sorting
Expanded CB-MN cells were collected and centrifuged at 300g for 5 min at 4°C. Cells were resuspended in PBS containing 1% BSA and 2 mM EDTA. CD34+ cells were enriched using MACS CD34+ Microbead Kit and MS Columns (Miltenyi Biotec) according to the manufacturer’s protocols. Resultant cells from CD34 positive selection were stained with PE-conjugated anti-CD34 mAb (Catalog No. 130-081-002; Miltenyi Biotec) and PE-Cy7-conjugated anti-CD38 mAb (Catalog No. 25-0389-71; eBioscience). CD34+CD38−/low cells were further sorted by flow cytometry (BD FACSAria) to obtain maximal purity.
OP9-DL1 Conditioned Medium (CM)
OP9-DL1 cells were a gift from Dr. Zúñiga-Pflücker (Toronto, Canada). 3.5 × 105 OP9-DL1 cells were seeded into T75 tissue-culture treated flasks and expanded in α-MEM supplemented with 20% FBS (StemCell Technologies), sodium bicarbonate (2.2 g/L), penicillin (100 U/mL), and streptomycin (100 µg/mL). Cells were grown at 37°C and 5% CO2. Media was replaced when the cells were 80% confluent. For the next 3 days thereafter, OP9-DL1 CM was collected, filtered through a 0.22 µm sterile membrane, and stored at 4°C.
Fc-DLL1 Plate Coating
Protein A (10 µg/mL) (Sigma) diluted in PBS was adsorbed on non-tissue culture treated 96-well plates (100 µL/well) for 30 minutes at 37°C and 5% CO2. Wells were washed twice with PBS and blocked with HBSS/2% BSA at 25°C for 45 minutes. Residual blocking buffer was removed by washing twice with PBS. Human Fc-DLL1 (Enzo Life Sciences, 2.5 µg/mL or 5 µg/mL diluted in PBS), was incubated with Protein A-coated wells for 2 hours at 37°C and 5% CO2. Wells were washed twice with PBS prior to cell seeding.
Notch-DLL1 Induced T Cell Differentiation
Sorted CD34+CD38−/low cells were seeded onto Fc-DLL1 coated plates at a density of 3.0×104 cells/well in OP9-DL1 CM supplemented with human recombinant Flt3L (10 ng/mL) and IL-7 (5 ng/mL) (Peprotech). After 13 days, all cells were collected and transferred to 24-well plates coated with Fc-DLL1 (5×105 cells/well). Cells were cultured with DLL1 for a total of 25 days. Media and cytokines were replenished every 3 days.
Antigen-Specific T Cell Differentiation
At day 25 of differentiation on DLL1-coated plates, CMVpp65 (NLVPMVATV) HLA-A*0201 tetramers (3 µg/mL) or irrelevant control GIL HLA-A*0201 Influenza M1 (GILGFVFTL) tetramers (3 µg/mL) (Baylor College of Medicine) were added to the cultures in OP9-DL1 CM supplemented with human recombinant Flt3L (10 ng/ml), IL-7 (5 ng/ml), IL-2 (10 ng/mL) and co-stimulatory molecules anti-CD3 (1 µg/mL) (Catalog No. 317304; Biolegend) and anti-CD28 (1 µg/mL) (Catalog No. 302914; Biolegend). Cells were cultured with tetramers for a total of 7 days. Tetramers, peptide, media, cytokines, and co-stimulatory molecules were replenished every 3 days.
Flow Cytometry
At days 16, 22, and 25 of differentiation, cells were collected to quantify the expression of lymphoid and myeloid markers. Briefly, cells were harvested and resuspended in PBS containing 1% BSA and 2 mM EDTA. FcR blocking reagent (Catalog No. 130-059-901; Miltenyi Biotec) was added at 1:100 for 15 minutes at 4°C prior to staining. Cells were then labeled with a combination of monoclonal antibodies against the lymphoid markers CD1a, CD7, CD4, CD8 and CD3 (CD1a-APC (Catalog No. 555807; BD Biosciences), CD7-PE-Cy5 (Catalog No. 555362; BD Biosciences), CD4-PercP (Catalog No. 550631; BD Biosciences), CD8-FITC (Catalog No. 555366; BD Biosciences), CD3-APC (Catalog No. 555335; BD Biosciences)) and myeloid markers CD11c, HLA-DR and HLA-ABC (CD11c-FITC (Catalog No. 11-0116-71; eBioscience), HLA-DR-APC (Catalog No. 559866; BD Biosciences), HLA-ABC-PE-Cy5 (Catalog No. 15-9983-41; eBioscience)) for 30 minutes at 4°C. 7 days after adding HLA tetramers to the differentiation cultures (day 32), cells were harvested to determine the percentage of CMV-antigen specific CD8+ cells in the cultures. Similarly, cells were stained with monoclonal antibodies against CD8, CD3, CD4, and for antigen-specificity using APC-conjugated tetramers (CMV or GIL HLA-A*0201) (Baylor College of Medicine). Isotype controls were included for each staining. To determine whether CMV-specific CD8+ T cells generated from this system were monoclonal or polyclonal, a similar staining was done at day 32 using IOTest® Beta Mark TCR Vβ Kit (Beckman Coulter, CA). All samples were analyzed on a BD Accuri C6 Flow Cytometer (BD Biosciences) or BD FACSAria (BD Biosciences). Data was analyzed using FlowJo Software (Tree Star, Inc.).
T Cell Functionality Assays: CD107a Mobilization and IFNγ Production
The functionality of antigen-specific T cells generated from CD34+CD38−/low HSCs was assessed in a CD107a mobilization assay as previously described.23 Differentiated cells were harvested and co-cultured with CMVpp65 peptide-loaded T2 target cells (HLA-A*0201) in a CTL assay. All viable cells obtained from differentiation cultures were counted as effector cells. T2 cells (174xCEM.T2; ATCC CRL-1992) were loaded with 50 µg/ml of CMVpp65 peptide for 2 hours at 37°C in RPMI containing 10% human serum (Sigma Aldrich). Cells were washed twice with PBS to remove un-bound peptide. Co-incubation of effector and target cells was carried out for 5 hours at 37°C at a 5:1 (E:T, effector:target) ratio. PE-conjugated anti-CD107a mAb (Catalog No. 555801; BD Biosciences) was added at the beginning of co-culture. BFA (Brefeldin A; BD Biosciences), a secretion inhibitor, was added after the first hour. Cells were collected and then surface-stained with FITC-conjugated anti-CD8 mAb and APC-conjugated tetramers (CMV or GIL HLA-A*0201) (Baylor College of Medicine). In some experiments, following surface staining, collected cells were fixed in Cytofix/Cytoperm solution (BD Biosciences) and stained with PE-Cy7-conjugated anti-mouse IFNγ (Catalog No. 557844; BD Biosciences). Finally, cells were analyzed on a BD Accuri C6 Flow Cytometer (BD Biosciences). Data was analyzed using FlowJo Software (Tree Star, Inc.).
