Abstract
France, Germany, Sweden, and the United Kingdom each use different types of policies for controlling prescription drug spending. Until recent years, these policies have relied heavily on regulating prices charged by drug manufacturers, with different systems providing varying degrees of pricing freedom. While these policies appear to have brought some degree of price restraint, they have not prevented continued growth in prescription drug spending. As a result, each country is supplementing its policies with measures aimed at physicians and consumers and targeted at reducing a perceived over-utilization of pharmaceutical products.
Introduction
While the United States has traditionally allowed the free market to determine drug prices, the rising cost of prescription drugs has increased the financial burden on vulnerable segments of the U.S. population. In addition, widely reported disparities in prescription drug prices between the United States and other industrialized countries have heightened interest in policies to control pharmaceutical prices. In response to this situation, some members of Congress have proposed Federal regulations limiting prescription drug prices. However, critics of pharmaceutical price regulation, within and outside the pharmaceutical industry, have asserted that U.S. adoption of regulations that reduce drug prices would cripple U.S. pharmaceutical companies' ability to develop life-saving and life-improving drugs. Because the United States has not regulated drug prices in the past, our country's experience does not provide the evidence necessary to evaluate the potential effectiveness of drug price regulations. Several European countries, however, have employed government policies to control pharmaceutical prices and, indirectly, expenditures.
Four European countries that have research-based pharmaceutical industries—France, Germany, Sweden, and the United Kingdom—have each developed a set of government controls to limit the growth of prescription drug prices and expenditures. As part of their national health insurance systems, these four countries provide universal prescription drug benefits, and each faces a continuing challenge to restrain increases in national spending on pharmaceuticals. In this persistent struggle, each country has developed spending control strategies consistent with two premises: first, that drug manufacturers can, if left unchecked by regulation, charge prices substantially above their marginal (or incremental) costs, because patents and marketing efforts protect them from competitors; and second, that insurance coverage and physician responsibility for prescribing discourage comparison shopping by consumers, who lack incentives to seek out the most cost-effective drugs and have limited knowledge about alternative medications. In designing approaches to dampen pharmaceutical spending, governments have tended to rely more on regulations and sanctions than on policies to strengthen competition and sharpen incentives.
The scope of pharmaceutical cost containment strategies is broad, targeting not only price but other determinants of drug spending. At least until the late 1980s, however, efforts to restrain drug prices had focused largely on controls at the point of sale—that is, at the prices charged, for example, by drug manufacturers to drug wholesalers, or by pharmacists to consumers. These traditional policies seem to have restrained prices, but increases in drug utilization and higher prices for new drugs have pushed up drug spending. Faced with this further stress on their national health care budgets, government officials in the countries we studied have concluded that, as a tool for restraining pharmaceutical spending, controls on prices alone are not sufficient. As a result, each country has introduced or is developing a distinctive set of policies to supplement its traditional regulatory approach. These policies are designed to reduce the growth in prescriptions written, encourage the use of drugs that are more cost-effective, and shift some of the burden of higher drug spending from the national health insurance system to consumers, physicians, and drug manufacturers.
This article reviews the cost-containment policies of these four countries and their effects on prescription drug prices and spending levels. The analysis is drawn from a more extensive review of these countries' policies done by the U.S. General Accounting Office (1994a). This article focuses on the pharmaceutical prices and spending control policies that have been adopted by these countries. It does not evaluate the potential effect of these policies on drug research and development such issues are examined in the larger U.S. General Accounting Office report.
Drug Prices and Affordability
The United States, while the world's leader in new drug development, is also a leader in drug prices. Drug prices have driven much of the increase in total outpatient expenditures on prescription drugs in the United States. Drug expenditures nearly doubled between 1980 and 1991 (from $15.8 billion to $29.2 billion), even after adjusting for inflation.1 Much of this increase was driven by increases in prescription drug prices, which rose by more than twice the rate of inflation between 1980 and 1991.2
American health care consumers in general are particularly sensitive to these increases because of the high proportion of drug expenditures that are not covered by health insurance. While outpatient prescription drugs are a relatively small amount of total health care costs—less than 5 percent in 1991—more than one-half of this amount is paid out of pocket (compared with 18.1 percent of spending for physician services and 3.4 percent for hospital care [Letsch, 1993]). The greatest burden of these out-of-pocket costs is likely to fall on the elderly, who as a group both use more drugs and are less likely to have insurance coverage for those drugs, because the Federal Medicare program does not offer outpatient prescription drug coverage.
As several recent studies show, prescription drug prices in other countries are generally lower than in the United States (U.S. Government Accounting Office, 1992, 1994b; Association Belge des Consommateurs, 1989; Reekie, 1984). Some of these countries have relatively little drug research and development, but others have relatively strong innovative drug industries. For example, France, Germany, Sweden, and the United Kingdom are home to firms that developed more than 25 percent of new drug entities from 1970 to May 1992 (Redwood, 1993).
