Abstract
Efficient neural communication between premotor and motor cortical areas is critical for manual motor control. Here, we used high-density electroencephalography to study cortical connectivity in patients with Parkinson's disease (PD) and age-matched healthy controls while they performed repetitive movements of the right index finger at maximal repetition rate. Multiple source beamformer analysis and dynamic causal modeling were used to assess oscillatory coupling between the lateral premotor cortex (lPM), supplementary motor area (SMA), and primary motor cortex (M1) in the contralateral hemisphere. Elderly healthy controls showed task-related modulation in connections from lPM to SMA and M1, mainly within the γ-band (>30 Hz). Nonmedicated PD patients also showed task-related γ-γ coupling from lPM to M1, but γ coupling from lPM to SMA was absent. Levodopa reinstated physiological γ-γ coupling from lPM to SMA and significantly strengthened coupling in the feedback connection from M1 to lPM expressed as β-β as well as θ-β coupling. Enhancement in cross-frequency θ-β coupling from M1 to lPM was correlated with levodopa-induced improvement in motor function. The results show that PD is associated with an altered neural communication between premotor and motor cortical areas, which can be modulated by dopamine replacement.
Keywords: dynamic causal modeling (DCM), effective connectivity, electroencephalography (EEG), neural communication
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by slowness of movement (akinesia), rigidity, tremor at rest and postural instability (Lang and Lozano 1998a,b). The core pathophysiological mechanism is degeneration of dopaminergic neurons in substantia nigra pars compacta (SNc), which is thought to cause abnormal modulation of cortico-basal ganglia-thalamo-cortical pathways (Hammond et al. 2007). While in healthy people, phasic movements are modulated in the γ-band (Pfurtscheller et al. 2003; Miller et al. 2007), pathological firing patterns in the β-band (13–30 Hz) in the subthalamic nucleus (STN) have been linked to akinesia and rigidity in PD patients (Marsden et al. 2001; Kuhn et al. 2008). In PD patients receiving therapeutic deep brain stimulation (DBS), modulation of STN activity leads to changes in oscillatory activity and coupling in motor cortical areas (Devos et al. 2004; Silberstein et al. 2005). Further, it has been shown that oscillatory activity in distinct connections between the cortex and basal ganglia is specifically linked to different frequency bands (Hirschmann et al. 2011, Litvak et al. 2011a, Timmermann and Fink 2011). These findings are in good agreement with the concept that altered firing patterns in basal ganglia lead to abnormal activation of cortical motor areas impairing their respective function (Timmermann et al. 2003; Redgrave et al. 2010).
Accordingly, functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies have provided converging evidence for abnormal activation of core motor regions comprising the supplementary motor area (SMA), lateral premotor cortex (lPM), and primary motor cortex (M1) underlying motor impairment in PD (Stoessl et al. 2011; Rowe and Siebner 2012). While a decreased activity in SMA and an increased activity in lPM during motor tasks has repeatedly been reported when patients were off medication (Sabatini et al. 2000; Haslinger et al. 2001; Wu et al. 2010), normal activity patterns can—at least partially—be restored by dopaminergic medication (Haslinger et al. 2001; Buhmann et al. 2003; Rowe et al. 2010). However, the observed neural activations vary significantly depending on the specific paradigm and the amount of attention that is assigned to the actions (Rowe et al. 2002a, b; Lau et al. 2004). Recent neuroimaging studies suggest that a functional disconnection between prefrontal, premotor, and motor areas might account for the observed hypoactivation of different neural regions (Rowe et al. 2010; Wu et al. 2011). However, little is known about the electrophysiological dynamics underlying altered communication between premotor and motor areas in PD. We hypothesized that during fast repetitive finger movements, neural communication between mesial and lateral premotor areas and the motor cortex would be expressed as abnormal oscillatory coupling in patients OFF medication. Furthermore, we expected that levodopa would at least partially normalize premotor-motor cortical connectivity in PD.
