Abstract
Vertebrates, including humans, can experience adverse effects from mercury consumed in fish. Humans often prefer large predatory fish that bioaccumulate high mercury levels. Recent attention has focused on the role of selenium countering mercury toxicity, but there is little research on the selenium:mercury molar ratios in freshwater fish. We examine selenium:mercury molar ratios in freshwater fish from Tennessee at Poplar Creek which receives ongoing inputs of mercury from the Department of Energy’s Oak Ridge Y-12 facility. Our objective was to determine variation of the ratios within species that might affect the protectiveness of selenium against mercury toxicity. Within species, the ratio was correlated significantly and positively with fish length only for two species. There was great individual variation in the selenium:mercury molar ratio within each species, except striped bass. The lack of a clear relationship between the selenium:mercury molar ratio and fish length, and the intraspecific variation, suggests that it would be difficult to use the molar ratio in predicting either the risk from mercury toxicity or in devising consumption advisories.
Keywords: mercury, selenium, molar ratios, fish, interspecific variability, risk management
Introduction
A primary goal of environmental management is to protect ecological and human health within a context of ecosystem sustainability. Restoration has become a central tool in ecosystem management, as has environmental remediation to remove or reduce the effects of toxic chemicals or radionuclides (Peterson et al. 2011). Determining potential effects of a contaminant on eco-receptors and humans involves understanding not only immediate lethality but also the effects of chronic exposure. While vulnerability, susceptibility, and bioavailability (Cabanero et al. 2007) affect response of organisms to toxic chemicals, other factors can reduce toxicity, such as the species or form of the contaminant, and the presence of other substances. Understanding these factors is an important aspect of understanding ecological health and risk.
Mercury is one of the most prevalent contaminants of concern at contaminated sites in the United States (ATSDR 2007). The Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA) requires that the National Oil and Hazardous Substances Pollution Contingency Plan develop a National Priorities List (NPL) of sites, also known as “Superfund Sites” with known or threatened releases of hazardous substances, pollutants, or contaminants throughout the United States (Federal Register 2012). CERCLA required the Environmental Protection Agency (EPA) and the Agency for Toxic Substances & Disease Registry (ATSDR) to prepare a list of priority substances. This list is prioritized based on frequency at NPL sites, toxicity, and potential for human exposure at these sites. The top three priority hazardous substances are arsenic, lead, and mercury (ATSDR 2007).
The primary source of mercury exposure for wildlife and humans is eating fish (Rice et al. 2000). Fish provide high quality protein as well as nutrients, yet many Americans are faced with deciding whether the benefits of eating fish out-weigh the risks from contaminants (Gochfeld and Burger 2005). High fishing rates occur in many cultures, in rural and urban areas (Burger et al. 2001a, b; Bienenfeld et al. 2003), among Native Americans (Harris and Harper 2000; Burger et al. 2007), and in other regions of the world (Burger et al. 2003; Lu et al. 2008; Hsiao et al. 2011). Fish are a low-fat source of protein that contributes to low blood cholesterol (Anderson and Wiener 1995), positive pregnancy outcomes, and better child cognitive test performances (Oken et al. 2008). Fish contain omega-3 (n-3) fatty acids that reduce cholesterol levels and the incidence of heart disease, blood pressure, stroke, and pre-term delivery (Kris-Etherton et al. 2002; Daviglus et al. 2002; Patterson 2002; Virtanen et al. 2008; Ramel et al. 2010). Presumably, other vertebrates also acquire benefits from fish consumption.
Yet, levels of methylmercury (MeHg), polychlorinated biphenyls (PCBs), and other contaminants in some fish are high enough to cause effects on the fish themselves, and on top-level predators, including humans (Eisler 1987; WHO 1989; NRC 2000; IOM 2006). Effects of high mercury exposure in humans include neurodevelopmental deficits (Steuerwald et al. 2000; NRC 2000), behavioral deficits in infants (JECFA 2003), poorer cognitive test performance (Oken et al. 2008; Freire et al. 2010), promotion of cardiovascular disease (Choi et al. 2009), and neurological and locomotory deficits (Hightower and Moore 2003). Mercury may counter some of the cardioprotective effects of omega-3 fatty acids (PUFAs) (Guallar et al. 2002; Roman et al. 2011). People with high fish intake may be at risk from chronic, high exposure to methylmercury (Grandjean et al. 1997). Trasande et al. (2005) estimated that 7.8–15% of fetuses in the US are in jeopardy from mercury exposure. Similarly, wildlife are affected adversely by high levels of mercury (Eisler 1987), especially piscivorous birds (Henry et al. 2002; Frederick and Jayasena 2010).
Methods of reducing the risks from mercury in the food chain include removal of contamination sources, blocking the movement through media, and modifying behavior of the receptors (by altering fish consumption patterns in the case of mercury exposure in humans). Thus, risk management for mercury toxicity of fish involves source reduction, physical remediation and restoration, dilution, and blocking the pathway to receptors (through physical or behavioral means).
Recently, attention has focused on the protective effects of selenium on mercury, particularly with respect to fish consumption. Selenium–mercury interaction may reduce the bioavailability or toxicity of methylmercury, and conversely some mercury toxicity may be due to impaired selenium-dependent enzyme synthesis or activity (Watanabe et al. 1999; Ralston 2008; Ralston et al. 2008). Cell culture studies and animal experiments show adverse impacts of high methylmercury exposure on selenoenzymes (Beyrouty and Chan 2006; Cabanero et al. 2007; Pinheiro et al. 2009). Mercury and methylmercury are irreversible selenoenzyme inhibitors (Carvalho et al. 2008) that impair selenoprotein form and function. Mercury binds to selenium with a high affinity, and high maternal exposure inhibits selenium-dependent enzyme activity in the brain (Berry and Ralston 2008). Moreover, selenium binds other cations—essential elements such as iron and zinc as well as xenobiotics such as cadmium. Mozaffarian (2009) reported that lower levels of nonfatal heart attacks are associated with higher levels of selenium.