Affordability of drugs to individual consumers is not as much of a problem in these other industrialized countries as it is in the United States. Many of these countries have universal health insurance systems that provide pharmaceutical drug coverage at little or no out-of-pocket cost to consumers.3
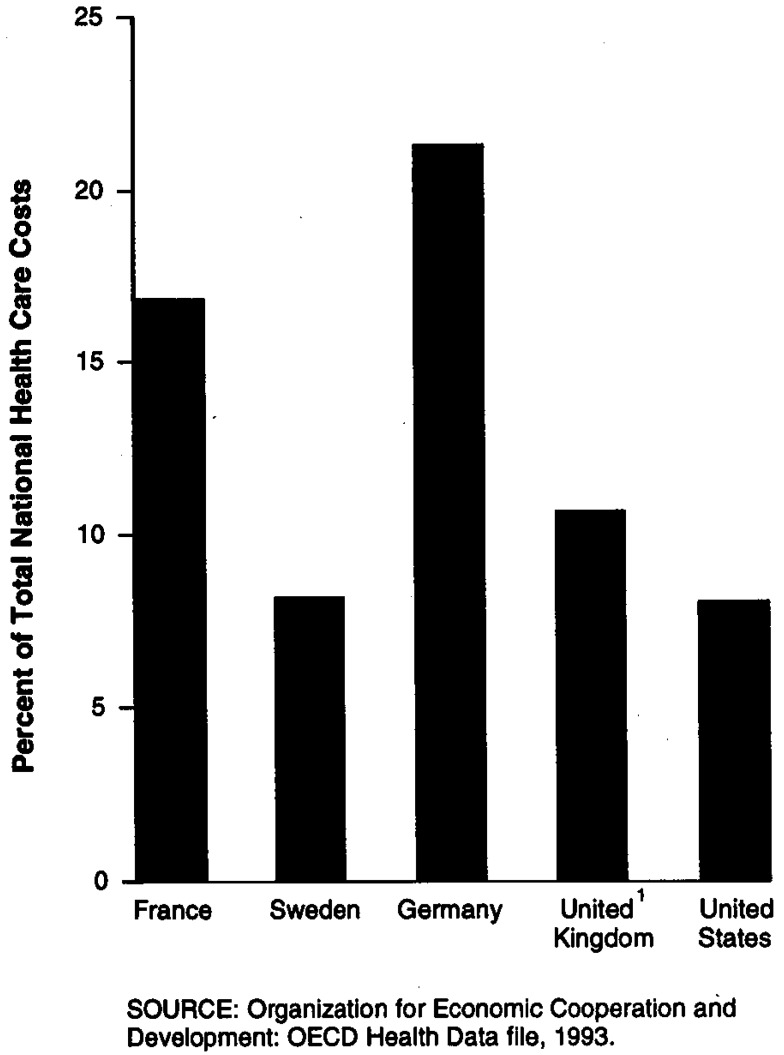
Universal drug coverage has shifted the burden of paying for drugs from the individual to the insurance system, thereby creating an incentive for the government to restrain spending growth and to maintain the fiscal stability of the health insurance system. In addition, the relatively high level of drug spending in several European countries has made restraining drug spending growth even more important to government officials. For example, while total pharmaceutical spending in 1990 composed about 8 percent of total health spending in the United States (as it did in Sweden), it accounted for almost 11 percent of health care costs in the United Kingdom, about 17 percent in France, and over 21 percent in Germany (Figure 1). Given the fiscal weight of the pharmaceutical sector, each of these countries has looked to this sector for a significant contribution to the national effort at slowing the growth of overall health care spending.
Figure 1. Pharmaceutical Expenditures as Percent of Total National Health Care Costs: 1990.
Drug Manufacturer Regulations
Each country has regulations that are designed to limit—either directly or indirectly—the price that drug manufacturers charge to wholesalers (or to retailers that buy directly from the manufacturer). These policies differ in the extent that manufacturers are free to set launch prices for new products as well as to increase prices on existing products. (Prices are also regulated at subsequent links in the distribution chain. The fees charged by wholesalers and pharmacists typically are not allowed to exceed a set ceiling.4 These fees can be calculated as a fixed amount per prescription or as a percentage of price.)
Regulations targeted at drug manufacturers' prices in the four countries embody one of three mechanisms: (1) product-by-product price controls; (2) limits on insurers' reimbursement levels; or (3) profit controls.
Product-By-Product Price Controls
These controls are the most direct form of price regulation, in that they largely bar manufacturers from selling their drug products at prices above those approved by the government (or other paying authority). In the two countries we studied where product-by-product price controls have been used for outpatient prescription drugs—France and (until 1993) Sweden—both new product prices and price increases were regulated by the government. New product prices emerge from negotiations between the government and each drug manufacturer. The criteria for setting these prices include the therapeutic value of the drug, the price of comparable treatments, and the contribution of the drug's sales to the national economy.5 Price increases in both countries are allowed only with prior government approval.6
Limits on Insurer Reimbursement Prices
These price controls set an upper limit (or reference price) on the amount the insurer can pay for groups of identical or equivalent drugs. Drug manufacturers are free to set any launch price or price increase that they choose, but consumers must pay the difference between that price and the reference price. Manufacturers' ability to charge a price that is higher than the reference price is limited by consumers' willingness to incur out-of-pocket costs for pharmaceuticals.
Germany and Sweden illustrate different ways that reimbursement prices can be calculated. In Germany, a drug's reference price is computed essentially as the average of the prices of that drug and similar products.7 In Sweden, the reference price for a drug is set at 10 percent above the price of the least expensive generic equivalent In Germany, drugs are not covered under the reference price system (RPS) if they do not have a sufficient number of comparable products, while in Sweden, only one generic equivalent is needed to set a reference price. In Germany, the statutory health insurers (known as sickness funds) pay the price that manufacturers set for drugs without a reference price (less the required patient copayment).8 By contrast, in Sweden the government negotiates the prices that can be charged for these drugs with manufacturers.9
Profit Controls
This regulatory method, used in the United Kingdom, is a more indirect form of drug spending control. A manufacturer that introduces a drug product into the U.K. market may freely set its launch price at any level, as long as company profits do not exceed a negotiated target. More precisely, the National Health Service (NHS), which in effect is the national health insurer, negotiates a profit ceiling with most drug manufacturers.10 Through this process, the government relates each manufacturer's profits and hence, indirectly, their prices, to the level of investment in pharmaceutical production and research and development in the country for the purpose of supplying drugs to NHS.11 Even under this profit control scheme, however, drug manufacturers are still subject to drug price regulations. While manufacturers freely set prices when introducing new drugs—so long as profits do not exceed the target level—they cannot increase drug prices without prior government approval.
Manufacturer Pricing Regulation Effects
The drug spending controls applied in these four countries have had mixed success at restraining the level of pharmaceutical expenditures. On the one hand, drug prices in these countries have grown relatively slowly under the price and profit controls—less than the rate of general inflation.12 But while price restraint has probably kept total drug spending lower than it would have been otherwise, total drug spending—which is affected by the quantity of drugs sold as well as their prices—has continued to rise faster than the countries' governments are willing to accept.
