Abstract
BACKGROUND
The 2014 Eighth Joint National Committee panel recommendations for management of high blood pressure (BP) recommend a systolic BP threshold for initiation of drug therapy and a therapeutic target of <150 mm Hg in those ≥60 years of age, a departure from prior recommendations of <140 mm Hg. However, it is not known whether this is an optimal choice, especially for the large population with coronary artery disease (CAD).
OBJECTIVES
This study sought to evaluate optimal BP in patients ≥60 years of age.
METHODS
Patients 60 years of age or older with CAD and baseline systolic BP >150 mm Hg randomized to a treatment strategy on the basis of either atenolol/hydrochlorothiazide or verapamil-SR (sustained release)/trandolapril in INVEST (INternational VErapamil SR Trandolapril STudy) were categorized into 3 groups on the basis of achieved on-treatment systolic BP: group 1, <140 mm Hg; group 2, 140 to <150 mm Hg; and group 3, ≥150 mm Hg. Primary outcome was first occurrence of all-cause death, nonfatal myocardial infarction (MI), or nonfatal stroke. Secondary outcomes were all-cause mortality, cardiovascular mortality, total MI, nonfatal MI, total stroke, nonfatal stroke, heart failure, or revascularization, tabulated separately. Outcomes for each group were compared in unadjusted and multiple propensity score–adjusted models.
RESULTS
Among 8,354 patients included in this analysis with an accumulated 22,308 patient-years of follow-up, 4,787 (57%) achieved systolic BP of <140 mm Hg (group 1), 1,747 (21%) achieved systolic BP of 140 to <150 mm Hg (group 2), and 1,820 (22%) achieved systolic BP of ≥150 mm Hg (group 3). In unadjusted models, group 1 had the lowest rates of the primary outcome (9.36% vs. 12.71% vs. 21.32%; p < 0.0001), all-cause mortality (7.92% vs. 10.07% vs. 16.81%; p < 0.0001), cardiovascular mortality (3.26% vs. 4.58% vs. 7.80%; p < 0.0001), MI (1.07% vs. 1.03% vs. 2.91%; p < 0.0001), total stroke (1.19% vs. 2.63% vs. 3.85%; p <0.0001), and nonfatal stroke (0.86% vs 1.89% vs 2.86%; p<0.0001) compared with groups 2 and 3, respectively. In multiple propensity score–adjusted models, compared with the reference group of <140 mm Hg (group 1), the risk of cardiovascular mortality (adjusted hazard ratio [HR]: 1.34; 95% confidence interval [CI]: 1.01 to 1.77; p = 0.04), total stroke (adjusted HR: 1.89; 95% CI: 1.26 to 2.82; p = 0.002) and nonfatal stroke (adjusted HR: 1.70; 95% CI: 1.06 to 2.72; p = 0.03) was increased in the group with BP of 140 to <150 mm Hg, whereas the risk of primary outcome, all-cause mortality, cardiovascular mortality, total MI, nonfatal MI, total stroke, and nonfatal stroke was increased in the group with BP ≥150 mm Hg.
CONCLUSIONS
In hypertensive patients with CAD who are ≥60 years of age, achieving a BP target of 140 to <150 mm Hg as recommended by the JNC-8 panel was associated with less benefit than the previously recommended target of <140 mm Hg.
Keywords: blood pressure, coronary artery disease, elderly, systolic, target
The panel members appointed to the Eighth Joint National Committee (JNC-8 panel) recently published recommendations for the management of high blood pressure (BP) in adults that recommended a systolic BP (SBP) threshold ≥150 mm Hg for initiation of drug therapy and a therapeutic target of <150/90 mm Hg in patients ≥60 years of age, one of the few “grade A” recommendations (1). The recommendations state that “setting a goal SBP of lower than 140 mm Hg in this age group provides no additional benefit compared with a higher goal SBP of 140 to 160 mm Hg or 140 to 149 mm Hg” (1). However, 5 of the 17 JNC-8 panel members did not agree with this viewpoint (2). The optimal BP for initiation of treatment target and for use as a therapeutic target in hypertensive patients ≥60 years of age is unknown. In HYVET (Hypertension in the Very Elderly Trial), a study of patients 80 years of age or older with baseline SBP of ≥160 mm Hg, patients randomized to diuretic agent–based therapy had a significant decrease in stroke, all-cause mortality, cardiovascular mortality, and heart failure compared with those given placebo (3). The target SBP in the active-treatment group was <150 mm Hg, with an achieved systolic pressure of approximately 144 mm Hg. In addition, there were fewer serious adverse events in the active-treatment group than in the placebo group (3).
Because the data regarding the best BP goal among the elderly are controversial and the 2014 recommendations have not been tested in a cohort of patients with coronary artery disease (CAD), we categorized patients ≥60 years of age with hypertension and CAD enrolled in INVEST (INternational VErapamil SR Trandolapril STudy) on the basis of their on-treatment SBP. We sought to assess the impact of SBP ≤150 mm Hg compared with lower achieved SBPs.