Ralston and colleagues (Raymond and Ralston 2004; Ralston et al. 2007; Kaneko and Ralston 2007; Peterson et al. 2009a; Ralston 2008) have argued strongly that a molar excess of selenium in salt water fish, offers protection against mercury. They indicates that Se:Hg molar ratio are important considerations for risk assessment and management. Ralston (2010) argues that selenium level should be incorporated into risk evaluation for mercury. Although there is probably consensus that an excess of mercury over selenium is potentially hazardous, there is no consensus about what level of selenium in fish will reduce its toxicity. The question then becomes—what levels of selenium are necessary to protect against mercury? Ralston and colleagues (Ralston 2008; Peterson et al. 2009a) suggested that selenium:mercury molar ratios above 1 are probably protective for adverse mercury affects. But this assumes that the selenium and mercury are completely mutually bioavailable and do not interact with other substances.
We examine the intraspecific, interspecific, and geographical variability in selenium:mercury molar ratios in four species of fish from eastern Tennessee. We were interested in the degree of variation within and among species as it relates to risk assessment and risk management, and in whether the molar ratios differed between reference sites and heavily mercury-contaminated site (East Fork Poplar Creek).
The Oak Ridge Reservation (ORR, 14,200 ha) is one of the four main Department of Energy sites, with both an ongoing mission and a remediation mission. It is located along the Clinch River arm of Watts Bar Reservoir in eastern Tennessee. The majority of contaminants were released from the site prior to 1980, primarily in the 1950s and 1960s (Turner and Southworth 1999). Mercury also comes from atmospheric deposition from coal-burning electrical generation plants (Nichols et al. 2002). Elemental mercury was used extensively at Oak Ridge in lithium separation process, and huge quantities of waste mercury were released. Mercury drains off the site into streams, particularly the Upper Reach of the East Fork of Poplar Creek, where high levels have been found in fish. Eventually, the mercury reaches the Clinch River (Peterson et al. 2011). Although there has been over an 80% reduction in mercury concentration in the water, levels in fish have not declined as much (Southworth et al. 2011). Our paper does not address water quality or chemistry, but rather the potential for amelioration of the effects of mercury toxicity by selenium content of the fish in terms of the selenium: mercury ratios in fish tissue available for consumption by predators and people.
Methods
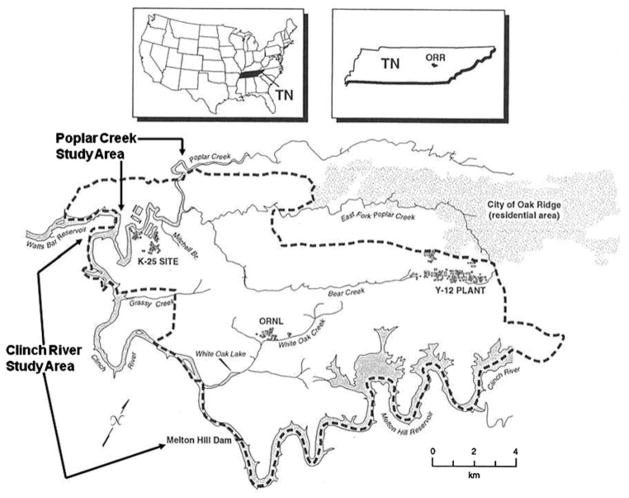
Fish were collected with fishing poles and seines in 2001–2002. We collected fish from three locations in Tennessee: (1) the lower 4-km reach of Poplar Creek within the ORR (contaminated site, Southworth et al. 2011), (2) the 1.6-km reach of the Clinch River below the Melton Hill Dam (Clinch River Mile 23.1 to 22.1), adjacent to ORR, and, (3) Little River, just downstream from the Great Smoky Mountains National Park near Townsend (reference site) (Fig. 1). Fish were collected under appropriate state fishing licenses or permits, and with protocol approvals from Rutgers University Animal Review Committee. All white bass (Morone chrysops) collected were large enough to be eaten by people (at least 22 cm in length). Stonerollers (Campostoma anomalum) are small fish that are eaten whole by a number of piscivorous birds and mammals. Further, stonerollers are used as bioindicators on ORR (Birge et al. 2000). Scientific names for species are given in Table 1. Once collected, fish were weighed and their total lengths measured before edible fillets were removed. One entire fillet was removed from each crappie and white bass, and a portion (at least 10 g) of a fillet was removed from each striped bass. Stonerollers were analyzed whole because of their small size, to be consistent with past methods for this species, and they are eaten whole by eco-receptors. The fillets were frozen and subsequently transported to the Environmental and Occupational Health Sciences Institute (EOHSI) for metals analysis.
Figure 1.
Map showing the location of Oak Ridge Reservation in Tennessee and the United States, with a larger map of Oak Ridge Reservation (within dotted line). The Y-12 plant was the source of much of the mercury to Poplar Creek. Fishing occurs on the Clinch River.
Table 1.
Mercury and selenium levels (ppm, wet weight) (μg/g) of freshwater species collected from the Department of Energy’s Oak Ridge Reservation and from Little River, Tennessee
| Common name | Scientific name | Location | n | Total length (Mean ± SE) | Mercury (Mean ± SE) | Se (Mean ± SE) | Hg (nmol/g wet wt.) | Se (nmol/g wet wt.) | Se:Hg molar ratio (Means) | Se:Hg ratio correlation with Hg τ (P) | Se:Hg ratio correlation with length τ (P) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oak Ridge Reservation | |||||||||||
| Central stonerollers | Campostoma anomalum | Poplar Creek | 20 | 10.8 ± 0.63 | 0.37 ± 0.05 | 0.61 ± 0.05 | 1.84 | 7.76 | 4.22 | −0.53 (0.001) | 0.42 (0.009) |
| Striped Bass | Morone saxatilis | Clinch River | 15 | 77.7 ± 3.07 | 0.30 ± 0.04 | 0.41 ± 0.03 | 1.48 | 5.21 | 3.53 | −0.49 (0.01) | −0.02 (NS) |
| White Bass | Morone chrysops | Poplar Creek | 15 | 29.4 ± 0.97 | 0.17 ± 0.02 | 0.57 ± 0.03 | 0.84 | 7.23 | 8.63 | −0.77 (< 0.0001) | 0.12 (NS) |
| Central stonerollers | Campostoma anomalum | Little River | 20 | 11.8 ± 0.30 | 0.12 ± 0.02 | 0.36 ± 0.05 | 0.61 | 4.60 | 7.50 | −0.68 (<0.0001) | 0.01 (NS) |
| White Bass | Morone chrysops | Clinch River | 15 | 31.9 ± 0.81 | 0.06 ± 0.01 | 0.73 ± 0.06 | 0.31 | 9.22 | 29.36 | −0.36 (0.07) | 0.05 (NS) |
| Crappie | Pomoxis spp. | Clinch River | 14 | 27.8 ± 0.59 | 0.05 ± 0.02 | 0.42 ± 0.03 | 0.25 | 5.26 | 21.09 | −0.62 (0.002) | −0.06 (NS) |
| Kruskal Wallis X2 (P) | 63.3 (<0.0001) | 43.6 (<0.0001) | 65.2 (<0.0001) | ||||||||
Given are arithmetic means ± SE.