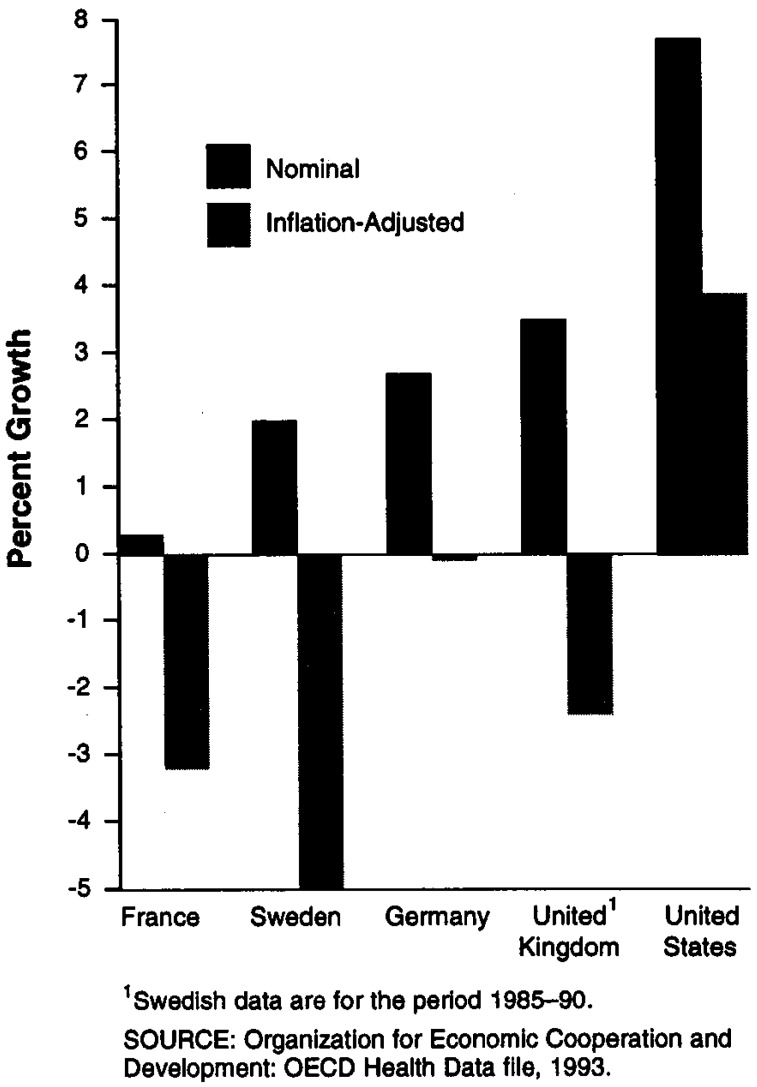
From 1985 to 1991, the countries with the most direct types of price controls—France and Sweden13—had the lowest average rates of increase in drug prices (Figure 2).14 In the United Kingdom, which has the most indirect type of price control, nominal drug price increases were the highest of the countries we reviewed; nonetheless, even U.K. drug prices rose relatively slowly—at about one-half the general rate of inflation. By contrast, during the same period (1985-91), pharmaceutical prices in the United States increased at an average annual rate that was more than twice the general inflation rate.
Figure 2. Pharmaceutical Price Changes: 1985–91.
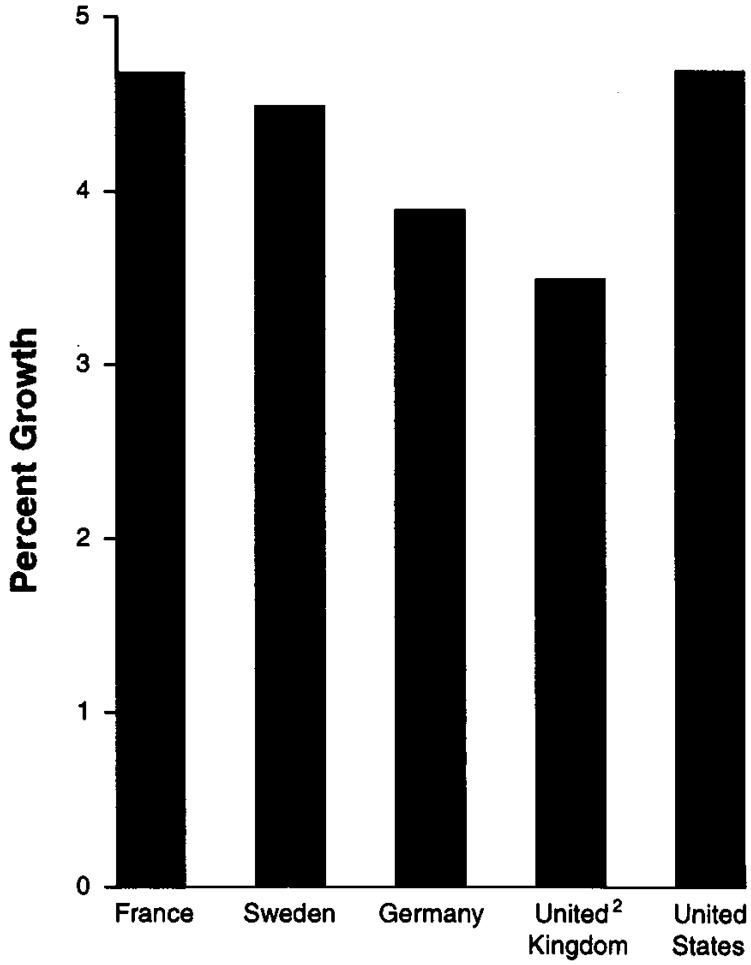
While the price restraint may have helped achieve some moderation in the growth of drug spending, the countries we examined had limited success in restraining the growth in total pharmaceutical expenditures during the same time period (Figure 3). The relative increases in pharmaceutical spending were greater for countries with direct price controls than for those with more indirect approaches. In France and Sweden, the countries that employed direct price controls, the average annual growth in pharmaceutical spending from 1985 to 1990 was comparable to that in the United States. In Germany and in the United Kingdom, pharmaceutical spending grew at a slightly slower rate than in the United States. However, pharmaceutical spending in Germany and the United Kingdom grew more rapidly than overall inflation.15
Figure 3. Pharmaceutical Expenditure Growth1:1985–90.