To specifically test this, we used dynamic causal modeling (DCM) of movement-related cortico-cortical oscillatory coupling (Chen et al. 2008) to investigate effective connectivity between core motor regions in the precentral cortex, namely M1, SMA, and lPM (Picard and Strick 2001). Electroencephalography (EEG) was recorded while PD patients and healthy age-matched individuals performed fast self-paced extension-flexion movements of the right index finger both before and after application of levodopa. This enabled us to assess physiological and disease-related changes in cortico-cortical communication between premotor and motor areas and to investigate how dopamine replacement modulates cortico-cortical oscillatory coupling in PD.
Participants and Methods
Participants
Thirteen patients with clinical diagnosis of idiopathic PD without dementia and 13 healthy individuals participated in the study. Exclusion criteria were as follows: age ≥80 years, neurological disease other than PD, abnormal MRI, and treatment with DBS. Two PD patients and 1 control participant were later excluded (see “source analysis”), leaving 11 patients (3 females; age 60.5 ± 9.4 years, mean ± SD) and 12 healthy control participants (6 females; age 63.8 ± 7.2 years). Clinical details are summarized in Table 1. All participants were right-handed as revealed by self-report. In accordance with the declaration of Helsinki, all participants gave their written informed consent to the study, which was approved by the local ethics committee of the Faculty of Medicine at the University of Cologne (study-nr: 08 067).
Table 1.
Patient clinical details
| Case | Age/sex | Disease duration (years) | Parkinsonism | UPDRS (OFF/ON) | LEDD (mg/day) |
|---|---|---|---|---|---|
| 1 | 60 f | 6 | Left | 32/26 | 910 |
| 2 | 58 f | 4 | Right | 24/15 | 860 |
| 3 | 64 f | 1 | Right | 17/8 | 240 |
| 4 | 50 m | 2 | Left | 11/6 | 150 |
| 5 | 46 m | 5 | Left | 31/15 | 260 |
| 6 | 67 m | 20 | Left | 38/31 | 1040 |
| 7 | 71 m | 11 | Right | 57/44 | 1600 |
| 8 | 53 m | 13 | Left | 22/9 | 950 |
| 9 | 75 m | 9 | Left | 34/16 | 650 |
| 10 | 69 m | 10 | Left | 30/15 | 1000 |
| 11 | 53 m | 7 | Right | 14/12 | 965 |
m, male; f, female; LEDD, levodopa-equivalent daily dose; LEDDs were calculated according to (Tomlinson et al. 2010).
Experimental Conditions
Subjects were seated in a comfortable chair with their eyes closed and asked to perform repetitive fast extension-flexion movements of the right index finger in the metacarpophalangeal joint. Participants were instructed to perform the movements at maximal repetition rate, while the hand was resting on a desk. The movement range was ∼30° in the horizontal plane. Each trial lasted for 10 s and was repeated 20 times. To avoid fatigue, we included small breaks (5–10 s) between trials. We also included a baseline condition without movement, where subjects had to keep still with their eyes closed (rest condition) for ∼5 min. Two examiners monitored the task performance during the motor task and controlled that participants did not fall asleep during the rest condition. Additionally, all participants performed a second motor task. The results related to the additional task will be reported separately, because it addresses a different experimental question, and therefore, different DCMs were constructed and compared. All patients were tested in the morning in the practical OFF state at least 12 h after withdrawal of their dopaminergic medication. Immediately prior to the experiment, a movement disorders specialist (MTB) assessed the Unified Parkinson's disease rating scale III (UPDRS-III) (Fahn et al. 1987). After completing the testing in the OFF state, patients received 200 mg of fast-released soluble levodopa (Madopar LT®, La Roche, Basel, Switzerland) and motor improvement was assessed consecutively every 15 min until a marked improvement of akinesia and rigidity was observed (at least 15% difference between UPDRS-ON and UPDRS-OFF). We then conducted EEG recordings as previously in the OFF state. PD patients did not perform any motor tasks during the break to avoid interference effects. One patient developed severe dyskinesias after application of levodopa and was therefore not tested in the ON state. The healthy participants performed the experiments only once without application of levodopa.