At EOHSI, tissues were washed vigorously in deionized water alternated with acetone to remove external contamination (Walsh 1990). A 2 g sample was digested in ultrex ultrapure nitric acid in a microwave (MD 2000 CEM), using a digestion protocol of three stages of 10 min each under 50, 100, and 150 (3.5, 7, and 10.6 kg/cm2) pounds per square inch at 70× power. Digested samples were subsequently diluted in 100 ml deionized water. All laboratory equipment and containers were washed in 10% HNO3 solution prior to each use.
Selenium was analyzed by graphite furnace atomic absorption, and mercury was analyzed by the cold vapor technique (HGS-4) (Burger and Campbell 2004). Detection limits in ng/g were Hg = 0.2, Se = 0.7. All tissue concentrations are expressed in parts per million (ppm, μg/g on wet weight). In another study, we found that the dry weight ranged from 23 to 33% of the corresponding wet weight (i.e., water content of 67–77%) for 11 species of fish from the Savannah River (Burger et al. 2001a, b). All specimens were run in batches that included blanks, a standard calibration curve, and spiked specimens. A DORM-2 Certified dogfish tissue was used as the calibration verification standard. The accepted recoveries for spikes ranged from 85 to 115%. The coefficient of variation (C.V.) on replicate samples ranged from 2 to 7%. Further descriptions of methods can be found in Burger and Campbell (2004) and Burger et al. (2005). In fish, 90% or more is of total mercury is methylmercury (Luten et al. 1980).
We calculated the molar ratio by dividing concentration (in μg/g) by the molecular weight (200.59 for mercury and 78.96 for selenium). For each species, a mean selenium: mercury molar ratio was calculated from the average selenium and average mercury levels (see Table 1). This is the usual method used to determine molar ratios. This gives a different result from calculating a ratio for each individual and then taking the average. We also computed the molar ratio for each individual fish. Note that some papers report the mercury:selenium ratio rather than the selenium:mercury reported in this paper. Selenium:mercury is the reciprocal of mercury:selenium.
Kruskal–Wallis non-parametric one-way analysis of variance (generating a Chi-Square statistic) was used to examine differences among and within species. Kendall correlations were used to examine relationships between molar ratio and body length of fish. The level for significance was designated as P < 0.05.
Results
Interspecific Variation
There were significant interspecific variations in mercury and selenium levels, and in the selenium:mercury molar ratio (Table 1). In this table, stonerollers and white bass from two different locations are treated separately because mercury levels were very different, and the selenium:mercury molar ratios did not necessarily act the same way. There was not a significant correlation between mean selenium:mercury ratio and mean total body length for the fish species (Table 1).
Intraspecific Variation
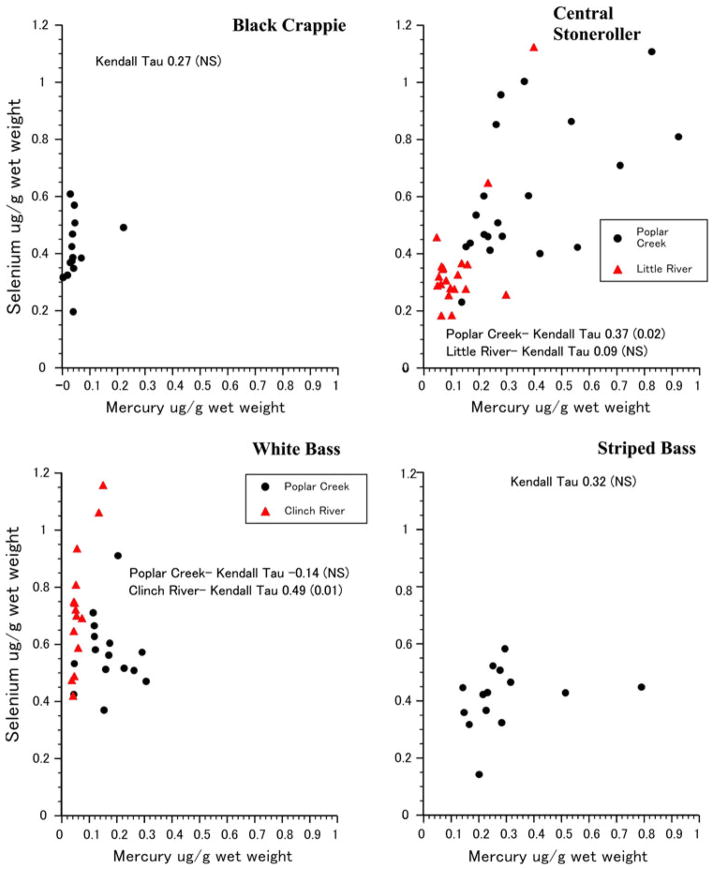
There was significant intraspecific variation in both the relationship of the selenium:mercury molar ratio and mean mercury levels (Fig. 2), and of the ratio with body length (Fig. 3). The relationship between selenium levels and mercury levels is shown in Fig. 2. For three species (crappie, white bass, striped bass), the relationship was not significant. There was a positive size:ratio relationship for stoneroller when all fish are considered together regardless of collection site (τ = 0.5, P < 0.0001). While the relationship of the two elements was significant for white bass from Clinch River (Fig. 2), it was not for all white bass combined (τ = −0.04).