1Inflation-adjusted.
2United Kingdom data are for the period 1985–89.
SOURCE: Organization for Economic Cooperation and Development: OECD Health Data file 1993.
Why has Pharmaceutical Spending Continued to Rise?
The increase in pharmaceutical spending does not necessarily imply that the controls were ineffective at restraining drug spending. Indeed, these policies may have kept drug expenditures from rising higher than they would have otherwise.16 However, the rise in drug spending suggests that factors outside the purview of these regulations outweighed any restraining impact that price and profit controls may have had on drug expenditures.
Increases in Drug Utilization
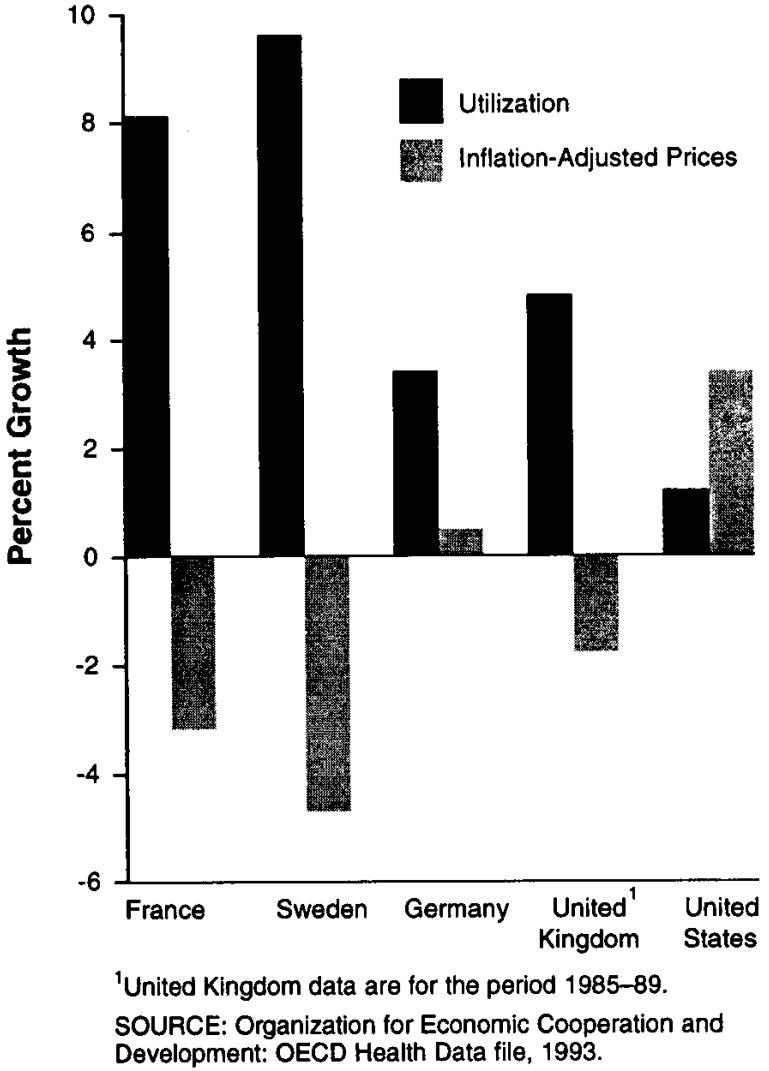
Increases in drug utilization likely provide one source of these spending increases. As Figure 4 shows, drug utilization grew more rapidly than drug prices in the four countries we reviewed, suggesting that greater utilization accounted for a large amount of the growth in drug spending. By contrast, in the United States drug utilization grew far less rapidly than drug prices, thereby suggesting a greater role for drug price increases in explaining spending growth.
Figure 4. Growth in Utilization and Prices: 1985–90.
Increases in utilization can come from both population growth and increases in the elderly population—both of which occur independently of price and profit controls. Increases in the elderly population can be of particular importance in explaining higher spending levels, since the elderly are more likely to have higher per capita drug use than the non-elderly. Each of the countries we reviewed has experienced increases in the elderly population, particularly in persons 75 years of age or over (Table 1).
Table 1. Growth of the Elderly Population as Percent of Total Population in the United States and Selected European Nations: 1985–91.
| Age Group and Year | France | Sweden | Germany | United Kingdom | United States |
|---|---|---|---|---|---|
| 65 Years of Age or Over: | |||||
| 1985 | 12.8 | 17.4 | 14.8 | 15.1 | 11.9 |
| 1991 | 14.1 | 17.7 | 15.4 | 15.8 | 12.7 |
| 75 Years of Age or Over: | |||||
| 1985 | 6.3 | 7.4 | 6.9 | 6.4 | 4.8 |
| 1991 | 7.0 | 8.1 | 7.2 | 7.0 | 5.2 |
SOURCE: Organization for Economic Cooperation and Development: OECD Health Data file, 1993.
Higher Prices for Newer Drugs
Increases in drug spending may also be caused by the use of newer, more expensive drugs. Despite the control mechanisms in place in these four countries, new drugs tend to have higher average prices than the drugs they replace, increasing the pressure on drug budgets even when consumption levels remain constant These new products, which can range from innovative treatments to modest improvements over existing products, can strain drug budgets when they replace less expensive medications.17 Higher new drug prices have been cited as a particular problem in the United Kingdom, where companies are free to set new drug prices so long as their profits remain within the target range.