CD8+ T cell-mediated Granzyme B Activity Assay
CD8+ T cell-mediated Granzyme B activity was analyzed using the GrantoxiLux PLUS! Kit (OncoImmunin, Inc.). Preparation of target T2 cells was done according to manufacturer’s specifications. Briefly, T2 cells were loaded with 10 µg/ml of CMVpp65 peptide for 2 hours at 37°C in RPMI containing 10% human serum. The fluorescent cell linker TFL2 dye (red) was added to the cells during the last 15 minutes of incubation. Cells were washed twice with PBS to remove un-bound peptide and TFL2 dye. Co-incubation of effector and target cells was carried out for 2 hours at 37°C at a 5:1 (E:T, effector:target) ratio in the presence of the cell permeable fluorogenic (green) Granzyme B substrate (OncoImmunin, Inc.). Cells were then collected and analyzed using FACSAria II (BD Biosciences). Data was analyzed using FlowJo Software (Tree Star, Inc.).
Statistics
Data are presented as mean ± standard error of mean. Statistical significances between experimental samples (p < 0.05) were determined with two-tailed unpaired Student’s t test.
Results
CD1a+CD7+ and CD3+CD4+CD8+ Early T Cells Can be Generated from In Vitro Expanded, Human CD34+38−/low HSCs Using Surface-Immobilized DLL1 and OP9-DL1 Conditioned Medium
To evaluate the ability of surface-immobilized DLL1 and OP9-DL1 conditioned media to induce T cell development, we evaluated whether human CD34+CD38−/low UCB-derived HSCs could be differentiated into early T cells when cultured on DLL1-functionalized plates (Figure 1). CD34+ UCB cells were expanded for 7 days. CD34+ cells were enriched using magnetic microbeads and CD34+CD38−/low cells were isolated by cell sorting (Figure 1). The resultant CD34+CD38−/low sorted population was > 95% pure. Purified CD34+CD38−/low cells were then plated on non-treated wells containing 2.5 µg/mL or 5 µg/mL Fc-DLL1 supplemented with Flt3L and IL-7.
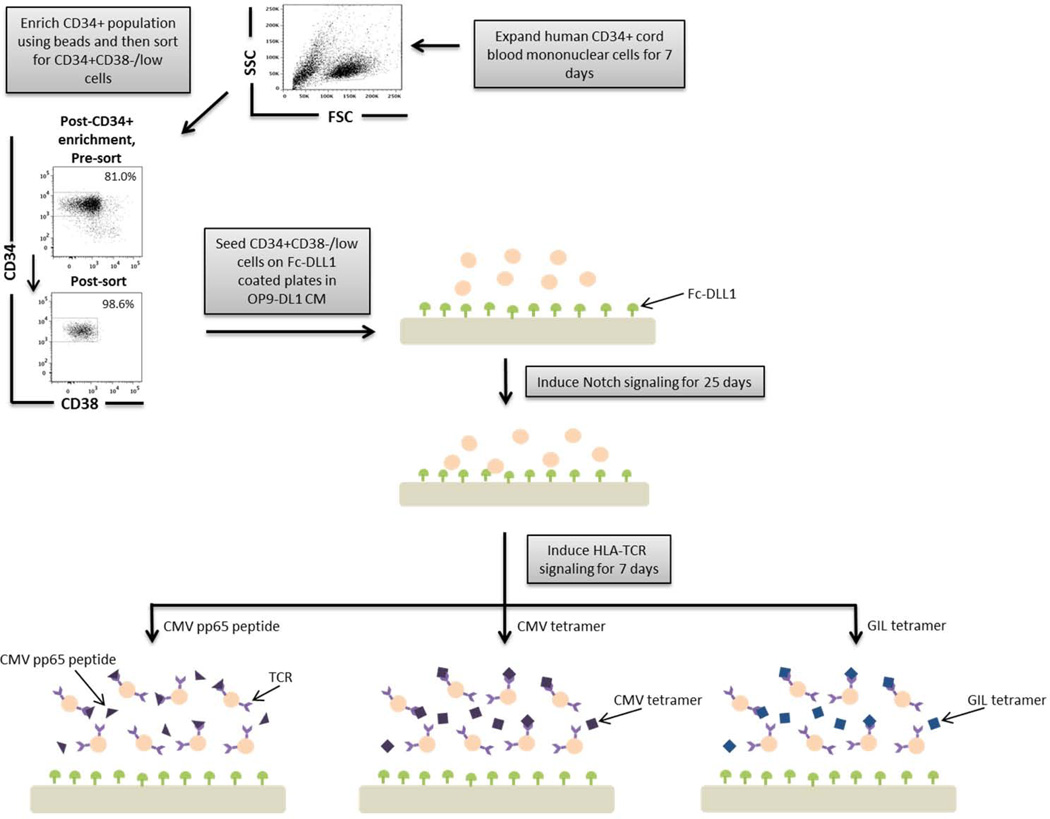
Figure 1. A schematic overview of our experimental design to generate antigen-specific CD8+ T cells.
CD34+ CB cells were expanded for 7 days and CD34+CD38−/low cells were isolated. After 7 days of CD34+ cell expansion, CD34+ cells were enriched using magnetic bead separation. The enriched cells were stained with anti-CD34 and anti-CD38 antibodies to isolate the CD34+CD38−/low HSC population. 81.0% of FSC vs SSC gated cells were CD34+CD38−/low. Gate was determined by the isotype staining control. Data are representative of at least 6 independent experiments. To induce Notch signaling and early T cell differentiation, CD34+CD38−/low cells were seeded onto DLL1-coated non-tissue culture treated plates. HLA-TCR signaling to direct antigen-specific differentiation was induced using CMVpp65 peptide or epitope-loaded HLA-A*0201 Class I tetramers or GIL epitope-loaded HLA-A*0201 Class I tetramers.
Our results show that by day 8 most of the CD34+CD38−/low cells differentiated into CD34-CD38+ cells (hematopoietic cells), independent of Notch induced signaling (results not shown). There is a modest population in all culture conditions (approximately 5 to 7%) of CD34+CD38−/low cells that remain CD34+ and gained CD38 (CD34+CD38+); however, by day 16 CD34+CD38−/low cells from the Notch-induced cultures (either 2.5 or 5 µg/mL Fc-DLL1) rapidly differentiate into CD34-CD38+ cells compared to no ligand control cultures that still contain a population of CD34+CD38+ cells (Supplementary Figure 1A). These results indicate that in our culture conditions, there is more rapid induction of hematopoiesis in the presence of Notch ligands consistent with previous results from other groups.14–17 By day 25, the majority of cells from Fc-DLL1 and no DLL1 conditions were CD34-CD38+ (Figure 2A).