Figure 2.
Individual variation in selenium levels as a function of mercury levels for four species of fish collected in Tennessee. The mean correlation for all stonerollers was 0.50 (P < 0.0001), and for white bass it was 0.04 (not significant).
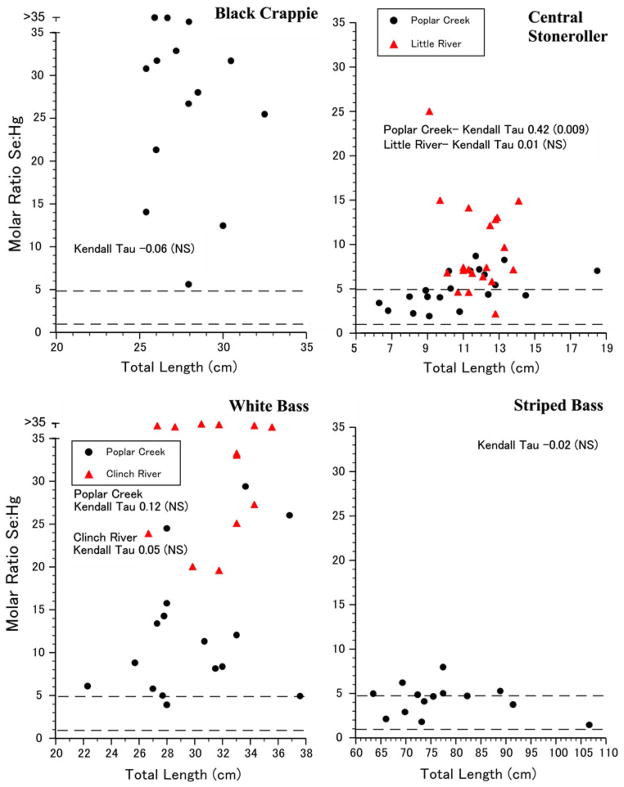
Figure 3.
Individual variation in selenium:mercury molar ratios as a function of total length for four species of fish collected in Tennessee. The mean correlation for all stonerollers was 0.28 (P < 0.01), and for white bass it was 0.26 (P < 0.05).
The mercury:selenium ratio was positively and significantly correlated with body length only when all white bass were considered together (τ = 0.26, P < 0.05, Fig. 3), for stonerollers from Poplar Creek (Table 1), as well as for all stonerollers (τ = 0.28, P < 0.01). All other correlations by locality were not significant perhaps due to small sample sizes.
For all species and locations, there were no individuals with a selenium:mercury molar ratio below 1. Despite the greater variation in length of striped bass (a range of over 40 cm) compared to the other species (ranges of less than 16 cm), the lowest variation in the selenium:mercury molar ratio occurred in this species. Both striped bass and stoneroller had a significant number of individuals with Se:Hg molar ratios ranging from 1 to 5.
Discussion
Physiological Limits of the Selenium:Mercury Ratio
Our intent in this paper is to examine how the selenium: mercury molar ratios varies in fish, as authors generally give only mean selenium:mercury molar ratios for each fish species. There are few data for these ratios in marine fish, and even fewer for freshwater fish.
It was not the intent of the paper to examine either the physiological limits of the selenium:mercury molar ratio, the toxicodynamics of either selenium or mercury in the body, or the relative protective value of particular selenium: mercury molar ratios to toxicity in fish. We comment only briefly on issue of the molar ratio, the meaning of 1:1, and current research issues with the use of these ratios. Ralston and colleagues (Raymond and Ralston 2004; Ralston et al. 2007; Ralston 2008; Berry and Ralston 2008, Peterson et al. 2009a; Ralston and Raymond 2010) have emphasized the importance of selenium as protective against mercury toxicity in fish and from fish, and have suggested that as long as there is excess selenium (molar ratio >1), toxicity is likely to be low, the protectiveness increasing as selenium rises, but not to the point of selenium toxicity (Ralston et al. 2007). Ralston (2010) suggested that selenium needs to be included in mercury risk assessment.
Although the protective effect of selenium against mercury has been known for more than 40 years, the mechanisms and quantitative aspects of their interaction are not well known. Ralston et al. (2007) observed that the molar ratio in the rat brain was not directly related to the molar ratio in the diet. Although some mercury toxicity is mediated by interfering with essential selenoenzymes and is countered by excess selenium, how much of an excess is needed is uncertain. Moreover, it is easy to forget that selenium itself can be toxic, and that mercury can actually confer some protection (Heinz et al. 2012). A 1:1 molar ratio is not a “bright line” between safety and harm. If there were universal and mutual bioavailability such that all selenium in the body could and did bind to all of the mercury (molar ratio = 1:1), there would be inadequate selenium to synthesize essential enzymes. So clearly the protective molar ratio must be >1. But how much greater is not at all clear, nor is it clear if complete protection against a high level of mercury could be conferred at any ratio. Studies of different forms of mercury and selenium will enrich our understanding of this important issue.
Interspecific Variation
There are very few papers that examine the mean selenium: mercury molar ratios in muscle of a range of freshwater fish (Cappon and Smith 1981), except for recent work in the Amazon basin where selenium levels are low in fish, but high in other foods such as Brazil nuts (Lemire et al. 2010). In this study of four species of fish from Tennessee, there were significant interspecific differences in the mean levels of mercury and selenium, and in the selenium:mercury molar ratio. The lowest ratios were in stonerollers and striped bass (the smallest and the largest species we examined), and the highest were in white bass and crappie.
The selenium:mercury molar ratio is most dependent upon the levels of mercury in the denominator, since selenium is an essential trace element and is physiologically regulated (Eisler 2000; ATSDR 2003). Usually, mercury levels are dependent upon size (both of the species generally, and of individuals within species) and trophic level relationships (Snodgrass et al. 2000; Burger et al. 2007; Burger 2009; Lowenstein et al. 2010). In our study, the top-level predator, striped bass, had the highest mercury levels, and not surprisingly the lowest selenium:mercury ratio of the four fish species.