METHODS
INVEST, a prospective, randomized, open, blinded-endpoint trial, involved 22,576 patients 50 years of age or older with hypertension that required drug treatment and coexisting CAD. Patients enrolled from 14 countries were randomized to a multidrug antihypertensive strategy on the basis of either verapamil-SR (sustained-release formulation; n = 11,267) or atenolol (n = 11,309). Details of the rationale, design, inclusion/exclusion criteria, and main outcomes were described previously (4,5). Briefly, the 2 treatment strategies were equivalent for the primary outcome (first occurrence of all-cause death, nonfatal myocardial infarction [MI], or nonfatal stroke). Between 1997 and 2003, 61,835 patient-years of follow-up were accumulated, with a total of 568 patients lost to follow-up for the overall trial.
For this non–pre-specified post-hoc analysis, patients ≥60 years of age with baseline SBP of ≥150 mm Hg at the time of entry into the trial were chosen. Patients were randomized to either a verapamil-SR/trandolapril– or an atenolol/hydrochlorothiazide-based strategy. Trandolapril and hydrochlorothiazide were added if needed for BP control and/or end-organ protection. In both strategies, trandolapril was recommended for heart failure, diabetes, or renal impairment (4,5). Titration of drug and dose were recommended to achieve the JNC-5/6 BP goals (<140/90 mm Hg, or <130/85 mm Hg in the presence of diabetes and/or renal impairment) (6). Each treatment strategy provided excellent BP control (>70% of patients achieved BP <140/90 mm Hg at 24 months) without differences in BP between the strategies, and therefore, for this analysis, the 2 treatment arms were combined. Patients were then divided into 3 groups on the basis of achieved on-treatment SBP: group 1, SBP <140 mm Hg; group 2, SBP 140 to <150 mm Hg; and group 3, SBP ≥150 mm Hg.
Data were collected by use of an Internet-based system, which provided for individualized prescribing of BP medications using a flexible treatment algorithm. Follow-up visits were scheduled every 6 weeks for the first 6 months and then every 6 months until 2 years after the last patient was enrolled.
STUDY OUTCOMES
The primary outcome for this analysis was the first occurrence of death (all-cause), nonfatal MI, or nonfatal stroke. The secondary outcomes for this analysis were all-cause mortality (all-cause), cardiovascular mortality, total MI (fatal and nonfatal), nonfatal MI, total stroke (fatal and nonfatal), nonfatal stroke, heart failure, and revascularization, considered separately. A 3-member clinical event committee, blinded to treatment assignment, adjudicated the outcomes. The definitions of the outcomes have been described previously (4,7).
STATISTICAL ANALYSIS
After dividing the cohort into 3 groups on the basis of on-treatment achieved SBP, analysis was performed. Baseline characteristics were compared between the groups by chi-square test for categorical variables or analysis of variance for continuous variables. The Kaplan-Meier method and the log-rank test were used to compare the time to event among the 3 groups.
The 3 groups differed in regard to baseline characteristics (Table 1). To adjust for these baseline differences, we used a multiple propensity score adjustment approach (8). Although propensity score matching has been used to assemble patient cohorts that are similar for 2 treatment comparisons, this approach is problematic when there are more than 2 groups (9). In such cases, a multiple propensity score approach has been proposed as a solution to the “dimensionality problem” (8,10).
TABLE 1.
Baseline Characteristics of the Cohort on the Basis of Achieved On-Treatment Systolic Pressure
| Group 1 <140 mm Hg (n = 4,787) | Group 2 140 to <150 mm Hg (n = 1,747) | Group 3 ≥150 mm Hg (n = 1,820) | All (n = 8,354) | p Value | Multiple PS Adjusted p Value | |
|---|---|---|---|---|---|---|
| Age, yrs | 70.1 ± 7.1 | 71.2 ± 7.1 | 71.6 ± 7.6 | 70.7 ± 7.3 | 0.001 | 0.99 |
|
| ||||||
| Age >70 yrs | 45.1 | 52.4 | 53.1 | 48.3 | <0.0001 | 0.99 |
|
| ||||||
| Female | 53.5 | 57.0 | 59.6 | 55.6 | <0.0001 | 0.99 |
|
| ||||||
| Race/ethnicity | ||||||
| White | 49.8 | 59.2 | 56.6 | 53.2 | <0.0001 | 0.97 |
| African American | 8.4 | 12.8 | 19.0 | 11.6 | ||
| Hispanic | 39.6 | 24.3 | 21.9 | 32.5 | ||
| Other/multiracial | 2.2 | 3.7 | 2.4 | 2.6 | ||
|
| ||||||
| Body mass index, kg/m2 | 28.2 ± 7.7 | 28.8 ± 5.3 | 28.7 ± 6.7 | 28.4 ± 7.1 | <0.0001 | 0.99 |