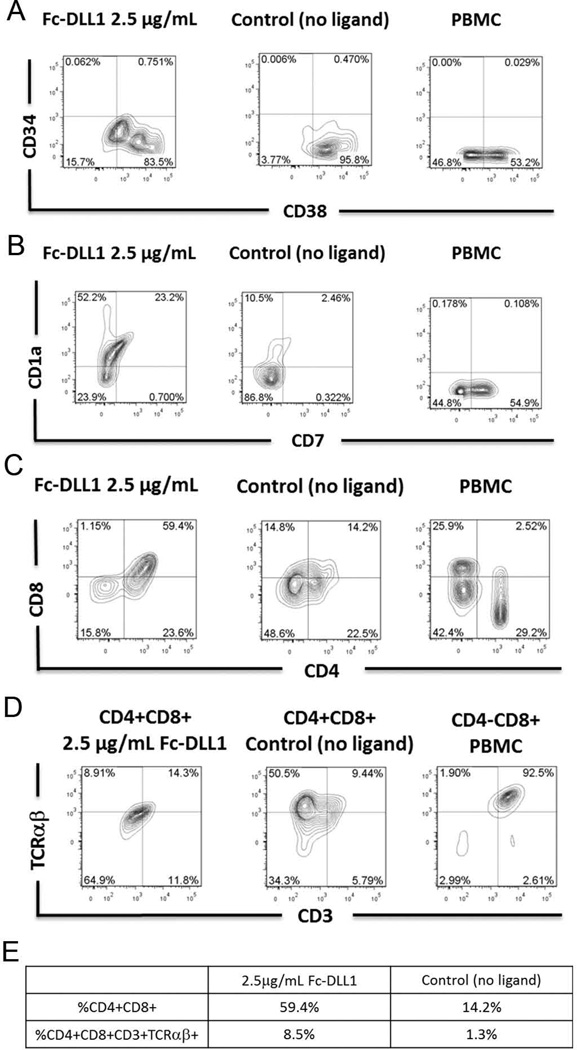
Figure 2. Notch-induced differentiation of CD34+CD38−/low human cord blood HSCs into early T cells.
Flow cytometric analysis of CD34 and CD38 on FSC vs SSC gated cells at day 25 of culture with OP9-DL1 conditioned medium in the presence of immobilized human Fc-DLL1 Notch ligand (2.5 µg/mL) or absence of Notch ligand (negative control). B) Flow cytometric analysis of CD1a and CD7 expression on FSC vs SSC gated cells cultured for 25 days with OP9-DL1 conditioned medium and in the presence of immobilized human Fc-DLL1 Notch ligand (2.5 µg/mL) or absence of Notch ligand (negative control). C) Flow cytometric analysis of CD4 and CD8 expression at day 25 on FSC vs SSC gated cells. PBMC-derived lymphocytes were included in the FACS staining as a positive control for CD4, CD8 and CD3 staining. D) Flow cytometric analysis of CD3 and TCRαβ expression at day 25 on CD4+CD8+ cells cultured in the presence or absence of immobilized Notch ligand (2.5 µg/mL). Isotype staining controls were included in each experiment to determine quadrant gates. E) Table indicating the percentage of the total population that are CD4+CD8+ or CD4+CD8+CD3+TCαβ+.
At days 16, 22, and 25, we determined if immobilized Notch ligands are capable of directing T lineage differentiation and generating early T lymphocytes. Cultures were analyzed for the presence of CD1a+CD7+ cells, previously identified as early T cells, and CD4+CD8+ DP T cells (Supplementary Figure 1B, 1C, and Figure 2B, 2C).10,12 At day 16, we found about twice as many CD1a+CD7+ cells in the Notch ligand conditions (2.5 µg/mL Fc-DLL1) compared to the no ligand control (Supplementary Figure 1B). After 25 days of culture with Fc-DLL1, the cultures had about 10 times as many CD7+CD1a cells compared to the control (Figure 2B). Furthermore, we observed a significantly higher number of total CD7+ cells in Notch ligand cultures compared to no ligand control at both time points, indicating progression of T cell differentiation. As expected, this CD7+ population is much higher in PBMC (Figure 2B). To investigate whether more mature T cells were present in the culture, cells were analyzed for the expression of CD4 and CD8 (Supplementary Figure 1C and Figure 2C). At day 22, conditions employing Fc-DLL1 had twice as many CD4+CD8+ cells compared to the no ligand control, indicating progression through the double negative stages to the double positive (DP) stage of T cell development. By day 25, we observed four times as many CD4+CD8+ early T cells in the DLL1 cultures compared to the control (Figure 2C). At day 22, there is a slight increase in CD4+CD8− cells in the no ligand control cultures compared to Notch ligand cultures. By day 25, the percentage of CD4+CD8− cells in the Notch ligand culture was similar to that of the no ligand control (Figure 2C). We hypothesized that CD4+CD8− cells may have differentiated due to the presence of Class II HLA-presenting myeloid cells. This was confirmed by the observation of CD11c+HLA-DR+ cells found in all conditions (Supplementary Figure 2).
In order to gauge T cell differentiation in the presence of immobilized DLL1, CD3 and TCRαβ expression was evaluated. Although CD4+CD8+ populations were present in all conditions at day 22, DP cells from the no ligand cultures expressed lower levels of CD3 compared to cells differentiated with Fc-DLL1 (Supplementary Figure 1D). We hypothesize that a small population of DP cells present even in the no ligand control is due to the presence of Notch ligands on various other cells within the culture. It has been shown by Karanu, et al. that human CD34+CD38− cells, myeloid cells, and mature CD3+ T cells express DLL1 and/or DLL4.24 At day 25, approximately 10–15% of the CD4+CD8+ DP cells generated from DLL1 and control cultures expressed CD3 and TCRαβ (Figure 2D and 2E). Out of the starting population, 8.5% of the cells differentiated with Notch ligand were CD4+CD8+CD3+TCRαβ+, compared to only 1.3% from the no ligand control. The expression of CD3 and TCRαβ on CD8+CD4− populations from PBMC is shown as staining control. As expected the majority of the CD8+CD4− PBMC express CD3 and TCRαβ, as these cells are mature T cells (Figure 2D and 2E).