Differences in mercury levels also depend upon season, especially in freshwater fish where water levels vary (Hylander et al. 2000), or in fish species that migrate (Burger 2009). Mercury levels (as well as selenium) can also vary as a function of differences in discharges, spills or changes in water flow or movement on contaminated sites. For example, on East Fork Poplar Creek on Oak Ridge Reservation, mercury levels in sunfish varied by size and season (Southworth et al. 2011). In turn, remedial actions, such as have occurred at Oak Ridge, have resulted in decreased mercury levels in fish (Ryan 2011).
Stonerollers are small minnows that serve as prey for many other species, and might be expected to be at the bottom of the food chain. In this case, however, the stonerollers with the lowest mean selenium:mercury molar ratio (and the highest mean mercury level) were from Poplar Creek, a site known for its mercury contamination (ORR 2011). Since stonerollers are eaten by a wide range of predatory fish, it could account for relative high mercury levels in their predators. Another explanation for the low selenium:mercury levels as a function of high mercury concentration in stonerollers is that if food is limiting (as it might be in a contaminated site such as Poplar Creek), growth may be slower allowing longer time for a fish to accumulate mercury (Downs et al. 1998).
In contrast, the mean selenium:mercury molar ratios were rather high for crappie and white bass from Clinch River, although the ratio was low for the latter species from Poplar Creek. This suggests that in general, these ratios (together with the relatively low mercury levels) would produce few toxic effects from mercury. The lower ratio in white bass from Poplar Creek, however, might be a problem except that mercury levels were also relatively low (only a very few individuals had levels above 0.3).
Intraspecific Variation
While there are papers that examine the molar ratios in fish (saltwater and fresh), there are none that present data for individual fish. The mean selenium:mercury molar ratio for individual fish is useful, especially for species that are either below a postulated level thought to be protective against mercury toxicity, or those that are well above a protective level. It is useful to know the number of individual fish with molar ratios either above (protective) or below some postulated protective level, even though the protective level is unclear for either eco-receptors or humans (see following section). Molar ratios may vary substantially by water body due to mercury contamination (this study) or selenium contamination (Reash 2012).
The results from this paper show variation in the selenium:mercury ratios for individuals of the same species, suggesting that even within a given species, eco-receptors and humans could be exposed to differences in relative mercury toxicity because of the protective effects of selenium. Size itself is not a sufficient indicator of the selenium: mercury ratio because it does not always predict the ratio. However, for both stonerollers (eaten mainly by eco-receptors such as other fish or birds) and white bass, the selenium:mercury ratio increased with fish size. Further, it is clear that most of the fish in this study had selenium: mercury ratios above 1.
Freshwater Versus Saltwater Fish and Selenium:Mercury Molar Ratios
Considerable attention has been devoted to mercury levels and associated potential toxicity. Large predatory fish such as shark, swordfish, and tuna can bioaccumulate high levels of mercury. Attention is now focusing on co-exposure to selenium (Ralston and Raymond 2010; Burger and Gochfeld 2011). Because there are both small herbivorous fish and large predatory fish in bays, estuaries, and the ocean, the range in the ratios for marine fish varies markedly: (1) 0.46–17.6 for 15 species from Hawaii (Kaneko and Ralston 2007), (2) 0.58–12.5 for 11 commercial species (Cappon and Smith 1981; but they selected mainly predatory species and evaluated only 1–4 individuals/species, (3) 3–22 for four species from Spain and Portugal (Cabanero et al. 2004), (4) 2.0 to 17.3 for three species from Spain (Cabanero et al. 2007), and (5) 0.36 to over 60 for 19 recreationally caught species from New Jersey (Burger and Gochfeld 2011).
Mercury is a contaminant of concern for freshwaters throughout the US. Coal-fired power plants are a major source leading to atmospheric transport and wet deposition, and the acidification of freshwater increases methylation (Gilmour and Henry 1991). Since mercury levels also can be high in freshwater fish, and nearly all states in the US have consumption advisories for wild-caught fish (most because of mercury, USEPA 2009), it is useful to consider the selenium:mercury molar ratios as a possible mechanism for reduction of mercury toxicity.
Data on selenium:mercury molar ratios from freshwater fish are limited, partly because the emphasis has been on mercury levels that pose a risk to human consumers, and many contaminant studies of freshwater fish report only mercury levels. Studies in freshwater fish have found selenium:mercury ratios to vary from: (1) 0.21–3.17 for seven species from the Madeira River, Amazon Region (Dorea et al. 1998), (2) 2.22–54.33 for ten species from western US streams (Peterson et al. 2009a), (3) 4.16–6.25 in three species from Finland (Kari and Kauranen 1978), (4) 0.51–3.70 for six species from lakes in New York (Cappon and Smith 1981), 0.68–12.51 in 11 species from the Savannah River in the southern US (Burger 2012). In this study of four species, the ratios ranged from 3.53 to 39.4. In a study of nine species from three fly ash contaminated sites in the Ohio River drainage, where selenium was the main contaminant of concern, Reash (2012) found Se:Hg ratios of 0.56–68.3 in two highly contaminated sites, 0.25–43.4 in three species at two medium contaminated sites, and 0.12–0.31 at two sites and 0.12–0.33 at three sites with low or no selenium contamination. In these studies the ranges of molar ratios across species was rather low, except for the study of Peterson et al. (2009b) and Reash (2012). The large variation in the Peterson study was no doubt due to the large geographical area studied (several states) and large number of species. The Reash study was conducted in waters with high selenium contamination.
Three features are apparent from the present study and from the data reported above for freshwater fish: (1) the ranges of selenium:mercury molar ratios are smaller than those from saltwater fish, (2) both freshwater and marine fish can have mean species selenium:mercury molar ratios above and below 1:1, and (3) in our studies, the selenium: mercury molar ratio for saltwater species was inversely related to fish size (Burger and Gochfeld 2011), while for freshwater fish, the relationship was positive only for two species (this study). The latter observation requires considerably more study from different freshwater systems.
The important point, however, is that for both freshwater and saltwater fish the mean selenium:mercury molar ratio for some species is <1, and for all species there is a great deal of individual variation. While attention is needed to the ways in which selenium may confer protection and the ratios that might be protective, it is valuable to understand the intra- and interspecific variability in the ratios in edible fish tissue. Variability will impact any risk communication about selenium and mercury.
