Abstract
Asymmetric, articulated jaws support active predation in vertebrates; they derive from the first pharyngeal arch (PA1) which generates both maxillary and mandibular components. PA1 is colonized by cranial neural crest cells (CNCCs) which give rise to most bones and tendons of the jaws. The elements formed by different CNCCs contingents are specified by the combinatorial expression of Dlx genes. Dlx5 and Dlx6 are predominantly expressed by mandibular CNCCs. Analysis of the phenotype of Dlx5 and Dlx6 double mutant mice has suggested that they are necessary and sufficient to specify mandibular identity. Here, using 3D reconstruction, we show that inactivation of Dlx5 and Dlx6 does not only affect the mandibular arch, but results in the simultaneous transformation of mandibular and maxillary skeletal elements which assume a similar morphology with gain of symmetry. As Dlx5- and Dlx6-expressing cells are not found in the maxillary bud, we have examined the lineage of Dlx5-expressing progenitors using an in vivo genetic approach. We find that a contingent of cells deriving from precursors transiently expressing Dlx5 participate in the formation of the maxillary arch. These cells are mostly located in the distal part of the maxillary arch and might derive from its lambdoidal junction with the olfactory pit. Our findings extend current models of jaw morphogenesis and provide an explanation for the maxillary defects of Dlx5 and Dlx6 mutants. Our results imply that Dlx5 and Dlx6 model the upper and the lower PA1 components through different morphogenetic mechanisms which are, however, coordinated as they give rise to functional, articulated jaws.
Introduction
The vertebrate skull is characterized by the presence of articulated, asymmetric jaws which support the function of a muscularized oral cavity essential for predation. During embryonic development, the upper and lower jaws derive from the maxillary and mandibular processes of the first pharyngeal arch (PA1). Most cartilaginous and dermatocranial derivatives of PA1 are formed by Cranial Neural Crest Cells (CNCCs) emigrating from the prosencephalic and anterior mesencephalic neural folds 1– 6. During migration, signals emanating from the endoderm and possibly other PA1 components instruct the CNCCs to unfold the morphogenetic process of the jaws 5, 7, 8. The nested expression of Dlx homeobox genes, vertebrate homologues of Drosophila Distal-less, has a fundamental role in the specification of the dorsoventral patterning of PA1 derivatives 9, 10. The six Dlx genes found in mammals are arranged as closely associated bigenic clusters: Dlx1, Dlx2; Dlx3, Dlx4; Dlx5, Dlx6; these pairs of genes are often coregulated and display a partially redundant function. While Dlx1 and Dlx2 are expressed by CNCCs of the maxillary and mandibular components of PA1, Dlx5 and Dlx6 transcripts are present only in mandibular CNCCs. Targeted simultaneous inactivation of Dlx5 and Dlx6 results in the transformation of lower jaw into upper jaw-like structures, underlining the importance of these genes for lower jaw identity 11– 14. Interestingly it has been observed 15, 16 that, after inactivation of Dlx5 and Dlx6, the maxillary component is also affected despite the fact that these genes are not expressed in maxillary CNCCs. This observation could be accounted for by the presence of shared Dlx5/6-dependent signalling centres in proximity to the extremities of both the mandibular and maxillary arches; this notion gave rise to the so-called “hinge and caps” model of jaw organization 17. In its original formulation this model predicts the presence of two opposing morphogen gradients, one emanating from the region of the upper/lower jaw articulation (hinge) and one from the distal extremities of PA1 (caps). While the origin and nature of these signals remain elusive, the possibility that transient Dlx expression in a contingent of cells populating the maxillary arch could play a role in its morphogenesis has not been yet analyzed. Here we revisit the effects of Dlx5 and Dlx6 double inactivation on jaw development and, using a transgenic lineage tracing approach, we reveal that the maxillary arch harbours a cellular contingent derived from Dlx5 progenitors.
Material and methods
Mouse strains and breeding
All animal experimentation was performed in accordance to French national regulations and approved by the MNHN ethical committee (approval n° 68–028r1). For this study we used about 35 dams (including 10 WT, 5 Dlx5 lacZ/+; 3 Dlx5/6+/-; 12 B6.129S4-Gt(ROSA)26Sor tm1Sor/J; 5 B6(Cg)-Dlx5 tm1(cre/ERT2)Zjh/J) and analyzed about 120 embryos, the exact record of animals used, litters obtained, embryos genotyped and number of embryos per litter is on record in our animal house. WT animals were form Charles River France and were maintained in the MNHN mouse facility which is officially certified by the French National Animal well being committee.