|
| ||||||
| SBP, mm Hg | 163.5 ± 12.6 | 165.7 ± 13.3 | 170.3 ± 16.0 | 165.4 ± 13.8 | <0.0001 | 0.95 |
|
| ||||||
| DBP, mm Hg | 91.5 ± 10.7 | 88.3 ± 11.0 | 88.9 ± 11.9 | 90.2 ± 11.1 | <0.0001 | 0.95 |
|
| ||||||
| Heart rate, beats/min | 75.8 ± 9.5 | 75.9 ± 9.8 | 75.5 ± 9.9 | 75.8 ± 9.7 | <0.0001 | 0.99 |
|
| ||||||
| Verapamil SR strategy | 50.9 | 49.7 | 47.5 | 49.9 | 0.05 | 0.99 |
|
| ||||||
| Medical history | ||||||
| Diabetes* | 26.8 | 30.2 | 33.6 | 29.0 | <0.0001 | 0.99 |
| Hypercholesterolemia* | 53.5 | 56.7 | 55.7 | 54.7 | 0.04 | 0.99 |
| Smoking (ever) | 42.7 | 42.6 | 43.2 | 42.8 | 0.92 | 0.99 |
| Myocardial infarction | 33.5 | 33.1 | 35.5 | 33.9 | 0.24 | 0.99 |
| Angina pectoris | 71.0 | 61.9 | 62.3 | 67.2 | <0.0001 | 0.98 |
| CABG or PCI | 23.7 | 30.7 | 30.3 | 26.6 | <0.0001 | 0.99 |
| Stroke or TIA | 7.4 | 8.9 | 9.8 | 8.3 | 0.004 | 0.99 |
| Left ventricular hypertrophy | 27.3 | 21.6 | 25.8 | 25.8 | <0.0001 | 0.96 |
| Unstable angina | 10.8 | 12.3 | 12.8 | 11.6 | 0.04 | 0.99 |
| Arrhythmia | 7.8 | 7.3 | 7.8 | 7.7 | 0.78 | 0.99 |
| Heart failure (class I to III) | 5.9 | 5.0 | 7.5 | 6.1 | 0.01 | 0.95 |
| PVD | 12.2 | 10.8 | 14.0 | 12.3 | 0.02 | 0.98 |
| Renal impairment† | 1.6 | 2.4 | 3.8 | 2.2 | <0.0001 | 0.96 |
| Cancer‡ | 3.3 | 5.1 | 4.9 | 4.0 | 0.001 | 0.99 |
| Well-being | ||||||
| Excellent | 6.7 | 8.4 | 6.1 | 7.0 | <0.0001 | 0.99 |
| Good | 58.8 | 62.8 | 58.7 | 59.6 | ||
| Fair | 31.3 | 26.3 | 31.3 | 30.2 | ||
| Poor | 3.1 | 2.4 | 3.8 | 3.1 | ||
|
| ||||||
| Baseline medications | ||||||
| Lipid-lowering agent | 34.8 | 36.3 | 32.9 | 34.7 | 0.09 | 0.99 |
| Nitrate | 41.9 | 31.5 | 29.8 | 37.1 | <0.0001 | 0.98 |
| Aspirin or other antiplatelet agent | 59.5 | 60.2 | 54.9 | 58.7 | 0.001 | 0.99 |
| Other NSAIDs | 14.7 | 16.4 | 17.2 | 15.6 | 0.03 | 1.00 |
| Potassium supplement | 4.9 | 8.8 | 9.8 | 6.8 | <0.0001 | 0.97 |
| Antidiabetic medication§ | 21.0 | 23.2 | 26.0 | 22.5 | <0.0001 | 0.99 |
| Hormone replacement | 6.7 | 8.4 | 9.2 | 7.6 | 0.001 | 0.99 |
Values are mean ± SD or %.
History of or currently taking antidiabetic or lipid-lowering medications.
History of or currently have elevated serum creatinine level but <4 mg/dl.
Patients with history of skin, prostate, and other cancers with long survival expectancy were not excluded.
Insulin and/or oral hypoglycemic agent.
CABG = coronary artery bypass graft surgery; DBP = diastolic blood pressure; NSAID = nonsteroidal anti-inflammatory agent; PCI = percutaneous coronary intervention; PS = propensity score; PVD = peripheral vascular disease; SBP = systolic blood pressure; SR = sustained release; TIA = transient ischemic attack.
For this study, a multiple propensity score was estimated with a nonparsimonious multinomial logistic regression model with on-treatment achieved systolic pressure (groups 1, 2, and 3) as the dependent variable and the baseline covariates outlined in Table 1 as independent variables. The baseline covariates were then adjusted for the propensity scores. A Cox proportional hazards model was used to estimate the effect of comparator groups on the primary and secondary outcomes after adjustment for the propensity scores (p1, p2, and p3), their products (e.g., product of any 2 of the 3 probabilities), and baseline covariates. In a sensitivity analysis, the primary results were rerun with a traditional Cox proportional hazards regression model adjusted to baseline covariates. The group with on-treatment achieved SBP of <140 mm Hg was used as the reference. A stepwise Cox proportional hazards model was used to adjust for the baseline difference with models that included variables for treatment strategy (verapamil-SR vs. atenolol), age, race, sex, prior heart failure, and additional considerations, listed in Table 1, that were selected if p ≤ 0.20. A p value of <0.05 was used to denote statistical significance. All analyses were performed with SAS 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Among the patients enrolled in INVEST, 8,354 were ≥60 years of age, had baseline systolic pressure of at least 150 mm Hg, and fulfilled inclusion criteria for this analysis. At the end of 24 months of follow-up, 4,787 patients (57%) achieved on-treatment SBP of <140 mm Hg (group 1); 1,747 patients (21%) achieved on-treatment SBP of 140 to <150 mm Hg (group 2); and 1,820 patients (22%) achieved on-treatment SBP of ≥150 mm Hg (group 3).