Implications for Ecological Health
It is important to mention that the fish represented in this study were collected 10 years ago. Thus, they do not represent the current levels of mercury in fish from East Fork Poplar Creek on ORR. An extensive biomonitoring program has tracked decreases in contaminants in this creek (Peterson et al. 2011). In general, most contaminants have decreased markedly with remediation, especially in the water column and in sediments. Mercury levels in fish, however, have not decreased as much as might be expected by the decreased levels in water and sediment (Southworth et al. 2011). It should be mentioned, however, that the mercury levels in fish from East Fork Poplar Creek decreased from 1988 to about 2000, and then have remained stable (ORR 2011). Thus, there is still a risk of mercury to the fish themselves, and to other eco-receptors within that eco-system, including predatory birds and mammals.
It was thus not the intent of this study to comment on current levels of mercury in fish from ORR or elsewhere in Tennessee, but rather to examine selenium:mercury molar ratios and variation of these ratios within fish species that might have implications for other fish, birds or mammals that consume them. Implications of the selenium:mercury molar ratio relate to protection against mercury toxicity because of the presence of excess selenium if the molar ratios are over 1:1 (Ralston 2008; Berry and Ralston 2008, Peterson et al. 2009a). Leaving aside the issue of the molar ratio that is protective against mercury toxicity, and the issue of mercury toxicity that occurs independently of levels of selenium (Lemire et al. 2010), sufficient selenium provides some protection from mercury toxicity in laboratory animal studies (Lindh and Johansson 1987; Berry and Ralston 2008). There are no animal studies in the wild of the protective effects of selenium on mercury, although there are many studies of adverse effects of high levels of selenium (Dalton and Bird 2003). However, it should be noted that stonerollers, with a relatively low selenium: mercury ratio (4.22 at Poplar Creek) are small minnows that are eaten by a many predators, including fish and birds (Burger et al. 2005).
The selenium:mercury molar ratio could have implications for people who consume large quantities of fish, particularly those with high mercury levels. Although fishing is prohibited in Poplar Creek (with posted signs), there is still some fishing there, and the Clinch River is a popular fishing and recreational site (Campbell et al. 2002). High income white recreational anglers had significantly higher consumption rates than did African American anglers interviewed on the Clinch River (Burger and Campbell 2008). Of the anglers interviewed, 64% ate crappie, 23% ate striped bass, and only 8% ate white bass (no one eats stonerollers as they are small minnows eaten by eco-receptors).
Ralston and colleagues (Ralston 2008; Peterson et al. 2009a) have suggested that even if people eat fish traditionally considered high in mercury, as the amount of selenium relative to mercury increases (selenium:mercury molar ratios above 1) mercury toxicity is likely to decrease. Partly their conclusion is based on recent animal studies that demonstrate such an effect (Berry and Ralston 2008). The implications are that saltwater fish that have high mercury levels (above the guidance of the FDA), but also have high selenium:mercury ratios, do not pose as great a problem for human consumption because the selenium will be protective against mercury toxicity. However, there is no consensus on the level of selenium required to protect against mercury toxicity. Moreover, protection conferred in one species or organ system may not be applicable to all organs or ages (Ralston et al. 2008). Peterson et al. (2009b) suggested the benchmarks in humans and wildlife for the toxicity of mercury alone may exaggerate mercury toxicity potentials compared to an assessment that is also based on selenium:mercury molar ratios. Further, the protective level for other eco-receptors (birds, mammals) is unclear and requires further study. As Ralston (personal communication) has recently noted, there are many fruitful areas of research that are essential before the ratios can be used effectively for risk communication.
Acknowledgments
The authors especially thank D. Mergler, A. Stern, R. Schoeny, M. Lemire, M. Peterson, E. Pierce, and N. Ralston for valuable discussions about selenium and mercury interactions. Several people contributed to the initial study examining contaminant levels in fish from Oak Ridge Reservation, including K.R. Campbell, T.S. Campbell, R.J. Dickey, and R. Sexton. This research was funded by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (AI # DE-FC01-95EW55084, DE-FG 26-00NT 40938, DE-FC01-06EW07053), NIEHS (P30ES005022), and EOHSI.
Footnotes
The results, conclusions, and interpretations presented in this paper are solely the responsibility of the authors, and should not in any way be interpreted as representing the funding agencies.
References
- Anderson PD, Wiener JB. Eating fish. In: Graham JD, Wiener JB, editors. Risk Versus Risk: Tradeoffs in Protecting Health and the Environment. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed Mar 24, 2012];Toxicological Profile:selenium. 2003 http://www.atsdr.cdc.gov/toxprofiles/tp92-p.pdf.