Dlx5 lacZ/+ knock-in mice were maintained on a mixed B6/D2 genetic background 18. Double Dlx5 and Dlx6 ( Dlx5/6) mutant mice were maintained and genotyped as reported 19. The inducible Cre driver strain B6(Cg)-Dlx5 tm1(cre/ERT2)Zjh/J (designed by Z. J. Huang 20), and the lacZ Cre reporter strain B6.129S4-Gt(ROSA)26Sor tm1Sor/J (R26R-lacZ) were purchased from Jackson Laboratory (#10705 and #003309 respectively; Maine, USA) through Charles River Laboratories (L’Arbresle, France) and maintained on a C57BL/6J genetic background through heterozygous mating. Double heterozygous embryos were obtained through bi-directional crosses. Induction of Cre recombinase activity was obtained upon single intraperitoneal injection of 5mg of tamoxifen (Sigma-Aldrich), in corn oil. Tamoxifen preparation and administration in pregnant dams followed the Jackson Laboratory’s Guidelines and CNRS/MNHN Animal Handling Guidelines. Dams were euthanized by cervical dislocation at indicated stages and embryos were collected in phosphate-buffered saline (PBS), then staged and fixed by immersion in ice-cold fixative (2% paraformaldehyde/0.2% glutaraldehyde) for 5 to 15 minutes (depending upon their developmental stage).
β-galactosidase detection
For lacZ expression, embryos were fixed for 15–30 min in 4% paraformaldehyde; X-gal staining was performed as described previously 18, 21. Vehicle (corn oil) injection in double heterozygous mice did not yield leaking ß-galactosidase activity.
Histology and 3D reconstruction
Heads from 18.5 dpc (days post coitum) Dlx5/6 -/- and wild type mouse embryos were fixed in Bouin’s solution (Sigma, France), embedded in paraffin and complete sets of frontal or parasagittal serial sections (12µm) were prepared. All sections were stained by Mallory’s trichrome as in 22 and photographed (Nikon Digital Site DS-FI1). Pictures were aligned, piled and registered using the Fiji plug-in of NIH ImageJ "Register Virtual Stack Slices" ( http://fiji.sc/wiki/index.php/Register_Virtual_Stack_Slices). 3D segmentation was performed with Mimics (Materialise, Belgium: http://biomedical.materialise.com/mimics) and visualized using Adobe Acrobat 9 pro.
Results
Dlx5/6 inactivation results in upper and lower jaw transformation with gain of symmetry
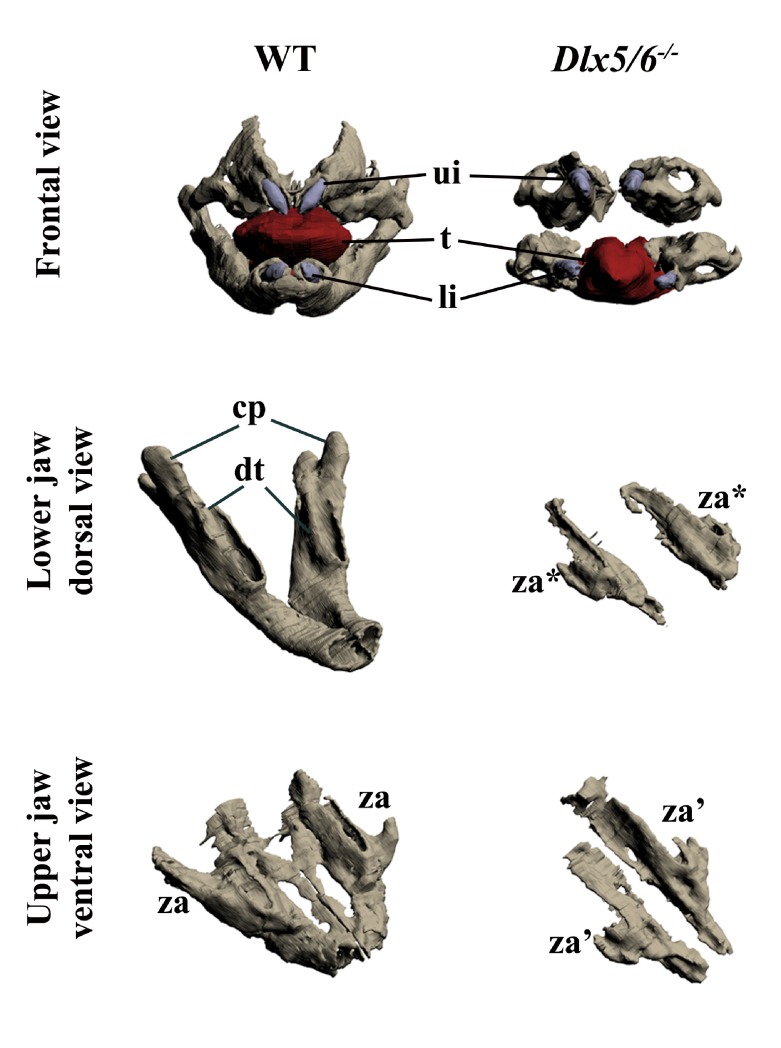
Previous reports suggest that double inactivation of Dlx5 and Dlx6 results in lower-to-upper jaw transformation; these reports also indicated that the upper jaw of these mice is not normal 15, 16. To better visualize the jaw phenotype of Dlx5/6 mutants, we performed 3D reconstruction of craniofacial elements of 18.5 dpc (days post coitum) embryos. Frontal view of the mutant jaws ( Figure 1, upper panel) shows an obvious gain of symmetry compared