Compared with the group with achieved BP <140 mm Hg (group 1; reference group), the groups with achieved SBP ≥140 mm Hg were older; had greater proportions of women and African Americans; had a higher prevalence of prior MI, coronary artery bypass graft surgery, percutaneous coronary intervention, stroke or transient ischemic attack, unstable angina, diabetes, renal impairment, hypercholesterolemia, and cancer; and had higher baseline SBP (Table 1). In addition, the groups with achieved SBP ≥140 mm Hg were less likely to be taking nitrates but more likely to be taking nonsteroidal anti-inflammatory drugs other than aspirin, potassium supplement, antidiabetes medication, or hormone replacement therapy (Table 1). After multiple propensity score adjustment, the differences in baseline characteristics were no longer significant (Table 1). The median and inter-quartile range for achieved SBP was 131 mm Hg (126 to 135 mm Hg) in group 1, 144 mm Hg (142 to 146 mm Hg) in group 2, and 158 mm Hg (153 to 166 mm Hg) in group 3.
PRIMARY OUTCOME
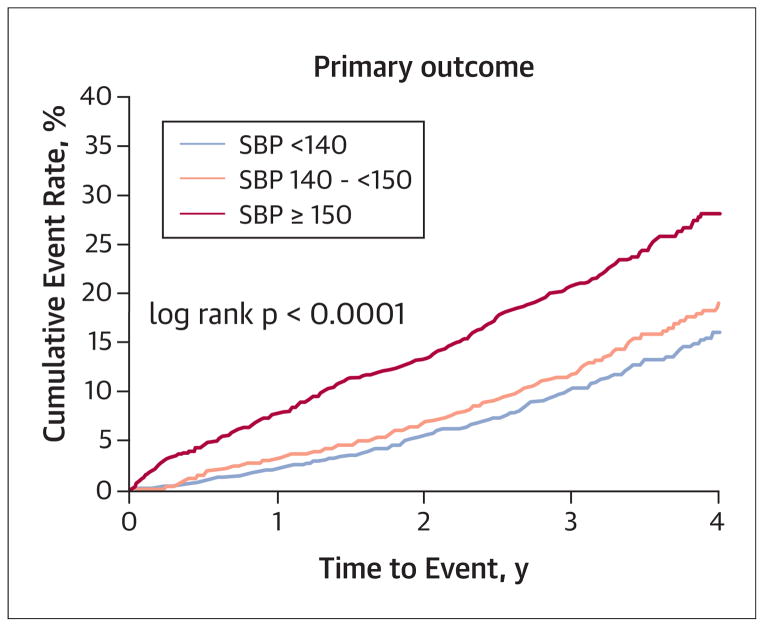
For this analysis, a total of 22,308 patient years of follow-up were accumulated. In the unadjusted model, group 1 had the lowest rate of the primary outcome compared with the other 2 groups (9.36% vs. 12.71% vs. 21.32%, respectively; p < 0.0001) (Table 2, Fig. 1). In the multiple propensity score–adjusted analysis, compared with group 1 (reference group; hazard ratio [HR]: 1.00), the risk of the primary outcome was no different in group 2 (adjusted HR: 1.12; 95% confidence interval [CI]: 0.95 to 1.32; p = 0.19), but the risk was significantly increased in group 3 (adjusted HR: 1.85; 95% CI: 1.59 to 2.14; p < 0.0001).
TABLE 2.
Event Rates (Unadjusted)
| Events | <140 mm Hg (n =4,787)
|
140 to <150 mm Hg (n = 1,747)
|
≥150 mm Hg (n = 1,820)
|
|||
|---|---|---|---|---|---|---|
| n (%) | Rate per 1,000 PY | n (%) | Rate per 1,000 PY | n (%) | Rate per 1,000 PY | |
| Primary outcome | 448 (9.36) | 35.26 | 222 (12.71) | 44.70 | 388 (21.32) | 83.68 |
|
| ||||||
| Death (all-cause) | 379 (7.92) | 29.56 | 176 (10.07) | 34.91 | 306 (16.81) | 63.62 |
|
| ||||||
| Cardiovascular death | 156 (3.26) | 12.16 | 80 (4.58) | 15.88 | 142 (7.8) | 28.94 |
|
| ||||||
| Total MI | 154 (3.22) | 12.06 | 73 (4.18) | 14.58 | 146 (8.02) | 30.32 |
|
| ||||||
| Nonfatal MI | 51 (1.07) | 3.99 | 18 (1.03) | 3.59 | 53 (2.91) | 11.01 |
|
| ||||||
| Total stroke | 57 (1.19) | 4.47 | 46 (2.63) | 9.21 | 70 (3.85) | 14.40 |
|
| ||||||
| Nonfatal stroke | 41 (0.86) | 3.21 | 33 (1.89) | 6.61 | 52 (2.86) | 28.47 |
|
| ||||||
| Revascularization | 101 (2.11) | 8.00 | 55 (3.15) | 11.12 | 44 (2.42) | 9.11 |
|
| ||||||
| Heart failure (class I to IV) | 91 (1.9) | 7.15 | 31 (1.77) | 6.19 | 45 (2.47) | 9.30 |
MI = myocardial infarction; PY = patient-years; Total MI = fatal plus nonfatal MI; Total stroke = fatal plus nonfatal stroke.