The price and profit controls used in these countries generally do not provide patients and physicians with an incentive to choose products that are less expensive. Of the systems that we reviewed, only the reference price systems, used in Germany and Sweden, create incentives for consumers to choose lower priced products. Under this system, a single reimbursement rate applies to drugs that are considered therapeutically equivalent or comparable to one another; if the price exceeds this level, then the consumer pays the remainder. By contrast, neither direct price controls nor profit controls create incentives for consumers or physicians to choose a
New Spending Control Mechanisms
Increased Reliance on Cost-Shifting Policies
The health care financing systems in the countries we reviewed have been strained by the pattern of increases in pharmaceutical spending that approach or outstrip the growth of gross domestic product These strains have resulted in the adoption of major changes in the drug reimbursement policy in Germany and Sweden, proposals for major changes in such policy in France, and modifications in both Germany and the United Kingdom that are intended to make physicians more aware of drug costs. These new policies—sometimes working within the context of existing price and profit controls, and sometimes not—are designed to meet two objectives: (1) to shift the burden of increased pharmaceutical spending from government to consumers, physicians, and drug manufacturers; and (2) to stimulate price competition in the pharmaceutical sector by encouraging consumers and physicians to choose more cost-effective medications.18
Increases In Consumer Cost Sharing
One approach used to reduce drug spending is to increase consumers' financial responsibility for prescription drugs. From 1989 to 1993, all four countries have increased the patient's share of drug costs (Table 2).
Table 2. Pharmaceutical Cost-Containment Policies in Selected European Nations: 1989-93.
| Country | 1989 | 1991 | 1993 |
|---|---|---|---|
| France | Copayment of 0, 30, 60, or 100 percent of drug cost, depending on the particular drug. | Copayment of 0, 30, 60, or 100 percent of drug cost, depending on the particular drug. | Copayment of 0, 35, 65, or 100 percent of drug cost, depending on the particular drug (effective summer 1993). |
| Germany | Copayment of 3 DM per prescription. Starting June 1, drugs under the reference price system: Patients pay the amount by which retail price exceeds the reference price. Drugs not under the reference price system: 3 DM per prescription. |
Drugs under the reference price system: Patients pay the amount by which the retail price exceeds the reference price. Drugs not under the reference price system: 3 DM per prescription. |
Copayment of 3-7 DM, depending on the price of the drug.1 In addition, the consumer pays any amount by which retail price exceeds the reference price. |
| Sweden | Flat copayment of 90 SEK for up to 10 drugs written on same prescription form. | Flat copayment of 90 SEK for up to 10 drugs written on same prescription form, for a maximum prescribing period of 90 days. | Copayment of 120 SEK for first prescription and 10 SEK for additional prescriptions obtained from the pharmacy at the same time, for a maximum prescribing period of 90 days. In addition, the consumer pays any amount by which the drug's price exceeds the reference price. |
| United Kingdom2 | Flat copayment of £2.80 for drugs covered by NHS.3 | Flat copayment of £3.40 for drugs covered by NHS.3 | Flat copayment of £4.25 for drugs covered by NHS.3 |
As of January 1994, the copayment in Germany is based on the size of the prescription rather than on the price of the drug.
Table lists copayment levels as of April 1 of each year cited. In addition, patients in the United Kingdom receiving frequent prescriptions may buy a season ticket covering the costs of all prescriptions for either 4 months or 12 months. In April 1989, the 4-month season ticket cost £14.50, and the 12-month season ticket cost £40. By April 1993, these costs increased to £22 for the 4-month ticket and £60 for the 12-month ticket.
Because of exemptions to cost sharing, about 80 percent of drugs dispensed in the United Kingdom have no consumer copayment.
NOTES: DM is Deutschmark. SEK is Swedish Kroner. NHS is National Health Service.
SOURCE: (U.S. General Accounting Office, 1994b).
Higher copayments may have the dual purpose of (1) shifting some of the financial burden of pharmaceuticals away from the national health insurance system and toward consumers, and (2) raising consumer cost-consciousness about their prescriptions, thereby reducing alleged overutilization of drugs.
Certain features of some copayment policies can be expected to limit their effectiveness at restraining drug spending. First, copayments that cover only certain drugs or certain segments of the population will reduce spending less than would more comprehensive cost sharing. For example, until 1993, there were no copayments for German pharmaceuticals covered under the reference price system (so long as the drug's price did not exceed the reference price). Therefore, consumers had no incentive to reduce consumption of those items. In the United Kingdom, copayment exemptions for the elderly, the poor, children, and pregnant women (among others) eliminate all cost sharing for about 80 percent of prescriptions written.
Second, copayments that are the same amount for every prescription cannot affect the choice between more and less expensive medications. If the consumer's copayment is identical for an expensive drug and for a cheaper substitute, the consumer has no reason to choose the less expensive medication.
Third, the small size of the copayments may also limit their ability to reduce the number of prescriptions filled. However, raising the copayment could present a financial barrier to poor households or to people who need to use a high volume of pharmaceuticals.
Encouraging Less Expensive Prescriptions
To an increasing extent, pharmaceutical payment reforms in the countries we reviewed—particularly in the United Kingdom and Germany—are designed to encourage economical prescribing by physicians and to emphasize the use of less expensive drugs. These policies recognize the vital role of the physician as the primary decisionmaker regarding choice of pharmaceuticals and, to varying degrees, tie financial incentives for physicians to the prescribing choices that they make. The United Kingdom uses a two-pronged strategy for encouraging physicians to be agents for lower pharmaceutical spending:
First, the government provides information to individual physicians about their prescribing habits (relative to those of their colleagues). Physicians receive a periodic report on the number and cost of the drugs they prescribed, compared with norms for physicians in their geographical area. The government also provides physicians with information on the safety and cost-effectiveness of alternative drug products. This information is intended to allow the physicians to make more responsible choices about prescribing.