Antigenic Peptide-Loaded HLA-A*0201 Tetramers Allow Generation of Antigen-Specific CD8+ T cells
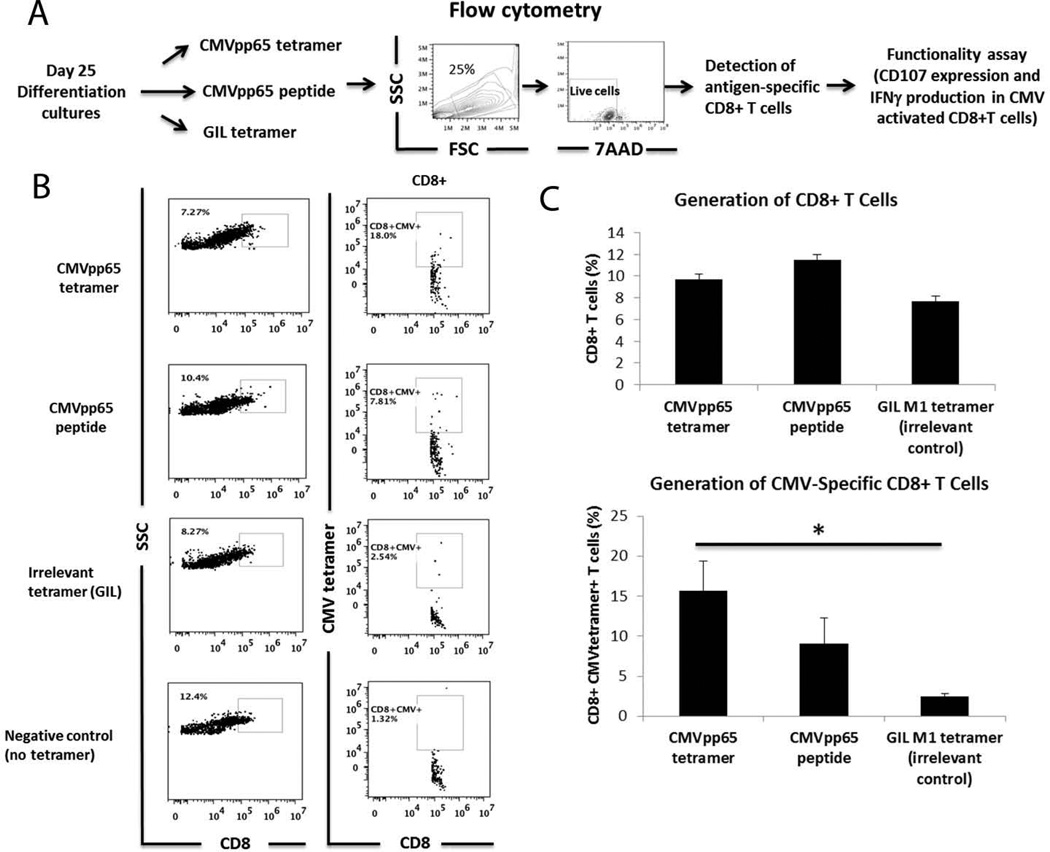
On day 25, 3 µg/mL CMVpp65 peptide, CMV (pp65) HLA-A*0201 Class I tetramers, or GIL HLA-A* 0201 Class I tetramers were added to the culture along with exogenous co-stimulatory molecules anti-CD3 and anti-CD28 (Figure 3A). 7 days after tetramer addition, cells were analyzed for antigen-specificity and functionality. CD8+ differentiated cells were primarily CD4 negative as shown in Supplementary Figure 3. There is no a significant number of CD4+CD8+ cells in the differentiation cultures 7 days after adding tetramers or peptides. The population of CD4+CD8− cells found in the culture could be due to the presence of Class II HLA-presenting myeloid cells (Supplementary Figure 2). Cells differentiated with CMV tetramer or CMVpp65 peptide resulted in 5.64% – 26.90% CD8+ T cells and 2.91% – 21.60% CD8+CMV+ T cells. T cells differentiated with CMV peptide or tetramer had a higher percentage of CMV-specific CD8+ T cells compared to the cells incubated with the GIL irrelevant tetramer control (Figure 3B). We observed consistent results over 5 independent samples (Figure 3C). Similarly, we were able to generate GIL-specific CD8+T cells after adding GIL tetramer in the differentiation cultures (Supplementary Figure 4). These results indicate that for the generation of antigen-specific T cells, our system is not restricted to a specific tetramer. Interestingly, incubation of differentiating cells with CMVpp65 peptide also initiated antigen-specific T cell development (Figure 3B). This may be attributed to the presence of differentiated DCs present in all culture conditions, as well as CMVpp65 peptide presentation by HLA Class I molecules expressed by most nucleated cells (Supplementary Figure 2).
Figure 3. Generation of CMV antigen-specific CD8+ T cells in the presence of CMV(pp65) tetramer or CMVpp65 peptide.
A) Experimental design. B) CMV antigen-specific CD8+ T cells were generated by adding CMVpp65 tetramer, CMVpp65 peptide or an irrelevant control tetramer (GIL M1 tetramer) at day 25 of differentiation. Cells differentiated with GIL tetramer were used as staining controls for CMV tetramer non-specific binding. Anti-CD3 (1 µg/mL) and anti-CD28 (1 µg/mL) and cytokines Flt3L (10 ng/mL), IL-7 (5 ng/mL), and IL-2 (10 ng/mL) were added in the culture medium to increase the number of antigen-specific CD8+ T cells. No significant CD8+CMV+ cells were generated using the irrelevant GIL tetramers or in no tetramer culture conditions. C) Bar graphs represent total percentage of CD8+ T cells in the different conditions and percentage of CD8+CMV+ cells in total CD8+ cells from 5 independent experiments. Staining with respective isotype controls was used to determine gates. *p<0.05
Human HSC-Derived Antigen-Specific CD8+ T Cells Are Functional
Functionality of CMV-specific CD8+ T lymphocytes was determined by CD107a surface mobilization, intracellular IFNγ production and Granzyme B mediated cytotoxicity in the presence of antigen-loaded target cells.23,25–27 During the process of cell killing, a CD8+ effector T cell fuses with the target cell membrane, releasing cytotoxic mediators such as perforins and granzymes. CD107a, also known as lysosomal-associated membrane protein-1, is a vesicle membrane protein that becomes transiently mobilized to the cell surface during this degranulation process, the initial event that takes place during target cell lysis. Additionally, intracellular expression of IFNγ was determined, as this critical cytokine is produced by CD8+ T cells upon antigen recognition. Functionality of antigen-specific T cells generated in our in vitro system was determined by CD107a expression upon co-culture of differentiated effectors with peptide-loaded target cells. This percentage of CD8+CMV+CD107a+ cells ranged from 0.287% to 0.5% of the total population. Moreover, we measured Granzyme B activity in live target cells, as this provides an extremely early quantitative assessment of cell-mediated cellular cytotoxicity.