FIGURE 1. On-Treatment Blood Pressure Categories and Risk of Primary Outcome.
Cumulative event rate was lowest in the group with achieved systolic blood pressure (SBP) <140 mm Hg.
SECONDARY OUTCOMES
In the unadjusted model, group 1 had the lowest rate of all-cause mortality (7.92% vs. 10.07% vs. 16.81% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2, Fig. 2A). In the multiple propensity score–adjusted analysis, compared with group 1, the mortality risk was no different in group 2 (adjusted HR: 1.03; 95% CI: 0.86 to 1.24; p = 0.74), but it was substantially increased in group 3 (adjusted HR: 1.64; 95% CI: 1.40 to 1.93; p < 0.0001).
FIGURE 2. On-Treatment Blood Pressure Categories and Risk of All-Cause and Cardiovascular Mortality.
Cumulative event rate was lowest in the group with achieved systolic blood pressure (SBP) <140 mm Hg for both all-cause mortality (A) and cardiovascular (CV) mortality (B).
In the unadjusted model, group 1 had the lowest rate of cardiovascular mortality (3.26% vs. 4.58% vs. 7.80% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2, Fig. 2B). In the multiple propensity score–adjusted analysis, compared with group 1, the risk of cardiovascular mortality was increased in group 2 (adjusted HR: 1.34; 95% CI: 1.01 to 1.77; p = 0.04) and group 3 (adjusted HR: 2.29; 95% CI: 1.79 to 2.93; p < 0.0001).
In the unadjusted model, group 1 had the lowest rate of total MI (fatal and nonfatal; 3.22% vs. 4.18% vs. 8.02% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2). In the multiple propensity score–adjusted analysis, compared with group 1, the risk of MI was no different in group 2 (adjusted HR: 1.20; 95% CI: 0.90 to 1.60; p = 0.21) but was substantially increased in group 3 (adjusted HR: 2.39; 95% CI: 1.87 to 3.05; p < 0.0001).
In the unadjusted model, group 2 had the lowest rate of nonfatal MI (1.07% vs. 1.03% vs. 2.91% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2, Fig. 3A). In the multiple propensity score–adjusted analysis, compared with group 1, the risk of MI was no different in group 2 (adjusted HR: 0.85; 95% CI: 0.49 to 1.46; p = 0.55) but was substantially increased in group 3 (adjusted HR: 2.45; 95% CI: 1.62 to 3.71; p < 0.0001).
FIGURE 3. On-Treatment Blood Pressure Categories and Risk of Nonfatal Myocardial Infarction, Total Stroke (Fatal and Nonfatal), Nonfatal Stroke, Heart Failure, and Revascularization.
Cumulative event rate for nonfatal myocardial infarction (A) was lower in the group with achieved systolic blood pressure (SBP) <140 mm Hg or 140 to <150 mm Hg. For risk of total stroke (B) and nonfatal stroke (C), cumulative event rate was lowest in the group with achieved SBP <140 mm Hg. For risk of heart failure (D) and risk of revascularization (E), cumulative event rate was similar among the 3 groups. Patients who achieved SBP <140 mm Hg had the lowest rate of the primary outcome, cardiovascular mortality, and fatal and nonfatal myocardial infarction compared with the groups with achieved SBP ≥140 mm Hg.
In the unadjusted model, group 1 had the lowest rate of total stroke (fatal plus nonfatal; 1.19% vs. 2.63% vs. 3.85% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2, Fig. 3B). In the multiple propensity score–adjusted analysis, compared with group 1, the risk of total stroke was increased in group 2 (adjusted HR: 1.89; 95% CI: 1.26 to 2.82; p = 0.002) and group 3 (adjusted HR: 2.93; 95% CI: 2.01 to 4.27; p < 0.0001).
In the unadjusted model, group 1 had the lowest rate of nonfatal stroke (0.86% vs. 1.89% vs. 2.86% for groups 1, 2, and 3, respectively; p < 0.0001) (Table 2, Fig. 3C). In the multiple propensity score–adjusted analysis, compared with group 1, the risk of nonfatal stroke was increased in group 2 (adjusted HR: 1.70; 95% CI: 1.06 to 2.72; p = 0.03) and group 3 (adjusted HR: 2.78; 95% CI: 1.80 to 4.30; p < 0.0001).