Second, physician spending targets are used to restrict pharmaceutical sales. Since 1991, physicians in the United Kingdom have been subject to the Indicative Prescribing Scheme (IPS), which sets financial targets for physician prescribing.19 Under IPS, doctors are given a financial benchmark, referred to as an indicative amount of prescribing. Physicians' indicative targets are based on several factors, including historical expenditures, demographic composition of their patients, and drug price inflation. These targets are not binding caps, although physicians who consistently prescribe more than their targeted amounts can be singled out for advice and detailed monitoring, and, in a last resort, cases of gross over-prescribing can be penalized.20
Germany also instituted pharmaceutical budgets on physicians, but these controls—implemented in 1993 as part of a comprehensive health financing reform—place more stringent financial controls on physicians than do the United Kingdom's policies. Since January 1993, Germany has had a global budget for pharmaceuticals, which, if exceeded, is offset by a reduction in the ambulatory care physician budget In 1993, the total pharmaceutical budget for office-based physicians was set at about 24 billion Deutschmarks (DM [about $15 billion]). While 1993 pharmaceutical spending did not exceed this level, any cost overrun up to 280 million DM (about $175 million) would have been offset by a Mark-to-Mark reduction in the 1994 ambulatory care physician budget (The cost overrun would also be borne by pharmaceutical manufacturers if it reached 280 million DM, up to another 280 million DM.) For most regions, the 1994 budget is set at the 1993 level, and all cost overruns will be borne by similar reductions in the ambulatory care physician budgets.21
The global budgets in Germany appear to have had an impact in the short time that they have been in effect.22 Total prescription drug costs for sickness funds declined by about 20 percent in the first half of 1993, compared with the same period in 1992, and total 1993 drug spending was actually less than the budgeted amount and, therefore, less than 1991's total. In addition, in the first half of 1993, physician prescribing fell below the 1992 level by about 17 percent.
Several reasons have been suggested for the drop in drug spending in Germany. First, physicians substituted cheaper generic drugs for more expensive, brand-name drugs. As a result, sales of the cheapest generic drugs increased in some cases by as much as 250 percent Second, many patients—especially those with long-term illnesses—obtained their prescriptions in December 1992 (before the law took effect) and thus did not need to acquire their drugs in the first few months of 1993. Third, physicians have been less willing to prescribe drugs with doubtful efficacy (e.g., anti-varicosis drugs) or drugs for conditions that can be treated in different ways (e.g., dietary drugs).23
Citizens and officials in both countries have been concerned about whether the budgets are reducing access to pharmaceuticals. In the United Kingdom, some observers believe that the budgets are constraining physicians' ability to prescribe the most effective drugs and respond to special patient needs, such as those of the elderly. However, government officials believe that the physician budgets could, instead, increase the quality and cost-effectiveness of prescribing, and so improve patient care. In Germany, some officials have expressed concern that the older drugs that physicians are prescribing in order to save costs may be less effective than newer, more innovative products. However, there is no firm evidence either to support or contradict this contention.
Stringent Drug Manufacturers' Controls
While many of the recent policy changes in the countries reviewed have applied to patient and physician practices, France, Germany, and the United Kingdom—to differing degrees—have also made efforts at reducing payments to manufacturers. These efforts have taken three forms: (1) across-the-board price cuts; (2) limits on total manufacturers' sales; and (3) limits on the types of drugs eligible for reimbursement.
Across-the-Board Price Cuts
One method used to reduce pharmaceutical spending is across-the-board cuts in payments to drug manufacturers. France, Germany, and the United Kingdom have used this measure in recent years. France's most recent price reductions occurred in 1991, when the government ordered that pharmaceutical prices be cut by 2.5 percent. Germany implemented across-the-board price cuts in 1993, when the government ordered a 5-percent reduction in the price of drugs not covered by the RPS, and a reduction in over-the-counter (non-prescription) drug prices to 2 percent below the May 1992 price level. The government also mandated a price freeze on affected drugs that will be in effect through 1994. The United Kingdom also implemented global price cuts in 1993, ordering a 2.5-percent price cut on all products, which is to be followed by a 3-year price freeze.
Budgets
Of the countries we reviewed, only Germany has imposed budgets that apply to manufacturers. As described in the previous section, Germany's 1993 global budget sets total limits on annual pharmaceutical spending. While physicians were to bear part of the budget overrun—the first 280/DM million in 1993—subsequent overruns (up to 280/DM million) would have come from the pharmaceutical manufacturers. However, under the 1994 budget, manufacturers will not have to bear the financial burdens of overruns if physicians exceed the budget.
France may adopt drug budgets for manufacturers. In 1991, the French government proposed a drug payment system in which manufacturers would each have a budget for total drug sales to the social insurance system. Under this framework, manufacturers would have been able to set prices freely, as long as their total revenues from sales to the national health system did not exceed the budget. This proposal was never enacted, due to political opposition. However, in January 1994, representatives of the pharmaceutical industry and French government reached an informal agreement that, if implemented, would include many aspects of this 1991 proposal.
Limiting Drugs Eligible For Reimbursement
Governments can limit the drugs eligible for reimbursement through lists that explicitly identify specific drugs as ineligible for reimbursement. Drugs may be excluded from the payment system because they (1) offer questionable therapeutic value or (2) have prices that are high relative to alternative medications of similar or equal therapeutic value.24
Three of the countries we studied are either establishing or expanding negative drug lists in an attempt to limit prescription drug dispensing. In January 1994, France established a list of 24 drugs and procedures which will not be reimbursed. The United Kingdom is in the process of excluding additional drugs from its reimbursable lists. Germany currently has a non-reimbursable drug list, but, after 1995, plans to replace this with a list of drugs that are eligible for reimbursement.