to a WT animal. Examining the defects of the lower and upper jaws separately ( Figure 1, middle and lower panels), it is evident that both are transformed. In the absence of Dlx5 and Dlx6 the dentary and the upper jaw bones do not form correctly and are replaced by remarkably similar skeletal structures. In the mutant embryos, both the upper and lower jaw skeletal elements are reduced in size, are not fused in the midline, and display a lateral process positionally homologous to the wild type zygomatic arch. Thus the upper and lower jaw mutant bones resemble each other more closely than usually found in their normal counterparts.
Figure 1. Three-dimensional reconstruction of the dentary and maxillary bones of 18.5 dpc wild type and Dlx5/6 -/- mouse embryos.
Upper row: Frontal view of WT and Dlx5/6 -/- oral apparatus. Skeletal elements are grey, the tongue is red and incisors are purple. Middle row: Dorsal view of the dentary bone of WT and Dlx5/6 -/- 18.5 dpc mice. Lower row: Ventral view of the maxillary components of WT and Dlx5/6 -/- 18.5 dpc mice. Note that the inactivation of Dlx5/6 results in the transformation of both lower and upper jaw skeletal elements into new structures which appear more similar to each other than to their WT counterpart. cp, coronoid processes; dt, dentary bone; li, lower incisor; t, tongue; ui, upper incisor; za, zygomatic arch; za*, zygomatic arch-like structure deriving from lower jaw transformation; za’, zygomatic arch-like structure deriving from upper jaw transformation.
Transient Dlx5 expression in maxillary arch progenitors
In Dlx5-lacZ heterozygous Theiler stage (ts) 19 (12 dpc) embryos the reporter is active in the olfactory pit and mandibular arch, but not in the maxillary arch; this pattern of expression does not change upon tamoxifen treatment of the pregnant dam ( Figure 2A,A’). To understand the origin of the Dlx5/6-dependent defect of the upper jaw we used a genetic approach to follow the lineage of Dlx5-precursors in the head. To this end we brought the R26R-lacZ reporter into the Dlx5-creERT2 driver background and we activated cre-recombinase activity by tamoxifen treatment of the pregnant dam at ts9 (6.25 dpc). We monitored ß-gal reporter activity from ts15 (10 dpc) to ts20 (12.5 dpc). At ts15 we observed a stream of ß-gal-positive cells extending from the lambdoidal junction, which joins the olfactory pit with the distal maxillary arch 17, 23, towards the body of the maxillary arch ( Figure 2B,B’). At ts19 and ts20 ( Figure 2C,C’; D,D’) reporter-expressing cells are found in the upper epithelial lining of the maxillary arch (arrowheads in Figure 2C’,2D’) and in two distinct proximal and distal territories of the arch body (red asterisk in Figure 2C’).