In the unadjusted model, the risk of heart failure was low and similar across the groups (1.90% vs. 1.77% vs. 2.47% for groups 1, 2, and 3, respectively; p = 0.18) (Table 2, Fig. 3D). Similarly, in the adjusted analysis, the risk was similar.
In the unadjusted model, the risk of revascularization was low and similar across the groups (2.11% vs. 3.15% vs. 2.42% for groups 1, 2, and 3, respectively; p = 0.21) (Table 2, Fig. 3E). Again, in the adjusted analysis, the risk was similar.
There were no significant increases in adverse experiences in group 1 compared with groups 2 and 3 (Table 3). A sensitivity analysis using a Cox proportional hazard regression model for the primary and secondary outcomes yielded largely similar results (Table 4).
TABLE 3.
Adverse Experiences
| Type | <140 mm Hg (n = 4,787) | 140 to <150 mm Hg (n = 1,747) | ≥150 mm Hg (n = 1,820) | p Value |
|---|---|---|---|---|
| Alzheimer’s disease | 31 (0.6) | 11 (0.6) | 11 (0.6) | 0.98 |
| Angina | 189 (3.9) | 109 (6.2) | 80 (4.4) | 0.0004 |
| AV block | 21 (0.4) | 16 (0.9) | 13 (0.7) | 0.07 |
| CABG/PCI | 257 (5.4) | 115 (6.6) | 96 (5.3) | 0.13 |
| Cancer | 167 (3.5) | 87 (5.0) | 74 (4.1) | 0.02 |
| Constipation | 76 (1.6) | 58 (3.3) | 49 (2.7) | <0.0001 |
| Cough | 153 (3.2) | 71 (4.1) | 78 (4.3) | 0.06 |
| Dizziness | 108 (2.3) | 68 (3.9) | 80 (4.4) | <0.0001 |
| Dyspnea | 78 (1.6) | 45 (2.6) | 40 (2.2) | 0.03 |
| Gastrointestinal bleeding | 76 (1.6) | 41 (2.4) | 29 (1.6) | 0.099 |
| Gout | 33 (0.7) | 21 (1.2) | 24 (1.3) | 0.025 |
| Headache | 39 (0.8) | 33 (1.9) | 33 (1.8) | 0.0001 |
| Hyperkalemia | 4 (0.1) | 4 (0.2) | 7 (0.4) | 0.03 |
| Hypokalemia | 11 (0.2) | 5 (0.3) | 5 (0.3) | 0.90 |
| Lightheadedness | 38 (0.8) | 27 (1.5) | 30 (1.6) | 0.0027 |
| Liver enzymes out of range | 16 (0.3) | 10 (0.6) | 3 (0.2) | 0.11 |
| Parkinson’s disease | 9 (0.2) | 9 (0.5) | 4 (0.2) | 0.068 |
| Peripheral edema | 31 (0.6) | 31 (1.8) | 27 (1.5) | <0.0001 |
| Peripheral vascular disease | 90 (1.9) | 41 (2.4) | 48 (2.6) | 0.13 |
| Renal failure | 35 (0.7) | 14 (0.8) | 23 (1.3) | 0.11 |
| Symptomatic bradycardia | 83 (1.73) | 46 (2.6) | 65 (3.6) | <0.0001 |
| Unstable angina | 108 (2.3) | 62 (3.6) | 53 (2.9) | 0.013 |
| Wheezing | 28 (0.6) | 14 (0.8) | 11 (0.6) | 0.61 |
Values are n (%).
AV = atrioventricular; CABG = coronary artery bypass surgery; PCI = percutaneous coronary intervention.
TABLE 4.
Sensitivity Analysis on the Basis of Traditional Cox Proportional Hazards Regression Model
| Primary Outcome
|
All-Cause Mortality
|
Cardiovascular Mortality
|
Total MI
|
Total Stroke
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Group 1 (<140 mm Hg) (Ref) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — |
|
| ||||||||||
| Group 2 (140 to <150 mm Hg) | 1.09 (0.93–1.29) | 0.27 | 1.00 (0.83–1.20) | 0.99 | 1.31 (1.00–1.73) | 0.05 | 1.14 (087–1.51) | 0.34 | 1.88 (1.27–2.78) | 0.002 |
|
| ||||||||||
| Group 3 (≥150 mm Hg) | 1.82 (1.58–2.09) | <0.0001 | 1.60 (1.37–1.86) | <0.0001 | 2.18 (1.73–2.76) | <0.0001 | 2.23 (1.78–2.81) | <0.0001 | 2.83 (1.98–4.04) | <0.0001 |
CI = confidence interval; HR = hazard ratio; MI = myocardial infarction; Ref = reference group.