Conclusions
Though price controls on prescription drugs have been prominent in Europe, they do not exhaust the variety of techniques and philosophical orientations that U.S. decisionmakers can consider. To control pharmaceutical expenditures, France, Germany, Sweden, and the United Kingdom employ an array of policies, some regulatory and some market-based. The balance struck varies from country to country—ranging from controlling corporate and physician actions by legal and administrative sanctions to strengthening competition by reshaping incentives. For example, France has emphasized the regulatory approach by imposing stringent product-by-product price controls. By contrast, the United Kingdom has evolved a more eclectic strategy: profit controls—a relatively flexible regulatory approach that allows companies considerable pricing freedom—are coupled with policies to sharpen competition among drug companies by encouraging physicians to prescribe less expensive medicines.
Yet none of the policy combinations used in these countries has yielded the degree of spending restraint that each government has desired. At best, these governments have seen mixed success—while pharmaceutical prices were restrained, total drug spending continued to grow (albeit at different rates in each country). It is too soon to tell whether the new policies aimed at consumers and physicians, in conjunction with existing price and/or profit controls, can further limit spending increases. Similarly, evidence is limited as to whether these new policies will limit consumers' access to important medicines.
What lessons, then, do these countries' experiences offer U.S. policymakers as they consider how to contain the costs of a universal prescription drug benefit? The evidence seems to support two different sets of lessons that, in terms of policy changes, point in opposite directions. At least in this case, the picture one sees depends on one's perspective.
From the perspective of the pragmatic regulator, two distinct messages emerge. First, when a country adopts a universal drug benefit, its policy goal necessarily shifts from restraining drug prices to restraining drug expenditures. In the United States, the current debate has centered on drug prices, because high prices can impede many people's access to prescription drugs. However, if the source of payment were to shift from consumers' pocketbooks to the public treasury, the potential obstacle to access would be the total expenditures that the political system can bear—that is, pharmaceutical price times quantity. As the French experience shows, strict controls on drug prices are not sufficient to restrain growth of drug utilization and total pharmaceutical spending.
The regulator's second lesson flows from the first Since control of overall spending implies two goals—limiting quantity as well as price—then the regulator needs distinct instruments to achieve those goals. Whether regulatory or market oriented, policies to restrain drug prices will usually differ from those used to reduce what is perceived as unnecessary drug consumption. Moreover, the pragmatic regulator will observe that new market incentives, which make physicians and consumers aware of the costs of prescription drugs, are not only useful additions to the policy menu, but may be necessary to achieving control of pharmaceutical spending.
Such are the lessons for the pragmatic regulator—but a different set of conclusions emerge for the advocate of market forces. This observer might draw three conclusions from the European efforts to control prescription drug prices and spending: first, that regulatory efforts have (at least in part) failed to meet their goal of spending control; second, that these failures have spurred additional controls and greater regulatory complexity; and third, that this pattern of regulatory ineffectiveness and growth reflects a failure to create a market environment that fosters appropriate cost consciousness among providers and consumers. Rather than rely on the European experience, proponents of a more market-based strategy may look to the United States, where the pharmaceutical market is undergoing major structural change. The increased role of managed care is unleashing competitive forces in the pharmaceutical sector that could potentially achieve many of the price and spending restraints desired by policymakers in both the United States and Europe.
This clash of regulatory and market-oriented perspectives echoes similar conflicts in other policy areas, from health insurance to housing. But these conflicts are not all ideological; in the present case, advocates for each approach can find some support for their position in the European experience. However, each side should bear in mind that the debate cannot be foreclosed on the basis of this evidence alone. Political considerations—based on different perceptions of the role of regulations versus reliance on market forces—play an important role in that debate. In addition, pharmaceutical pricing policies must reflect the potential impact that any regulations have on pharmaceutical research and development—a relationship that is uncertain at best, and in any event is beyond the scope of this article.
Footnotes
David J. Gross is with KPMG Peat Marwick. Jonathan Ratner, James Perez, and Sarah L. Glavin are with the U.S. General Accounting Office (GAO). The views presented in this article are those of the authors and do not necessarily reflect the opinions of KPMG Peat Marwick, GAO, or the Health Care Financing Administration.
Some portion of this increase may be attributable to a general movement of treatment from inpatient to outpatient settings over this period.
Some research indicates that prescription drug price indexes may over-sample medium-aged drugs that undergo above-average price increases, and under-sample younger products that experience less-than-average price increases, thereby overstating annual average drug price inflation (Berndt, Griliches, and Rosett, 1992). Alternatively, these indexes may understate annual changes in average drug prices because they generally do not measure the impact of new drugs, many of which enter the market at relatively high prices.
There are also fewer networks for buying prescription drugs in other countries than in the United States. For example, in the countries we studied, consumers generally purchase their pharmaceuticals from retail pharmacists. By contrast, while most Americans buy their pharmaceuticals at retail pharmacies, many purchase through mail order houses and managed care organizations (Schondelmeyer and Thomas, 1990).
The exception to this is in Sweden, where wholesaler fees are not subject to government regulation, but are negotiated between wholesalers and manufacturers.
In addition, Sweden based its allowable price on the price charged for the drug in other countries, and, in particular, on the price in the manufacturer's home country.
In France, the government prohibits price increases for drugs that have been on the market less than 2½ years. After that time, prices can only be increased through a directive, which raises or lowers the prices of all drugs on the market by a set percentage. In Sweden, the government tries to keep drug price increases within the rate of inflation.
Three different categories are used to define sets of similar drugs: (1) drugs with the same active ingredients (for example, brand name drugs and their generic equivalents); (2) drugs with therapeutically comparable active ingredients (for example, beta-blockers or H-2 antagonists); and (3) drugs with therapeutically comparable effects (for example, different aspirin combinations). The reference price for a particular drug is adjusted for variations from the average product's strength and package size.
In Germany, many single source products that lack comparable products cannot be assigned reference prices. Furthermore, other products do not yet have reference prices because of the technical difficulties in ascertaining which products have comparable therapeutic ingredients or actions. As of July 1993, about one-half of pharmaceutical products in Germany had reference prices. In 1993, the German government simplified the process by which drugs are put into comparable groups. The government hopes that this simplification will allow for the eventual inclusion of 70 percent of drugs into the RPS.