Figure 2. Lineage of Dlx5-expressing cells in the maxillary arch.
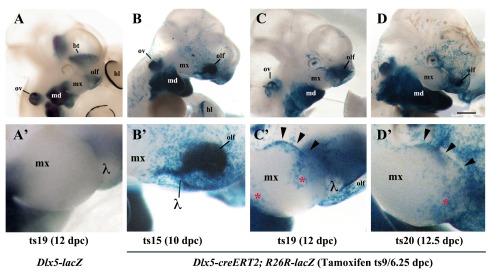
ß-Galactosidase activity in the cephalic region of Dlx5-lacZ ( A, A’) and Dlx5-creERT2; R26R-lacZ mouse embryos ( B– D’). In all cases pregnant dams were treated with tamoxifen at 6.25 dpc/Theiler stage 9 (ts9) and embryos were collected at the indicated Theiler stage. ( A, A’) As expected, even after tamoxifen treatment, Dlx5 is expressed in the mandibular arch (md), in the olfactory pit (olf), in the otic vesicle (ov), in the basal telencephalon (bt) and in the hind limb (hl), but not in the maxillary arch. ( B, B’) Permanent activation of lacZ reporter expression in derivatives of Dlx5-expressing early progenitors (ts9) reveals the presence of a positive cellular contingent in the ts15 lambdoidal junction (λ) between the olfactory pit and the maxillary process. ( C, C’; D, D’) At later developmental stages (ts19, ts20) a contingent of lacZ positive cells populates the distal domain of the maxillary arch. hl, hind limb; md, mandibular arch; mx, maxillary arch; olf, olfactory pit; ov, otic vesicle; bt, basal telencephalon; λ, lambdoidal junction; red asterisk/black arrowheads, territories of the maxillary arch colonized by derivatives of Dlx5-expressing progenitors. Bar: A– D 1mm; A’– D’ 250µm.
Discussion
In this study we have re-examined the skeletal jaw phenotype of Dlx5/6 mutant mice. We confirm that both the mandibular and maxillary arches are transformed. The profound change in the shape of the maxillary arch is difficult to explain as this region does not derive from a Dlx5/6-expressing territory. Lineage analysis to identify derivatives of Dlx5-positive progenitors reveals a new population of cells extending from the olfactory pit through the lambdoidal junction towards the maxillary arch 17, 23. These derivatives of Dlx5-positive cells have lost Dlx5 expression as seen by Dlx5 in situ hybridization (see for example Depew et al. (2002) 16, Acampora et al. (1999) 18 and Depew et al. (1999) 24) and by lacZ-Dlx5 knock-in 18, and Figure 2A’. We have shown that early Dlx5 and Dlx6 expression in the anterior neural fold is essential for nasal capsule patterning 25; our present findings suggest that the same population of cells could also contribute to maxillary patterning. This cell contingent might well exert a patterning role upon the maxillary arch providing either epithelial or mesenchymal cues. This observation fits with the prediction of the ‘hinge and caps’ model 17, and suggests that ’cap’ signals could originate from derivatives of Dlx5-expressing progenitors. Even if after migration in the maxillary arch these cells lose Dlx5 expression, it is still possible that the early expression of Dlx5 confers on them the capacity to pattern maxillary arch CNCCs, which do not themselves express Dlx5 and Dlx6. In contrast, in the lower jaw Dlx5 and Dlx6 are expressed by CNCCs; it appears, therefore, that Dlx5 and Dlx6 pattern the upper and lower jaw through very different mechanisms, which must be coordinated to generate the asymmetric, articulated, muscularized jaws of vertebrate predators.
Acknowledgements
This work was made possible thanks to the excellent technical assistance of Mss. Anastasia Fontaine, Aurélie Hagneau, Ocilia Fernandes and Gladys Alfama.