DISCUSSION
In this post-hoc analysis from INVEST, among patients ≥60 years of age with CAD and baseline SBP ≥150 mm Hg, those who achieved an SBP <140 mm Hg had the lowest rate of the primary outcome, all-cause mortality, cardiovascular mortality, total MI, nonfatal MI, total stroke, and nonfatal stroke in unadjusted models compared with those in the groups with achieved SBP ≥140 mm Hg, without any increase in adverse experiences (Central Illustration). Moreover, in the multiple propensity score–adjusted model, the group with the current 2014 JNC-8 panel–recommended BP target of 140 to <150 mm Hg was associated with an increased hazard for risk of cardiovascular mortality, total stroke, and nonfatal stroke compared with the group with achieved SBP <140 mm Hg.
The recent recommendation, by the majority of the JNC-8 panel members, of a more relaxed SBP threshold for initiation of treatment and a target of <150 mm Hg in patients ≥60 years of age created a “tempest in the teapot,” with some members of the panel disagreeing with the recommendation (2). Relaxing the BP target in this age group may have resulted from recent data suggesting that lower may perhaps not be better when it comes to BP targets (11–15). However, common themes in most of these analyses were 2-fold: 1) there was target organ heterogeneity in that although lower was not better for cardiac-related outcomes, lower was indeed better for stroke-related endpoints; 2) the optimal BP (the nadir BP) with the lowest event rate in many of these analyses was between 130 and 140 mm Hg. In the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial of patients with diabetes, targeting an SBP of <120 mm Hg compared with <140 mm Hg did not reduce the rate of a composite outcome of fatal and nonfatal major cardiovascular events, although there was a significant reduction in the risk of stroke (41% reduction) at the expense of a significant increase in serious adverse events attributed to antihypertensive treatment (16). Of note, the mean age in ACCORD was 62 years. Similarly, in INVEST, tight control of SBP among patients with diabetes and CAD was not associated with improved cardiovascular outcomes compared with usual control (17). Why then did the panel members choose a target of <150 mm Hg? The evidence document states that “setting a goal SBP of lower than 140 mm Hg in this age group provides no additional benefit compared with a higher goal SBP of 140 to 160 mm Hg or 140 to 149 mm Hg,” on the basis of 2 randomized trials in Japanese patients (18,19). The JATOS (Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients) enrolled patients ≥65 years of age with hypertension randomized to strict treatment (targe <140 mm Hg) versus mild treatment (target 140 to 160 mm Hg) of BP (19). The primary endpoint was no different between the 2 groups; however, the test for interaction was significant (p = 0.03) for age such that in those <75 years versus ≥75 years, there was a trend toward benefit of the strict treatment group for primary outcome (2.35 vs. 3.46; p = 0.10), driven by numerically lower stroke risk (1.33% vs. 2.04%; p = 0.15) (19). The second trial cited by the document is the VALISH (Valsartan in Elderly Isolated Systolic Hypertension) trial, which enrolled subjects 70 to 84 years of age with hypertension randomized to strict BP control (<140 mm Hg) versus moderate BP control (140 to <150 mm Hg); there was no statistically significant difference in any of the cardiovascular outcomes (18); however, as acknowledged by the authors, the analysis was underpowered to detect a difference (18). Thus, the evidence to support this 2014 JNC-8 panel grade A recommendation of a BP target of <150 mm Hg appears rather weak.
A commentary published by 5 of the 17 members appointed to the JNC-8 panel rejects the new target BP, arguing that the evidence does not support this target and that its liberalization could lead to harmful consequences, because a large proportion of these patients have established cardiovascular disease or are at high risk for cardiovascular disease (including African Americans and those with multiple risk factors) (2). This retrospective subgroup analysis demonstrated that in hypertensive patients ≥60 years of age with CAD who entered the study with BP >150 mm Hg and who had a protocol-defined treatment target of SBP <140 mm Hg, those who actually achieved the target had numerically lower rates of primary and secondary outcomes than those whose on-treatment BP remained higher. A prudent interpretation of the data suggests that patients ≥60 years of age with CAD who fail to achieve an on-treatment SBP of <140 mm Hg have a significantly increased risk of stroke and other adverse outcomes. Of additional concern, if the U.S. population demographic resembles that from INVEST, which is suggested from National Health and Nutrition Examination Survey data, the new recommendation places a disproportionately large number of elderly women and African Americans at increased risk. However, although the current analysis supports a target of <140 mm Hg, the data are based on achieved on-target BP, which is likely influenced by patients’ baseline characteristics. In addition, the current analysis was not designed to investigate whether “lower is better.” Prior analyses from INVEST have shown a J-curve relationship between BP and cardiovascular events, such that both a very low and very high BP were associated significantly with higher event rates (15). Furthermore, for more stringent BP targets such as that in the intensive arm of the ACCORD trial, trying to achieve a BP <120 mm Hg may have deleterious consequences, including an increase in serious adverse events attributed to antihypertensive treatment.