Data Acquisition and Preprocessing
Before the EEG experiment, T1-weighted structural magnetic resonance images (MRI) of the whole brain were acquired on a 3-Tesla Trio scanner (Siemens, Erlangen, Germany) using a 3D-modified driven equilibrium Fourier transform sequence (repetition-time = 1930 ms, echo-time = 5.8 ms, flip-angle = 18°, slice thickness = 1.25 mm) for the control group and on a 1.5-Tesla Intera scanner (Philips, Amsterdam, the Netherlands) using a 3D-turbo field echo sequence (repetition-time = 20 ms, echo-time = 4.6 ms, flip-angle = 25°, slice thickness = 2 mm) for patients. In 4 control subjects and 2 patients, MR images could not be acquired because of claustrophobia. The MR images were transformed to Talairach-space in Brainvoyager software (Brain Innovation, Maastricht, the Netherlands) and a mesh of the head was generated for electrode co-registration. If no structural MRI was available, we used a standard brain template for electrode co-registration and source analysis.
One hundred twenty-two electrodes were mounted on the head using an elastic cap in a spherical array (Easy-Cap, Herrsching, Germany). Optimal positioning of EEG electrodes was ensured using an ultrasound localization system (CMS20, Zebris, Isny, Germany) before starting the EEG recordings. EEG data were recorded with a 122-channel EEG system (Braintronics, Almere, the Netherlands) after assuring that impedances of all electrodes were ≤10 kΩ. EEG signals were amplified, band-pass filtered from 0.87 to 344 Hz and digitized at a sample rate of 1024 Hz. EEG data preprocessing was carried out on a personal computer using the brain electrical source analysis (BESA) software (BESA, Graefelfing, Germany). Default electrode positions delivered by the manufacturer (Easy-Cap) were co-registered to the individual MRI for each subject. In a next step, the data were average-referenced and artifact-corrected. A channel was classified as noisy, if the amplitude was larger than 120 μV, smaller than 0.07 μV, or showed a higher gradient than 75 μV to adjacent channels, which corresponds to the BESA default settings. Correction for eye-movement artifacts was carried out using the BESA eye-movement correction tool. The voltage threshold for horizontal and vertical eye movements was set at 150 and 250 μV, respectively (Ille et al. 2002). Additionally, the whole EEG recording was visually inspected for artifacts. Noisy trials were removed and excluded from the analysis. Noisy channels were extrapolated or interpolated (spherical spline interpolation) in case of a sufficient number of adjacent channels.
We simultaneously recorded activity of the right first dorsal interosseus (FDI) muscle using surface electromyography (EMG) electrodes (AMBU, Ølstykke, Denmark) to assess task performance. EMG signals were amplified and digitized at a sample rate of 1024 Hz. Analysis of EMG data was carried out using MATLAB 7.10.2 (MathWorks, Natick, MA, USA) and comprised 2 steps. In a first step, we analyzed the peak frequency in the EMG for each participant to assess the repetition frequency of the finger movements. The bandwidth was set to 1–7 Hz to include the typical frequency range of fast repetitive movements of the index finger (Aoki et al. 2003) and to avoid the dominant α-peak observed in EEG data. In a second step, we analyzed the power of the EMG data. Frequencies were divided into the θ- (4–7 Hz), α- (8–12 Hz), β- (13–30 Hz), and γ-band (31–48 Hz) (Timmermann et al. 2007) and normalized to the mean power. The aim of this analysis step was to assess putative group differences in EMG activity that could confound the EEG spectra. Tests for differences in EMG power and peak frequency between patients in the ON and OFF state and between patients and healthy controls were conducted using the Wilcoxon signed-rank test and Mann–Whitney U-test, respectively, and corrected for multiple comparisons using false-discovery rate.