- Agency for Toxic Substances and Disease Registry (ATSDR) CERCLA Priority List of Hazardous Substances that will be the subject of toxicological profiles and support documentations. ATSDR and EPA, U.S. Department of Health and Human Services; Atlanta, GA: 2007. [Google Scholar]
- Berry MJ, Ralston NV. Mercury toxicity and the mitigating role of selenium. Ecohealth. 2008;5:456–459. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Chan HM. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicology and Teratology. 2006;28:49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Bienenfeld LS, Golden AL, Garland EJ. Consumption of fish from polluted waters by WIC participants in East Harlem. Journal of Urban Health. 2003;80:349–358. doi: 10.1093/jurban/jtg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge WJ, Price DJ, Shaw JR, Spromberg JA, Wigginton C, Hogstrand C. Metal body burden and biological sensors as ecological indicators. Environmental Toxicology and Chemistry. 2000;19:1199–1212. [Google Scholar]
- Burger J. Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: size, season and geographical effects. Environmental Research. 2009;109:803–811. doi: 10.1016/j.envres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J. Selenium:mercury molar ratios in fish from the Savannah River: implications for risk management. Journal of Risk Research. 2012;2012:1–18. [Google Scholar]
- Burger J, Campbell Species differences in contaminants in fish on and adjacent to the Oak Ridge Reservation, Tennessee. Environmental Research. 2004;96:145–155. doi: 10.1016/j.envres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Burger J, Campbell KR. Fishing and consumption patterns of anglers adjacent to the Oak Ridge Reservation, Tennessee: higher income anglers ate more fish and are more at risk. Journal Risk Research. 2008;11:335–350. [Google Scholar]
- Burger J, Gochfeld M. Mercury and selenium levels in 19 species of salt water fish from New Jersey as a function of species, size and season. Science Total Environment. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M. Ethnic differences in risk from mercury among Savannah River fishermen. Risk Analysis. 2001;21:533–544. doi: 10.1111/0272-4332.213130. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Boring CS, Stephens WL, Jr, Snodgrass J, Gochfeld M. Mercury and selenium in fish from the Savannah River: species, trophic level, and locational differences. Environmental Research. 2001;87:108–118. doi: 10.1006/enrs.2001.4294. [DOI] [PubMed] [Google Scholar]
- Burger J, Fleischer J, Gochfeld M. Fish, shellfish, and meat meals of the public in Singapore. Environmental Research. 2003;93:254–261. doi: 10.1016/s0013-9351(03)00015-x. [DOI] [PubMed] [Google Scholar]
- Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environmental Health Perspectives. 2005;113:266–271. doi: 10.1289/ehp.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, et al. Mercury levels and potential risk from subsistence foods from the Aleutians. Science of the Total Environment. 2007;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cabanero AJ, Carvalho C, Madrid Y, Batoreu C, Camara C. Quantification and speciation of mercury and selenium in fish samples of high consumption in Spain and Portugal. Biological Trace Element Research. 2004;101:1–19. doi: 10.1385/BTER:103:1:017. [DOI] [PubMed] [Google Scholar]
- Cabanero AI, Madrid Y, Camara C. Mercury–selenium species ratio in representative fish samples and their bioaccessibility by an in vitro digestion method. Biological Trace Element Research. 2007;119:195–211. doi: 10.1007/s12011-007-8007-5. [DOI] [PubMed] [Google Scholar]
- Campbell KR, Dickey RJ, Sexton R, Burger J. Fishing along the Clinch River arm of Watts Bar Reservoir adjacent to the Oak Ridge Reservation, Tennessee: behavior, knowledge and risk perception. Science Total Environment. 2002;299:145–161. doi: 10.1016/s0048-9697(02)00276-0. [DOI] [PubMed] [Google Scholar]
- Cappon CJ, Smith JC. Mercury and selenium content and chemical form in fish muscle. Archives of Environmental Contamination and Toxicology. 1981;10:305–319. doi: 10.1007/BF01055632. [DOI] [PubMed] [Google Scholar]
- Carvalho CML, Chew EH, Hashemy LI, Lu J, Holmgren A. Inhibition of the human thiore-doxin system: a molecular mechanism of mercury toxicity. Journal of Biology and Chemistry. 2008;283:11913–11923. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen T, Murata K, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environmental Health Perspectives. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton C, Bird P. Risk assessment for the consumption of fish with elevated selenium levels. NSW Public Health Bulletin. 2003;14:174–176. doi: 10.1071/nb03050. [DOI] [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comments on Toxicology. 2002;8:345–374. [Google Scholar]
- Dorea JG, Moreira MB, East G, Barbosa AC. Selenium and mercury concentrations in some fish species of the Madeira River, Amazon Basin, Brazil. Biological Trace Element Research. 1998;65:211–220. doi: 10.1007/BF02789097. [DOI] [PubMed] [Google Scholar]
- Downs SG, Macleod CL, Lester JN. Mercury precipitation and its relation to bioaccumulation in fish: a literature review. Water Air Soil Pollution. 1998;108:149–187. [Google Scholar]
- Eisler R. Mercury hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service; Washington, DC: 1987. Report 85 (1.10) [Google Scholar]
- Eisler R. Selenium. Handbook of Chemical Risk Assessment: Health Hazards to Humans, Plants, and Animals. Vol. 3. CRC Press; Boca Raton: 2000. [Google Scholar]
- Federal Register. Environmental Protection Agency; 2012. [Accessed Mar 15, 2012]. National Priorities List, final rule No. 53. [Google Scholar]
- Frederick P, Jayasena N. Altered pairing behaviour and reproductive success in white ibises exposed to environmentally relevant concentrations of methylmercury. Proceedings of the Royal Society. 2010 doi: 10.1098/rspb.2010.2189. doi:10:1098/rspb.2010.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Ramos R, Lopez-Expinosa M, Diez S, Vioque J, Ballester F, et al. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environmental Research. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Gilmour C, Henry E. Mercury methylation in aquatic systems affected by acid deposition. Environmental Pollution. 1991;71:131–169. doi: 10.1016/0269-7491(91)90031-q. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit-risk by dose curve. Neurotoxicology. 2005;26:511–520. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology. 1997;19:418–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gómez-Aracena J, et al. Heavy Metals and Myocardial Infarction Study Group. New England Medical Journal. 2002;28:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Using eco-cultural dependency webs in risk assessment and characterization of risks to tribal health and cultures. Environmental Science and Policy Research. 2000;2:91–100. [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR. A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Archives of Environmental Contamination and Toxicology. 2012;62:519–528. doi: 10.1007/s00244-011-9717-4. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA. Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicology. 2002;11:213–231. doi: 10.1023/a:1016327602656. [DOI] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environmental Health Perspectives. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H, Ullrich SM, Tanton TW. Burdens of mercury in residents of Temirtau, Kazakhstan. 1: hair mercury concentrations and factors of elevated hair mercury levels. Science of the Total Environment. 2011;409:2272–2280. doi: 10.1016/j.scitotenv.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Hylander L, Pinto F, Guimaraes J, Meili M, Oliveira L, Castro E. Fish mercury concentration in the Alto Pantanal, Brazil: influence of season and water parameters. Science of the Total Environment. 2000;261:9–20. doi: 10.1016/s0048-9697(00)00591-x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Seafood Choices: Balancing benefits and risks. Washington, DC: National Academy Press; 2006. [Google Scholar]
- [Accessed March 2005];JECFA Joint FAO/WHO Expert committee on food additives. 2003 www.who.int/pcs/jecfa/jecra-htm.
- Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biological Trace Element Research. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- Kari T, Kauranen P. Mercury and selenium contents of seals from fresh and brackish waters in Finland. Bulletin of Environmental Contamination and Toxicology. 1978;19:273–280. doi: 10.1007/BF01685798. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Lemire M, Fillion M, Frenette B, Mayer A, Philbert A, Passos CJC, Guimaraes JRD, Barbosa F, Jr, Mergler D. Selenium and mercury in the Brazilian Amazon: opposing influences on age-related cataracts. Environmental Health Perspectives. 2010;118:1584–1589. doi: 10.1289/ehp.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein JH, Burger J, Jeitner CW, Amato G, Kolokotronis SO, Gochfeld M. DNA barcodes reveal species-specific mercury levels in tuna sushi that pose a health risk to consumers. Biology Letters. 2010;6:692–695. doi: 10.1098/rsbl.2010.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luten JB, Ruiter A, Ritskes MM, Rauchbaar AB, Riekwel-Body G. Mercury and selenium in marine and freshwater fish. Journal of Food Science. 1980;45:416–419. [Google Scholar]
- Lindh U, Johansson E. Protective effects of selenium against mercury toxicity as studied in the rat and kidney by nuclear analytical techniques. Biological Trace Element Research. 1987;12:109–120. doi: 10.1007/BF02796669. [DOI] [PubMed] [Google Scholar]
- Lu YZ, Yan BX, Wang MJ, Guo LY. The evolution rule and ecology risk assessment of mercury in fish of Gonghua River. Journal of Agro-Environmental Science. 2008;27:2430–2433. [Google Scholar]
- Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. International Journal of Environmental Research and Public Health. 2009;6(6):1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) Toxicological Effects of Methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Nichols AC, Murray TP, Richardson TD. Mercury accumulation in catfish (Ictalurus furcatus and I. punctatus) from southwestern Tennessee River Valley. Southeast Naturalist. 2002;1:159–168. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. American Journal of Epidemiology. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORR (Oak Ridge Reservation) Annual Site Environmental Report. Oak Ridge, TN: Oak Ridge Reservation; 2011. [Google Scholar]
- Patterson J. Introduction—comparative dietary risk: balance the risks and benefits of fish consumption. Comments in Toxicology. 2002;8:337–344. [Google Scholar]
- Peterson MJ, Efroymson RA, Adams SM. Long-term biological monitoring of an impaired stream: synthesis and environmental management implications. Environmental Management. 2011;47:1125–1140. doi: 10.1007/s00267-011-9665-9. [DOI] [PubMed] [Google Scholar]
- Peterson SA, Ralston NV, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS. How might selenium moderate the toxic effects of mercury in stream fish of the western U.S.? Environmental Science and Technology. 2009;43:3919–3925. doi: 10.1021/es803203g. [DOI] [PubMed] [Google Scholar]
- Peterson SA, Ralston NVC, Whanger PD, Oldfield JE, Mosher WD. Selenium and mercury interactions with emphasis on fish tissue. Environmental Bioindicators. 2009;4:318–334. [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, Aschner M. Mercury and selenium—a review on aspects related to the health of human populations in the Amazon. Environmental Bioindicators. 2009;4:222–245. doi: 10.1080/15555270903143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston NV. Selenium health benefit values as seafood safety criteria. Ecohealth. 2008;5:442–455. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston NV. Mercury in canned tuna: the importance of selenium. Environmental Toxicology and Chemistry. 2010;29:2133–2134. doi: 10.1002/etc.294. [DOI] [PubMed] [Google Scholar]
- Ralston NV, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Ralston NV, Blackwell JL, 3rd, Raymond LJ. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biological Trace Element Research. 2007;119:255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–811. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrition. 2010;26:168–174. doi: 10.1016/j.nut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Mercury:selenium interactions and health implications. Seychelles Medical and Dental Journal. 2004;17:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Reash RJ. Selenium, arsenic, and mercury in fish inhabiting a fly ash exposure gradient: inter-specific bioaccumulation patterns and elemental associations. Environmental Toxicology and Chemistry. 2012;31:739–747. doi: 10.1002/etc.1745. [DOI] [PubMed] [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of U.S. EPS’s oral reference dose (RfD) for methylmercury. Drug and Chemical Toxicology. 2000;23:41–54. doi: 10.1081/dct-100100101. [DOI] [PubMed] [Google Scholar]
- Roman HA, Walsh TL, Coull BA, Dewailly É, Guallar E, Hattis D, Mariën K, Schwartz J, Stern AH, Virtanen JK, Rice G. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environmental Health Perspectives. 2011;119:607–614. doi: 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MG. Recovery of fish communities in a warm water stream following pollution abatement. Environmental Management. 2011;47:1096–1111. doi: 10.1007/s00267-010-9596-x. [DOI] [PubMed] [Google Scholar]
- Snodgrass JW, Jagoe CH, Bryan AL, Jr, Burger J. Effects of trophic status, and wetland morphology, hydroperiod and water chemistry on mercury concentrations in fish. Canadian Journal of Fish Aquatic Science. 2000;57:171–180. [Google Scholar]
- Southworth GR, Peterson MJ, Roy WK, Mathews TJ. Monitoring fish contaminant responses to abatement actions: factors that affect recovery. Environmental Management. 2011;47:1064–1076. doi: 10.1007/s00267-011-9637-0. [DOI] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. Journal of Pediatrics. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methylmercury toxicity to the developing brain. Environmental Health Perspectives. 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RR, Southworth GR. Mercury-contaminated industrial and mining sites in North America: an overview of selected case studies. In: Ebinghaus R, Turner RR, editors. Mercury Contaminated Sites. Berlin: Springer; 1999. pp. 89–112. [Google Scholar]
- USEPA. The National Listing of Fish Advisories. 2009 http://water.epa.gov/scitech/swguidance/fishshellfish/fishadvisories/
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. American Journal of Clinical Nutrition. 2008;88:1618–1625. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PM. The use of seabirds as monitors of heavy metals in the marine environment. In: Furness RW, Rainbow PS, editors. Heavy Metals in the Marine Environment. Boca Raton, FL: CRC Press; 1990. pp. 183–204. [Google Scholar]
- Watanabe C, Yoshida K, Kasanuma Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environmental Research. 1999;80:208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Mercury-Environmental Aspects. Geneva: WHO; 1989. [Google Scholar]