STUDY LIMITATIONS
This post-hoc analysis from a randomized trial was not specifically designed to test various BP targets. In addition, the results are applicable only to patients ≥60 years of age with known CAD who had SBP >150 mm Hg at entry. However, patients with CAD are known to be more susceptible to adverse events with low BP than are patients without CAD. Although the group with SBP <140 mm Hg had numerically lower primary and secondary outcomes, in the multivariable analysis there was a statistically significant difference with the group with SBP 140 to ≤150 mm Hg only for the outcomes of cardiovascular mortality, total stroke, and stroke, even though the point estimate for the other outcomes also favored the group with BP <140 mm Hg. The present study was not designed to test whether patients ≥60 years of age with SBP of 140 to 150 mm Hg would benefit from antihypertensive treatment.
CONCLUSIONS
In hypertensive patients ≥60 years of age with CAD, an SBP <140 mm Hg is associated with numerically the lowest rate of primary and most secondary cardiovascular outcomes compared with SBP ≥140 mm Hg, without any increase in adverse experiences. Moreover, in the multivariable model, when INVEST patients who achieved on-treatment SBP <140 mm Hg were compared with those who achieved on-treatment BP of 140 to 150 mm Hg, the rates of the overall primary outcome and all-cause mortality were similar. However, patients in the group with BP <140 mm Hg had significantly fewer strokes and cardiovascular mortality. Patients whose on-treatment BP remained >150 mm Hg had significant increases in the primary outcome and all secondary outcomes tested. These data provide important information to focus the risk-benefit discussion for patients with on-treatment BP in the 140 to 150 mm Hg range clearly on cardiovascular mortality and stroke prevention and on overall reduction of mortality for patients with on-treatment BP >150 mm Hg.
FIGURE 4. CENTRAL ILLUSTRATION Study Design and Main Outcomes.
Patients who achieved a systolic blood pressure <140 mm Hg had the lowest rate of the primary outcome, cardiovascular mortality, and fatal and nonfatal stroke compared with the groups with achieved systolic blood pressure ≥140 mm Hg. BP = blood pressure; CV = cardiovascular; INVEST = INternational VErapamil SR Trandolapril STudy; MI = myocardial infarction; SBP = systolic blood pressure.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE
Some patients greater than 60 years of age who have hypertension and coronary artery disease may benefit from lowering systolic blood pressure below 140 mm Hg.
TRANSLATIONAL OUTLOOK
The relative risks and benefits of more aggressive treatment of hypertension on cardiovascular outcomes in older patients with coronary artery disease require further study in adequately powered randomized trials comparing various anti-hypertensive strategies and target blood pressure levels.
Acknowledgments
Drs. Gong and Cooper-DeHoff are supported by a grant from the National Institutes of Health/National Institute of General Medical Sciences (U01 GM074492). Dr. Bangalore serves on advisory boards for Daiichi Sankyo, Boehringer Ingelheim, Abbott, Gilead, and Abbott Vascular. Dr. Cooper-DeHoff is supported by a research grant from Abbott Laboratories. Dr. Meserli is an ad-hoc consultant for Daiichi Sankyo, Pfizer Inc., Takeda Pharmaceuticals, Abbott Laboratories, AbbVie, Servier, Medtronic Inc., and Ipca Laboratories Ltd.
ABBREVIATIONS AND ACRONYMS
- BP
blood pressure
- CAD
coronary artery disease
- MI
myocardial infarction
- SBP
systolic blood pressure
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC-8) [published correction appears in JAMA 2014;311:1809] JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 3.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 4.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. for the INVEST Investigators. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease: the International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–16. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 5.Pepine CJ, Handberg-Thurmond E, Marks RG, et al. Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol. 1998;32:1228–37. doi: 10.1016/s0735-1097(98)00423-9. [DOI] [PubMed] [Google Scholar]
- 6.The sixth report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure [published correction appears in Arch Intern Med 1998;158: 573] Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S, Messerli FH, Cohen JD, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156:241–7. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Spreeuwenberg MD, Bartak A, Croon MA, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care. 2010;48:166–74. doi: 10.1097/MLR.0b013e3181c1328f. [DOI] [PubMed] [Google Scholar]
- 9.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–10. [Google Scholar]
- 11.Bangalore S, Qin J, Sloan S, Murphy SA, Cannon CP for the PROVE-IT TIMI 22 Trial Investigators. What is the optimal blood pressure in patients after acute coronary syndromes? Relationship of blood pressure and cardiovascular events in the PRavastatin OR atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) 22 trial. Circulation. 2010;122:2142–51. doi: 10.1161/CIRCULATIONAHA.109.905687. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore S, Messerli FH, Wun CC, et al. for the Treating to New Targets Steering Committee and Investigators. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897–908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 13.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–93. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–810. doi: 10.1161/CIRCULATIONAHA.110.016337. [DOI] [PubMed] [Google Scholar]
- 15.Bangalore S, Kumar S, Volodarskiy A, Messerli FH. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601–13. doi: 10.1136/heartjnl-2012-301968. [DOI] [PubMed] [Google Scholar]
- 16.ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–8. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogihara T, Saruta T, Rakugi H, et al. for the Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension Study. Hypertension. 2010;56:196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 19.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31:2115–27. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]