Source Analysis
Details of the analysis procedure can be found in (Herz et al. 2012). In short, we defined a core motor cortical network of interest based on studies using fMRI (Sabatini et al. 2000; Haslinger et al. 2001; Rowe et al. 2010; Wu et al. 2010). This core network comprised M1, lPM, and SMA in the left hemisphere contralateral to the moving hand. To confirm that activity within the network was consistently present in our data, we defined a fronto-parietal cortical area comprising these regions (x: 8–−32, y: 0–−50) based on coordinates from Haslinger et al. (Haslinger et al. 2001) and conducted source analysis using BESA's multiple source beamformer (BESA, Graefelfing, Germany). We used the rest condition as baseline, that is, the power in the target time–frequency interval was referenced to the corresponding interval in the baseline condition. This approach allows detecting sources that are specifically related to movement, but can also lead to removal of sources that are modulated in frequencies that are related to resting EEG activity, particularly in the α-frequency band. Sources, as detected during beamformer analysis, were fitted on individual MRI and the corresponding stereotactic coordinates were registered in Talairach space with a 10-mm range to account for the low spatial resolution of EEG source analysis. This was done for the first 5 sources detected by the MSBF based on the number of sources in the study conducted by Haslinger et al. (2001). To avoid modeling of data unrelated to the motor task, we excluded 2 of the originally included 13 patients and 1 of 13 control subjects, because they failed to show activation in the predefined area.
Dynamic Causal Modeling
In the remaining 11 PD patients and 12 control subjects, we performed connectivity analysis of the EEG data using DCM of induced responses (Chen et al. 2008) as implemented in SPM8 (Update revision number: 4290; Wellcome Trust Centre for Neuroimaging, London, UK). DCM of induced responses enables modeling of spectral responses as the response of a set of interconnected electromagnetic sources to a spectral perturbation. The models are formulated in terms of differential equations including an A-matrix, which represents changes in spectral activity due to endogenous coupling between sources and a C-matrix, which represents changes induced by exogenous inputs. In this experiment, the exogenous input refers to the onset of the motor task, which induces changes in the coupling between the sources (A-matrix). Thus, even though the input is set to only one source, all other sources can express changes in spectral activity, because they are interconnected. For a more thorough explanation of DCM of induced responses, the reader is referred to (Chen et al. 2008, 2009). Before computing the DCM, EEG data were epoched to single trials, band-pass filtered from 0.5 to 48 Hz, and downsampled from 1024 to 200 Hz (Garrido et al. 2008). DCM was based on a modeling framework that included a core motor cortical network comprising the left M1, lPM, and SMA (Fig. 1A). We assumed reciprocal connections between these cortical regions based on anatomical studies in monkeys (Muakkassa and Strick 1979; Barbas and Pandya 1987; Fang et al. 2005). We used the identical coordinates as in our previous study (Herz et al. 2012) based on an fMRI study by Haslinger et al. (2001). The input induced by the experimental manipulation (onset of the motor task) was set to the lPM. Several considerations prompted us to include only a single input at the onset of the continuous movement rather than defining several inputs for each “submovement.” First, the repetitive movements represent a continuous motor pattern that is generated and controlled as an entity (Gerloff et al. 1998; Siebner et al. 2001), which cannot be partitioned into distinct segments (Kennerley et al. 2004). Second, we refrained from explicitly modeling discrete sensorimotor input generated by the movement itself, because the central motor control adopts a continuous “whole-field control” rather than monitoring particular aspects of each submovement during repetitive movements with a high repetition rate (Siebner et al. 2001). And finally, a wide range of previous studies have used constant intervals of continuous movements exceeding the duration of one “submovement” for analyzing cortical connectivity patterns (Gross et al. 2002; Timmermann et al. 2003; Serrien et al. 2004; Pollok et al. 2005; Lalo et al. 2008). This approach was confirmed by inspecting the predictions of time frequency plots, which revealed that adequate predictions of spectral responses were not restricted to the beginning of a trial but could be modeled for the whole trial duration (see Results section).