Funding Statement
This research was partially supported by the EU Consortium IDEAL (HEALTH-F2-2011-259679) to GL.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: approved with reservations 1
References
- 1.Tan SS, Morriss-Kay GM: Analysis of cranial neural crest cell migration and early fates in postimplantation rat chimaeras. J Embryol Exp Morphol. 1986;98:21–58 [PubMed] [Google Scholar]
- 2.Couly GF, Coltey PM, Le Douarin NM: The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117(2):409–29 [DOI] [PubMed] [Google Scholar]
- 3.Creuzet S, Couly G, Vincent C, et al. : Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129(18):4301–13 [DOI] [PubMed] [Google Scholar]
- 4.Kontges G, Lumsden A: Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122(10):3229–42 [DOI] [PubMed] [Google Scholar]
- 5.Noden DM: Vertebrate craniofacial development: novel approaches and new dilemmas. Curr Opin Genet Dev. 1992;2(4):576–81 10.1016/S0959-437X(05)80175-3 [DOI] [PubMed] [Google Scholar]
- 6.Trainor PA, Tam PP: Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development. 1995;121(8):2569–82 [DOI] [PubMed] [Google Scholar]
- 7.Ruhin B, Creuzet S, Vincent C, et al. : Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003;228(2):239–46 10.1002/dvdy.10380 [DOI] [PubMed] [Google Scholar]
- 8.Couly G, Creuzet S, Bennaceur S, et al. : Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129(4):1061–73 [DOI] [PubMed] [Google Scholar]
- 9.Merlo GR, Zerega B, Paleari L, et al. : Multiple functions of Dlx genes. Int J Dev Biol. 2000;44(6):619–26 [PubMed] [Google Scholar]
- 10.Depew MJ, Simpson CA, Morasso M, et al. : Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207(5):501–61 10.1111/j.1469-7580.2005.00487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clouthier DE, Hosoda K, Richardson JA, et al. : Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–24 [DOI] [PubMed] [Google Scholar]
- 12.Ozeki H, Kurihara Y, Tonami K, et al. : Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121(4):387–95 10.1016/j.mod.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Ruest LB, Xiang X, Lim KC, et al. : Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development. 2004;131(18):4413–23 10.1242/dev.01291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuhara S, Kurihara Y, Arima Y, et al. : Temporal requirement of signaling cascade involving endothelin-1/endothelin receptor type A in branchial arch development. Mech Dev. 2004;121(10):1223–33 10.1016/j.mod.2004.05.014 [DOI] [PubMed] [Google Scholar]
- 15.Beverdam A, Merlo GR, Paleari L, et al. : Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis. 2002;34(4):221–7 10.1002/gene.10156 [DOI] [PubMed] [Google Scholar]
- 16.Depew MJ, Lufkin T, Rubenstein JL: Specification of jaw subdivisions by Dlx genes. Science. 2002;298(5592):381–5 10.1126/science.1075703 [DOI] [PubMed] [Google Scholar]
- 17.Depew MJ, Compagnucci C: Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zoolog B Mol Dev Evol. 2008;310(4):315–35 10.1002/jez.b.21205 [DOI] [PubMed] [Google Scholar]
- 18.Acampora D, Merlo GR, Paleari L, et al. : Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126(17):3795–809 [DOI] [PubMed] [Google Scholar]
- 19.Merlo GR, Paleari L, Mantero S, et al. : Mouse model of split hand/foot malformation type I. Genesis. 2002;33(2):97–101 10.1002/gene.10098 [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi H, He M, Wu P, et al. : A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013 10.1016/j.neuron.2011.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gitton Y, Cohen-Tannoudji M, Wassef M: Specification of somatosensory area identity in cortical explants. J Neurosci. 1999;19(12):4889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato T, Kurihara Y, Asai R, et al. : An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci U S A. 2008;105(48):18806–11 10.1073/pnas.0807345105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamarin A, Boyde A: Facial and visceral arch development in the mouse embryo: a study by scanning electron microscopy. J Anat. 1977;124(Pt 3):563–80 [PMC free article] [PubMed] [Google Scholar]
- 24.Depew MJ, Liu JK, Long JE, et al. : Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126(17):3831–46 [DOI] [PubMed] [Google Scholar]
- 25.Gitton Y, Benouaiche L, Vincent C, et al. : Dlx5 and Dlx6 expression in the anterior neural fold is essential for patterning the dorsal nasal capsule. Development. 2011;138(5):897–903 10.1242/dev.057505 [DOI] [PubMed] [Google Scholar]