These negotiations are performed for patented drugs that do not have generic substitutes and for over-the-counter drugs that the manufacturer wants included under the reimbursement system. Factors going into the negotiations include the basis of the drug's therapeutic value, the price of the particular drug in other countries, the price of comparable products in other countries, and the extent to which the drug's usage substituted for more expensive treatments. No negotiations take place for non-reimbursable drugs (for example, drugs sold in hospitals); instead, manufacturers are able to price these drugs freely.
The United Kingdom's profit control scheme applies to all firms with sales to NHS of over £0.5 million (about $740,000) per year.
Under the United Kingdom's profit control scheme, which excludes generic drugs, manufacturers' profits are regulated in two ways, depending on their capital investment in the country. Manufacturers with sizeable capital investment are permitted to price drugs in line with target profit levels, based on their return on capital—current profit levels on sales to the NHS are set at 17 to 21 percent of the capital invested in the country, and devoted to supplying brand-name (i.e., non-generic) prescription drugs to NHS. Other manufacturers selling in the U.K.'s drug market also have target profit levels, but these are based on their return on sales. Manufacturers can justify keeping additional profits (up to 25 percent over their target level) if the additional profits are attributable to new products or to increased operating efficiency.
The general inflation rate is measured by the growth in the price deflator for gross domestic product (GDP) in each country.
Swedish data are for the period 1985-90.
Drug price inflation can occur even under regulatory schemes which largely restrict drug price increases, such as those in France and the United Kingdom. This is because the pharmaceutical price index, on which drug price inflation is based, is composed of a market basket of drugs that changes over time. As new drugs become part of this market basket, the cost of this basket can increase if the price of those new drugs exceeds the average cost of the other drugs in the previous market basket.
Data on pharmaceutical spending in the United Kingdom are for the period 1985-89.
Analyses of the effects of Germany's RPS suggest that drug prices and spending were lower after the imposition of reference pricing than they would have been otherwise. We were not able to identify any formal studies on how the policies used in France, Sweden, or the United Kingdom affected drug spending, nor were there sufficient data for doing a before-and-after analysis on the policies' effects.
Even when use of these medications replaces more expensive non-drug treatments, they can increase the pharmaceutical budget. Consider the hypothetical example of a new medication that costs $1,000, but reduces the need for surgery that would cost $25,000. Each time that the medication is prescribed in lieu of surgery, hospital costs would be reduced by $25,000, but prescription drug spending—accounted for in another budget—would be increased by $1,000.
Sweden's recent payment reform was imposed, to some extent, for an additional reason—to respond to a European Community directive that requires member countries to make public the rules governing pricing of prescription drugs. The directive does not interfere with the right of countries to control prices or reimbursement by any method they choose, provided the method used is “transparent” and does not discriminate between foreign and domestic drug manufacturers. Sweden is not a European Community member, but has applied for membership.
Some physicians in the United Kingdom—25 percent as of April 1993—are subject to an alternative budgeting scheme, known as the GP fundholding scheme. Under this scheme, which is voluntary, physicians who are in relatively large group practices are given a practice budget, which is intended to cover all prescribing costs for patients as well as the cost of some hospital services, outpatient services, administrative services, and visiting and district nurse services.
The provisions requiring physicians to justify this prescribing behavior are separate from and predate IPS.
Most regional physicians' associations chose to accept the 1994 budget set at the 1993 level rather than negotiate a budget based on real 1993 expenditures.
No systematic evidence exists on the effects of IPS in the United Kingdom.
There was a disproportionate decrease in the prescription of drugs that are considered to be therapeutically controversial and drugs that are considered to be therapeutically meaningful. For example, drugs in the former group include circulatory drugs and vitamins (which declined 29.9 percent and 29.1 percent, respectively). Drugs in the latter group include antibiotics and anti-diabetic drugs (which declined 5.2 percent and 0.7 percent, respectively).
In Germany, drugs will be excluded from reimbursement only if they have questionable therapeutic value; in France, Sweden, and the United Kingdom, reimbursement decisions take into account a drug's price as well as its therapeutic value.
Reprint Requests: David J. Gross, Ph.D., Policy Economics Group, KPMG Peat Marwick, 2001 M Street, NW., Washington, DC 20036.
References
- Association Belge des Consommateurs. Statement prepared for the United States Senate Special Committee on Aging. Washington DC.: 1989. [Google Scholar]
- Berndt ER, Griliches Z, Rosett J. Auditing the Producer Price Index: Micro Evidence from Prescription Pharmaceutical Preparations. Washington, DC.: National Bureau of Economic Research; 1992. Working Paper No. 4009. [Google Scholar]
- Letsch SW. National Health Care Spending in 1991. Health Affairs. 1993;12:94–110. doi: 10.1377/hlthaff.12.1.94. [DOI] [PubMed] [Google Scholar]
- Redwood H. Price Regulation and Pharmaceutical Research: The Limits of Coexistence. Suffolk, England: Oldwicks Press Limited; 1993. [Google Scholar]
- Reekie WD. Drug Prices in the UK, USA, Europe, and Australia. Australian Economic Papers. 1984 Jun;:71–78. [Google Scholar]
- Schondelmeyer S, Thomas J. Trends in Retail Prescription Expenditures. Health Affairs. 1990;9:131–145. doi: 10.1377/hlthaff.9.3.131. [DOI] [PubMed] [Google Scholar]
- U.S. General Accounting Office. Prescription Drugs: Companies Typically Charge More in the United States Than in Canada. Washington, DC.: 1992. Pub. No. GAO/HRD-92-110. [Google Scholar]
- U.S. General Accounting Office. Prescription Drugs: Companies Typically Charge More in the United States Than in the United Kingdom. Washington, DC.: 1994a. Pub. No. GAO/HEHS-94-29. [Google Scholar]
- U.S. General Accounting Office. Prescription Drugs: Spending Controls on Four European Countries. Washington, DC.: Apr, 1994b. Pub. No. GAO/HEHS-94-30. [Google Scholar]