For our assays, all cells from differentiation cultures were harvested and used as effector cells. T2 target cells (HLA-A*0201) were loaded with CMVpp65 peptide for 2 hours. Target and effector cells were co-cultured with peptide-loaded T2 target cells 5 hours (or 2 hours for Granzyme B measurement) at an E:T ratio of 5:1, in the presence of BFA and anti-CD107a mAb (or fluorogenic Granzyme B substrate for Granzyme B measurement). Following stimulation, we examined, by flow cytometry, the ability of tetramer-stained cells to degranulate and produce IFNγ (Figure 4A). The cells were washed, permeabilized and stained for CD8, intracellular IFNγ, and antigen-specificity (enumerated with fluorescently-labeled tetramers).
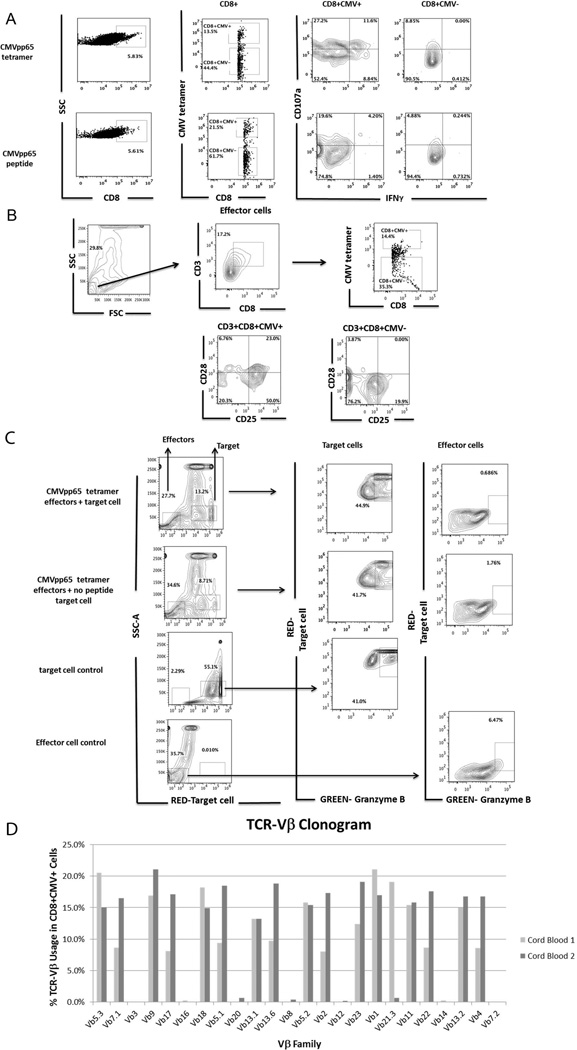
Figure 4. Functionality of CMV-antigen specific CD8+ T cells generated in the presence of CMVpp65 tetramer or CMVpp65 peptide.
A) Functional CD8+CMV+ T cells express CD107a and produce IFNγ after activation. CMVantigen-specific CD8+ T cells were generated by adding CMVpp65 tetramer or CMVpp65 peptide at day 25 of differentiation. Anti-CD3 (1 mg/mL) and anti-CD28 (1 mg/ml) and cytokines Flt3L (10 ng/mL), IL-7 (5 ng/mL), and IL-2 (10 ng/mL) were added in the culture medium to increase the number of antigen-specific CD8+ T cells. 7 days after adding CMV tetramer or CMVpp65 peptide to the differentiation cultures, cells were harvested and co-cultured with CMVpp65 peptide-loaded T2 target cells (HLA-A*0201). The CD107a mobilization assay was used for detection of CD8+CMV+ T cells with CTL activity. We show functional CD8+CMV+ T cells that express CD107a and produce IFNγ. Staining with relevant isotype controls was used to determine the gates. B) CD28 and CD25 expression on CMV antigen-specific CD8+ T effector cells (CD3+CD8+CMV+), and CD3+CD8+CMV− cells C) CMV antigen-specific CD8+ T cells mediated Granzyme B expression. CMVpp65 peptide-loaded T2 target cells (HLA-A*0201) were labeled with a red dye and incubated, in the presence of Granzyme B substrate, with cells harvested from CMVpp65 tetramer differentiation cultures (effector cells) at a 5:1 effector:target ratio for 2 hours. CTL assay was carried out using a modified cytotoxicity protocol (Materials and Methods). T2 (no peptide) target cells, T2 target cells alone, and effectors cells alone are shown as controls. Target cells were gated on FSChi cells and effector cells were gated on FSClo cells. The relative percentage of Granzyme B+ target cells within the entire population is higher in CMVpp65 peptide loaded target cells (top panel) compared to no-peptide target cells and target cells alone (control). As expected there is no significant detection of green-Granzyme B in the effector cells as Granzyme B exists in an inactive form in effector cells. D) Clonogram showing TCR Vβ repertoire of CMV antigen-specific CD8+ T cells.
Approximately 13% of CD8+ T cells generated, in this particular experiment, from CMV tetramer differentiation cultures were capable of binding to the CMV HLA-A*0201 MHC-class I tetramer (Figure 4A, top panel). After stimulation with tetramer, about 40% of the CMV+ cells express CD107a. From those, 11.6% produced IFNγ (Figure 4A, top panel). Similarly, CD8+CMV+ cells were generated from CMV peptide differentiation cultures (Figure 4A, bottom panel). About 25% of these cells express CD107a, but less than 5% produced IFNγ. Although stimulation with CMVpp65 peptide for 7 days results in a high number of antigen-specific CD8+ T cells, this time frame may not be sufficient to activate CMV-specific CD8+ T cells to become effector T cells that produce IFNγ. It is possible that more than 7 days is needed for activation. In contrast, these conditions work well for generation of antigen-specific CD8+ T cells in the presence of tetramers. We compared the expression of CD107a between CD8+CMV+ and CD8+CMV− cells generated from CMV tetramer cultures and CMV peptide cultures (Figure 4A). Although CMV-specific CD107a surface expression was higher in CD8+CMV+ T cells than in CD8-CMV+ T cells, we still found non-specific CD107a expression in CD8+CMV− cells. This could be due to several confounding factors. First, all cells from the differentiation cultures were used as effector cells (due to cell number limitations). In addition, the use of co-stimulatory molecules anti-CD3 and anti-CD28, as well as cytokine IL-2, to stimulate T cells proliferation may have induced a background level of activation, resulting in CD107a expression. It is also possible that some of the CD8+ T cells are not positively selected with the tetramer or peptide we add, but rather with peptides that are already present in the media, causing a high level of CD107a expression.
We also determined the surface-expression of CD28 and CD25, co-stimulation and activation markers relevant in the cytolytic process initiated by CD8+ T cells, in the antigen-specific in-vitro generated T cells. Our results (Figure 4B) show that CD3+CD8+CMV+ in vitro differentiated T cells are CD28+ and express CD25, similar to in vivo differentiated cells. In contrast, CD3+CD8+CMV− in vitro differentiated tetramer negative T cells are mostly CD28 negative and few of them express low levels of CD25.