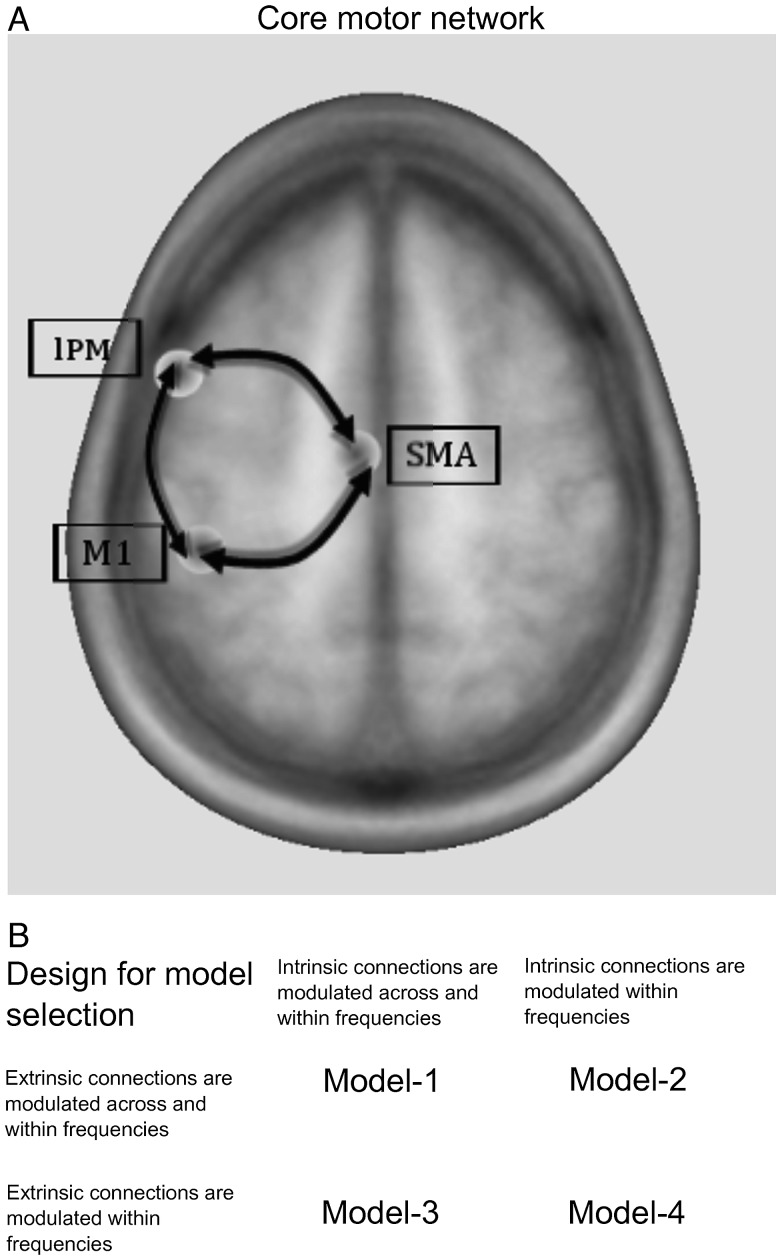
Figure 1.
Core motor cortical network. (A) Cortical areas that were analyzed using dynamic causal modeling. All areas share reciprocal connections. Note that all sources also exhibit intrinsic (self)-coupling, which are omitted for readability. Minimum distance between the single sources is 40 mm. (B) Design for Bayesian model selection. The critical difference between the models was whether extrinsic and/or intrinsic connections were modulated only within frequencies or additionally across frequencies.