Cytotoxic CD8+ T cells-mediated killing of target cells was evaluated by measuring Granzyme B activity inside live target cells (Figure 4C). T2 target cells were fluorescently labeled (red) and then co-incubated with CMVpp65 tetramer differentiated cells in the presence of a fluorogenic granzyme B substrate (green). Following incubation and washing (described in Materials and Methods), samples were analyzed by flow cytometry. Cleavage of the substrate results in increased green fluorescence in dying red target cells (Figure 4C, upper panel). Granzyme B activity was detected in about 44.9% of T2 CMVpp65 peptide loaded target cells (13.2% within entire population) after incubation with effector cells containing CMV-antigen specific CD8+ T cells, compared to 41.7% and 41% background levels detected in no peptide loaded target cells and no effector conditions, respectively. In summary, there do not appear to be significant differences in the percentages of Granzyme B+ effectors in the different conditions but notably, the data demonstrate that a significant proportion of the ex vivo differentiated CD8+ cells exhibit effector function. Staining of effector cells alone was evaluated as a control. As expected there is no significant detection of green-Granzyme B in the effector cells alone as Granzyme B exists in an inactive form in effector cells (Figure 4C, right panel). The relatively high level of background staining could be due to prolonged culture with the tetramers and co-stimulatory molecules as well as due to other peptides present in the culture medium. It could also be due to the presence of dead target cells from the beginning of the CTL experiment, prior to incubation of targets with effectors.
Antigen-Specific T Cells are Polyclonal
To provide insight about the clonality of the CMV-specific CD8+ T cells generated from our cultures, TCR Vβ analysis via flow cytometry was performed. Interestingly, we found that multiple Vβ families were represented in the CMV-specific CD8+ T cell population (Figure 4D). This finding was consistent over two independent sets of differentiated CB-MN cells.
Discussion
The ability of hematopoietic stem cells (HSCs) to differentiate into various blood lineages as well as their capacity to self-renew, makes them an attractive source from which potentially therapeutic T cells can be generated.29,30 HSCs can be isolated from the bone marrow, peripheral blood, or umbilical cord blood (UCB) and can be expanded, thereby providing a large, readily available cell source that could be differentiated into lineage-specific therapeutic cells as needed. In addition, problems associated with allogeneic T cell transfer, which is limited by a shortage of donors and the difficulty of finding HLA-matched donor cells, may be overcome through the use of HSCs as the source.31 In particular, human umbilical cord and placenta, which support the developing fetus during pregnancy, are delivered with the baby and can be easily retrieved after birth, is an attractive and rich source for HSCs. Numerous public cord blood banks have been developed worldwide to collect, type, and cryopreserve cord blood for potential future transplant.32 The development of an efficient and scalable differentiation system that could generate antigen-specific, functional T cells from cord blood HSCs could enable important therapeutic opportunities in a variety of diseases.
With advancements in in vitro stem cell differentiation technologies, stem-cell derived therapies are becoming more of a reality. Specifically, to induce T lymphoid lineage commitment, ES cells or HSCs can be co-cultured with OP9-DL1 cells, bone marrow-derived stromal cells retrovirally transfected to stably express the Notch ligand DLL1.10–13 The OP9-DL1 co-culture system is now an established method for differentiating mouse and human hematopoietic stem cells into early T cells as well as CD8+ SP T cells; however, the generation of human antigen-specific, functional T cells without retroviral transfection of specific TCRs has not been reported.10–13,21,22,33 Furthermore, large scale production of therapeutic T cells for eventual clinical application via co-culture with retrovirally transfected cells is difficult, both from pharmaceutical and regulatory perspectives. Therefore, in order for in vitro T cell differentiation to become clinically applicable, a system that is capable of producing antigen-specific T cells on a large scale, without relying on complicated co-culture methods or retroviral transfection, is necessary.
Here, we have developed a stromal cell contact-free system that facilitates the differentiation of human cord blood-derived HSCs into functional, antigen-specific T cells. Notch signaling for the generation of early T cells was mediated by plate-immobilized Notch ligands in the presence of soluble factors from stromal conditioned media. Recent studies have indicated the importance of Notch ligand endocytosis in Notch signaling and T cell development in mice.34,35 In the context of OP9-DL1 or OP9-DL4 co-culture, mutants that are unable to undergo Notch ligand endocytosis or recycling have a reduced capacity to induce Notch signaling and T cell development.34,35 While our system utilizes plate-immobilized Notch ligands that are unable to be endocytosed, we still observe the development of CD1a+CD7+ and CD4+CD8+ early human T cells. This observation is consistent with that of other groups utilizing immobilized Notch ligands for HSC expansion.13–17 While the frequency of T cell progenitors may increase by using Notch ligand-presenting cells, the goal here is to develop a cell contact-free system for inducing T cell development that can be easily scaled up and is clinically translatable.
Subsequent TCR signaling, induced by antigen-loaded HLA-A*0201 Class I tetramers, provided sufficient signals to generate a population of polyclonal, antigen-specific CD8+ T cells. Our results demonstrate that the antigen-specific T cells developed in our system exhibited specific effector function against peptide-loaded target cells, as indicated by the surface presentation of activated cytotoxic-specific marker, CD107a, and production of intracellular IFNγ, as well as Granzyme B activity inside dying target cells. For CD8+ T cell immunotherapy to be successful, such efficient T cell activation and cytotoxic activity must occur in response to foreign antigen. While the affinities of the T cells generated through our system may vary with different antigens and tetramers, the continued presence of HLA Class I tetramers, and the exogenous use of anti-CD28 and IL-2 may prime and increase the functional avidity of the in vitro-derived antigen-specific CD8+ T cells.36–39 Thus, our system could provide a platform for in vitro generation of cytotoxic T cells with therapeutic efficacy.