Four models were compared using DCM (Fig. 1B). The critical difference between the models was whether extrinsic connections and/or intrinsic connections were modulated only within the same frequency or within and across frequencies. Extrinsic connections refer to connections between areas, while intrinsic connections refer to “self-connections” of each source (i.e., how a connection influences itself). For each participant, the dimensionality of spectra was reduced to 4 frequency modes derived from a singular value decomposition of the spectra (Chen et al. 2009). The bandwidth for computing spectral densities was chosen from 4 to 40 Hz to account for θ-activity (4–7 Hz) that has been linked to large-scale integration during cognitive and motor events (Canolty and Knight 2010) and to avoid a potential 50-Hz electric current artifact. The time-window was set to −100 to 2000 ms with respect to task onset, which has been shown to be adequate in previous studies to reduce the amount of data for computation (Chen et al. 2010, Herz et al. 2012). We did not include the resting condition in the model, because in DCM resting data are pure noise that is best modeled by a flat line. Parameters of each model and each participant were estimated by minimizing the relative entropy defined via the data and model outcome using an expectation maximization algorithm (Chen et al. 2008). In DCM of induced responses, DCM does not model data features in sensor space. Instead, after inversion of the electromagnetic model, the power of the neural source is modeled (Litvak et al. 2011b). As the skull acts as a low-pass filter on intracerebral activity, power spectra in EEG recordings are dominated by activity in low-frequency bands (Schaul 1998). Thus, model optimization will more strongly rely on low-frequency power, because most variance of the data is explained by the low-frequency components of the frequency modes. This generally applies to DCM of neurophysiological data, but is less pronounced when using MEG or intracerebral recordings compared with EEG measurements. The different models were then compared with regards to their accuracy in explaining the data taking into account complexity of the model (Penny et al. 2004). Here, we compared the different models using Bayesian model selection for random effects (Stephan et al. 2009). The model with the highest posterior exceedance probability, that is, the model with the highest relative probability compared with any other model considered, was used to make inference on coupling parameters. The prior odds ratio assumed that all models were equally likely. We opted for comparing only a few models and focusing on changes in oscillatory coupling between left M1, lPM, and SMA, because the main goal of this study was to assess changes in task-dependent modulation within a core motor network in PD.
ANOVA was used to test the significance of oscillatory coupling within the most likely model. To test whether changes in effective connectivity could be confounded by power changes of the cortical sources, we compared time–frequency spectra between groups using ANOVA. We report significant coupling at a statistical threshold of P < 0.05 familywise error (FWE)-corrected at the cluster level. In an exploratory analysis, we extracted for each participant individual coupling values from connections that were significantly modulated during the task. Coupling values were averaged over the respective significant frequencies (e.g., γ-γ coupling from lPM to SMA). Positive values indicate that a source region exerts a positive influence on a target region (i.e., increases power in the target region) during the task, while negative values indicate a negative influence (i.e., a decrease in power). We then calculated the Spearman rank correlation coefficient (2-tailed) to test whether individual differences in coupling showed a linear relationship with differences in motor impairment (UPDRS-III scores), applying Bonferroni-correction for multiple comparisons. All data are given as mean ± standard deviation, if not specified otherwise.
Results
After a short training session of 5 min, all subjects were able to perform the repetitive extension-flexion task properly with their right index finger without showing signs of fatigue throughout the experiment. Two patients showed a predominantly left-sided resting tremor, which was present OFF and ON medication. No tremor of the right hand was observed during task performance. Application of levodopa alleviated motor symptoms in all patients as reflected by a consistent decrease in the UPDRS score (OFF state: 28.18 ± 12.9 vs. ON state: 17.91 ± 11.41; P ≤ 0.001 paired samples t-test).
EMG Recordings During the Repetitive Extension-Flexion Task
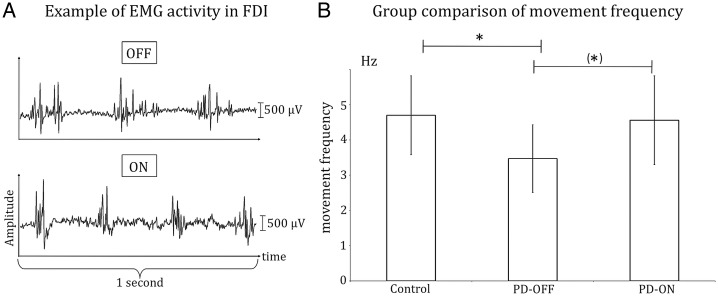
Patients OFF medication performed the task significantly slower than the control group with a mean repetition frequency of 3.47 Hz ± 0.96 compared with 4.7 Hz ± 1.12 (P < 0.05). There was a significant improvement in repetition frequency after levodopa application (ON state: 4.56 Hz ± 1.26 vs. OFF state: 3.47 Hz ± 0.96; P < 0.05) with an increase in repetition frequency in 9 of 11 patients (Fig. 2). No significant difference in repetition frequency was found when comparing PD patients ON medication and healthy controls.