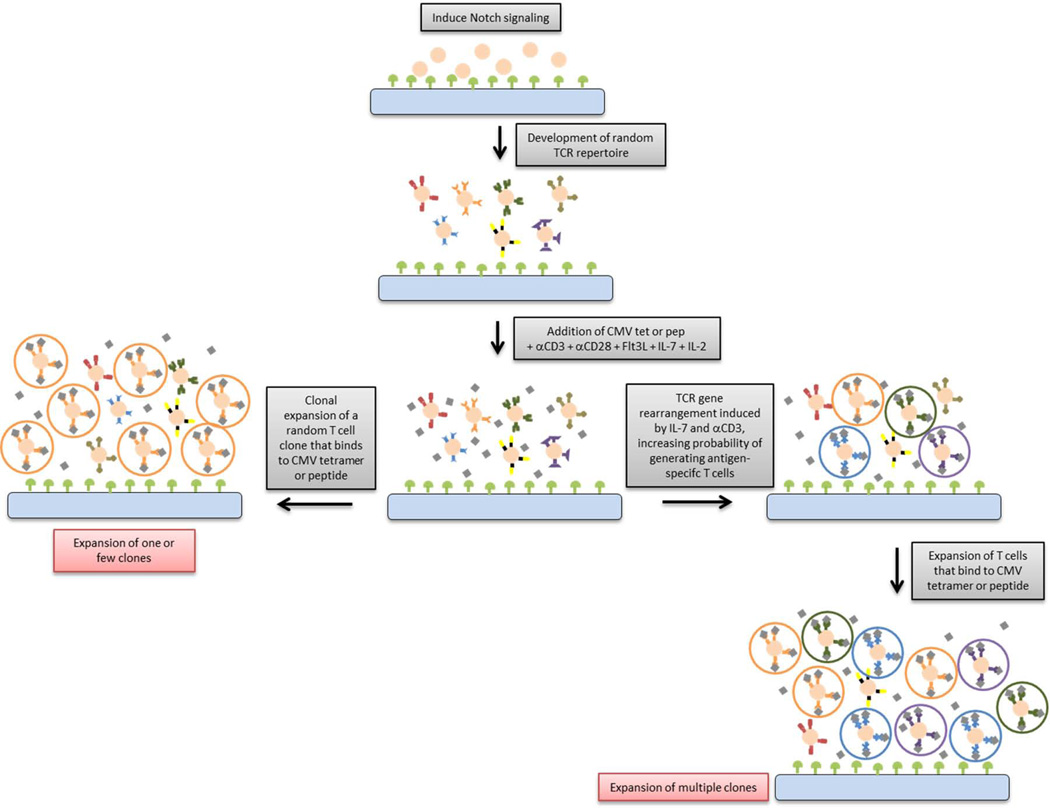
The TCRVβ repertoire of the antigen-specific CD8+ T cells were evaluated in order to elucidate how MHC/HLA-TCR signaling, induced by antigen-loaded HLA tetramers, facilitates the clonal development of antigen-specific T cells in vitro. We hypothesized two possible mechanisms for the in vitro generation of antigen-specific T cells (Figure 5): (1) Proliferation of a single random antigen-specific CD8+ T cell clone or (2) TCR gene rearrangement induced by co-stimulatory molecules and/or soluble factors within our culture system that increases the probability of generating antigen-specific T cells which are further expanded by the continued presence of tetramers and cytokines. Recent studies indicate that in vitro T cell differentiation systems using OP9-DL1 co-cultures are capable of producing a population of antigen-specific CD8+ mouse T cells that can be expanded by epitope-presenting DCs.19 Since CMV or GIL epitope-loaded HLA tetramers are added to the bulk of differentiated cells, it is possible that randomly generated T cell clones, specific for either antigen, are expanded. If this were the case, the clonal diversity of antigen-specific cells would be low. Our data indicate that several unique Vβ families were highly represented in the antigen-specific T cell repertoire, suggesting that the primary mechanism of tetramer-mediated generation of antigen-specific T cells may not be limited solely to clonal expansion of a single or few clones.
Figure 5. A schematic overview of our model.
We suggest that antigen-specific CD8+ T cells can be generated in vitro when TCR gene rearrangement is triggered by the presence of specific antigen-loaded tetramers or soluble co-stimulatory factors, followed by T cell expansion in the continued presence of tetramers.
Given the diverse clonality of CMV-specific T cells generated from our system, we hypothesize that another, or an additional mechanism, may be responsible for the generation of antigen-specific T cells. In the periphery, if a T cell cannot recognize a presented antigen, it can either undergo anergy and death or TCR revision which may allow the cell to respond to an antigen.40 During TCR revision, T cells lose surface expression of the TCR, up-regulate recombinase machinery, and rearrange genes to produce an extrathymically generated TCR that is then expressed on the cell surface.41–43 This phenomenon was first identified in Vβ5 transgenic mice that lost Vβ5 expression and gained an endogenous Vβ repertoire similar to wild type mice as they aged.44 Vβ5low peripheral T cells were found to have down-regulated surface TCR expression and high levels of RAG1, RAG2, and TdT indicating gene rearrangement of the TCR loci.44
Recently, it has been demonstrated that treatment of mouse DP thymocytes with antigen-loaded H-2Kb MHC Class I tetramers, anti-CD28, anti-CD3, IL-2, and IL-7 results in RAG-1 up-regulation, suggesting that TCR gene rearrangement is initiated or continued due to the presence of MHC tetramers.20 Furthermore, an in vitro model of TCR revision in mature human CD8+ T cells, induced by the presence of anti-CD3 and IL-7, was recently described.43 In particular, IL-7 has been shown to induce T cell survival and facilitate chromatin remodeling at the TCR loci in developing, immature T cells.46–48 Thus, due to the presence of HLA-A*0201 Class I tetramers and other soluble stimulatory factors within our culture, we cannot rule out the possibility that TCR gene rearrangement may be occurring in our in vitro system. While more in-depth studies on clonality are required to confirm this hypothesis, our findings present important insights into the mechanism behind in vitro T cell development.
One concern with this in vitro differentiation system is that while it provides a means for selection of T cell specific for one antigen, there is no mechanism that ensures negative selection to eliminate self-reactive cells. We have found that our CMVpp65-specific T cells display significantly more cytolytic activity against CMVpp65 peptide-displaying target cells compared to target cells loaded with no peptide. We hypothesize that a pure, antigen specific CD8+ functional T cell population could be easily sorted at the end of the differentiation and would eliminate or reduce the possibility of recovering cells which would be host-reactive. To increase the yield of desired cells, antigen-specific CD8+ T cells, several rounds of tetramer stimulation can be done by continuously pulsing the cells with Class I tetramers and co-stimulatory molecules anti-CD3, anti-CD28, and IL-2. It has been shown by using bead-based artificial antigen-presenting cells displaying pMHC tetramers along with co-stimulatory molecules (anti-CD3, anti-CD28, and 4-1BB) that mature, antigen-specific T cells can be activated and expanded.49 Furthermore, Savage et al. have shown that soluble Class I MHC tetramers can be used to expand CD8+ T cells in vitro.50 Given the previous success of these groups, we expect that the antigen-specific CD8+ T cells generated by our in vitro differentiation system can be expanded easily.
In summary, we describe an in vitro system which can be used to generate antigen-specific T cells from human hematopoietic progenitors. By utilizing plate-immobilized and soluble ligands, we eliminate the need for feeder cells or retroviral transfection, thereby providing a practical and scalable technology that could facilitate the high-throughput production of therapeutic T cells from hematopoietic stem cells.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant HL089843 and partially by EB011666.
Footnotes
Author Contributions:
Irina Fernandez: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Tracy Ooi: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Krishnendu Roy: Conception and design, Manuscript writing, Financial support, Administrative support, Final approval of manuscript
References
- 1.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 2.Laky K, Fleischacker C, Fowlkes BJ. TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol Rev. 2006;209:274–283. doi: 10.1111/j.0105-2896.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Rosenberg S. Adoptive-cell-transfer therapy for the treatment of patients with cancer. NatRevCancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong MC, Latouche JB, Krause A, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1210. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 8.Plautz GE, Bukowski RM, Novick AC, et al. T-cell adoptive immunotherapy of metastatic renal cell carcinoma. Urology. 1999;54:617–623. doi: 10.1016/s0090-4295(99)00303-9. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clincal path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awong G, Herer E, La Motte-Mohs RN, et al. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol. 2011;12 doi: 10.1186/1471-2172-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes R, Zúñiga-Pflücker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc. 2009;2 doi: 10.1101/pdb.prot5156. [DOI] [PubMed] [Google Scholar]
- 12.La Motte-Mohs RN, Herer E, Zúñiga-Pflücker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 14.Dallas MH, Varnum-Finney B, Delaney C, et al. Density of notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallas MH, Varnum-Finney B, Martin PJ, et al. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney C, Varnum-Finney B, Aoyama K, et al. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varnum-Finney B, Wu L, Yu M, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 18.Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J Biomed Mater Res A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- 19.Dervovic DD, Ciofani M, Kianizad K, et al. Comparative and functional evaluation of in vitro generated ex vivo CD8 T cells. J Immunol. 2012;189:3411–3420. doi: 10.4049/jimmunol.1200979. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Nie H, Tucker PW, et al. Controlled major histocompatibility complex-T cell receptor signaling allows efficient generation of functional, antigen-specific CD8+ T cells from embryonic stem cells and thymic progenitors. Tissue Eng Part A. 2010;16:2709–2720. doi: 10.1089/ten.tea.2009.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Parkhurst M, Zheng Z, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Lent AU, Nagasawa M, van Loenen MM, et al. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2008;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 24.Karanu FN, Murdoch B, Miyabayashi T, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–1967. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 25.Lacey SF, Martinez J, Gallez-Hawkins G, et al. Simultaneous reconstitution of multiple cytomegalovirus-specific CD8+ cell populations with divergent functionality in hematopoietic stem-cell transplant recipients. J Infect Dis. 2005;191:977–984. doi: 10.1086/428136. [DOI] [PubMed] [Google Scholar]
- 26.Mittendorf EA, Storrer CE, Shriver CD, et al. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 27.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 28.Trapani JA, Smyth MJ. Functional significant of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 29.Emerson SG. Ex vivo expansion of hematopoietic precursors, progenitors, and stem cells: the next generation of cellular therapeutics. Blood. 1996;87:3082–3088. [PubMed] [Google Scholar]
- 30.Flores-Guzmán P, Gutiérrez-Rodríguez M, Mayani H. In vitro proliferation, expansion, and differentiation of a CD34+ cell-enriched hematopoietic cell population from umbilical cord blood in response to recominant cytokines. Arch of Med Res. 2002;33:107–114. doi: 10.1016/s0188-4409(01)00368-x. [DOI] [PubMed] [Google Scholar]
- 31.Opelz G, Wujciak T, Döhler B. Is HLA matching worth the effort? Collaborative Transplant Study. Transplantation Proc. 1999;31:717–720. doi: 10.1016/s0041-1345(98)01620-0. [DOI] [PubMed] [Google Scholar]
- 32.Bart T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. ClinicoEcon Outcomes Res. 2010;2:141–147. doi: 10.2147/CEOR.S11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zúñiga-Pflücker J. T-cell development made simple. Nat Rev Immunol. 2004;4:67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
- 34.Shah DK, Mohtashami M, Zúñiga-Pflücker JC. Role of Recycling, Mindbomb1 Association, and Exclusion from Lipid Rafts of Delta-like 4 for Effective Notch Signaling to Drive T Cell Development. J Immunol. 2012;189:5797–5808. doi: 10.4049/jimmunol.1202469. [DOI] [PubMed] [Google Scholar]
- 35.Heuss SF, Ndiaye-Lobry D, Six EM, et al. The intracellular region of Notch ligands Dll1 and Dll3 regulates their trafficking and signaling activity. Proc Natl Acad Sci U S A. 2008;105:11212–11217. doi: 10.1073/pnas.0800695105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Essen MR, Kongsbak M, Geisler C. Mechanisms behind functional avidity maturation in T cells. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/163453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluestone JA. New perspectives of CD28-B7 mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 38.Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol. 2009;21:69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagerstrom CG, Kerr EM, Allison JP, et al. Activation and differentiation requirements of primary T cells in vitro. Proc Natl Acad Sci U S A. 1993;90:8987–8991. doi: 10.1073/pnas.90.19.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner DH Jr. Re-shaping the T cell repertoire: TCR editing and TCR revision for good and for bad. Clin Immunol. 2007;123:1–6. doi: 10.1016/j.clim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Ali M, Weinreich M, Balcaitis S, et al. Differential regulation of peripheral CD4+ T cell tolerance induced by deletion and TCR revision. J Immunol. 2003;171:6290–6296. doi: 10.4049/jimmunol.171.11.6290. [DOI] [PubMed] [Google Scholar]
- 42.Hale JS, Ames KT, Boursalian TE, et al. Cutting Edge: Rag deletion in peripheral T cells blocks TCR revision. J Immunol. 2010;184:5964–5968. doi: 10.4049/jimmunol.1000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahan CJ, Fink PJ. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637–647. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- 44.McMahan CJ, Fink PJ. Receptor revision in peripheral T cells creates a diverse Vbeta repertoire. J Immunol. 2000;165:6902–6907. doi: 10.4049/jimmunol.165.12.6902. [DOI] [PubMed] [Google Scholar]
- 45.Lantleme E, Orlando L, Porcedda P, et al. An in vitro model of T cell receptor revision in mature human CD8+ T cells. Mol Immunol. 2008;45:328–337. doi: 10.1016/j.molimm.2007.06.153. [DOI] [PubMed] [Google Scholar]
- 46.Durum SK, Candéias S, Nakajima H, et al. Interleukin 7 receptor control of T cell receptor gamma gene rearrangement: role of receptor-associated chains and locus accessibility. J Exp Med. 1998;188:2233–2241. doi: 10.1084/jem.188.12.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Muegge K. Control of chromatin accessibility for V(D)J recombination by interleukin-7. J Leukoc Biol. 2001;69:907–911. [PubMed] [Google Scholar]
- 48.Muegge K, Vila MP, Durum SK. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993;261:93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 49.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 50.Savage P, Millrain M, Diimakou S, et al. Expansion of CD8+ cytotxic T cells in vitro and in vivo using MHC class I tetramers. Tumour Biol. 2007;28:70–76. doi: 10.1159/000099152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.