Figure 2.
Movement frequency during the motor task. (A) Individual EMG data of the first dorsal interosseus muscle of a representative PD patient before (OFF) and after application of levodopa (ON). Note that movement frequency increased by ∼1 Hz after application of levodopa. (B) Results of the group comparison of movement frequency. Error bars indicate standard deviation. *P < 0.05; ns, not significant.
There were no significant differences between groups (PD-OFF, PD-ON, Control group) when comparing EMG power for the θ-, α-, β-, and γ-bands.
Bayesian Model Selection and Model Fit
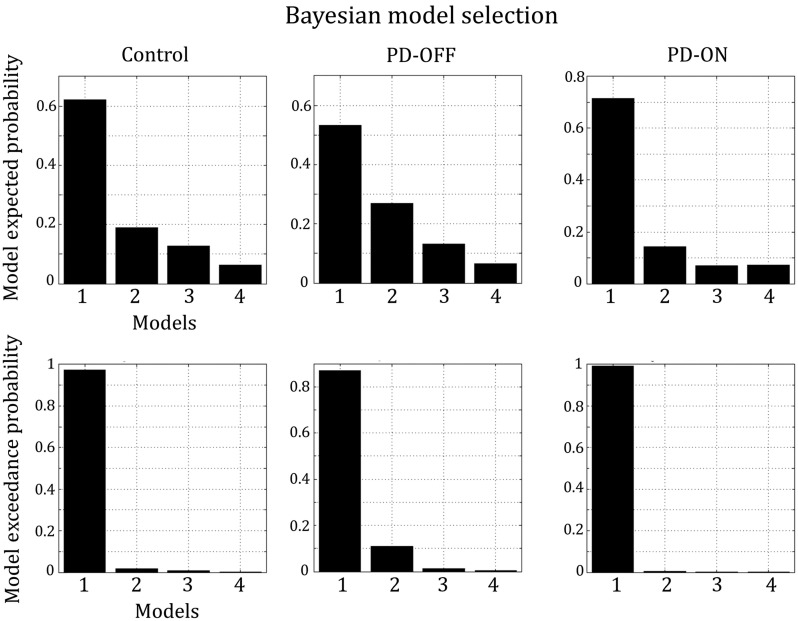
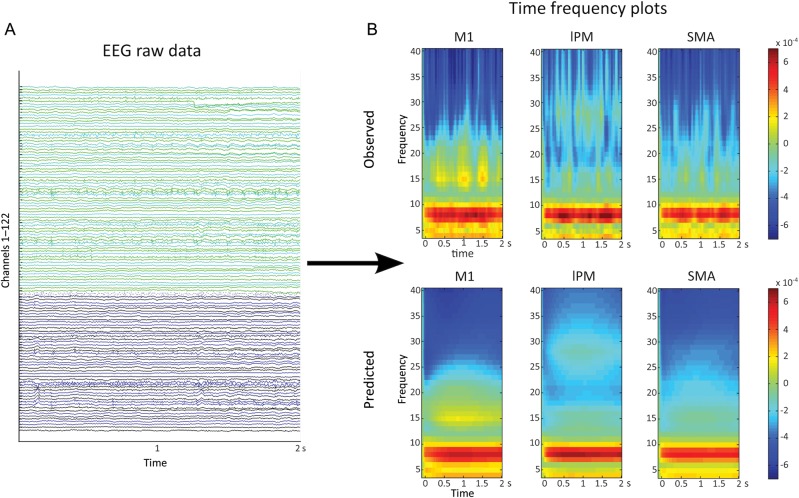
Bayesian model selection for random effects strongly favored a model postulating cross- and within-frequency coupling in both extrinsic and intrinsic (self-) connections (model 1) in all groups (Fig. 3). Exceedance probability was almost 1 in the control group and patients ON medication and ∼0.87 in patients OFF medication, highly outranking all other models. We therefore based our statistical inferences on coupling parameters as revealed by model 1. Figure 4 shows that model 1 satisfyingly predicted the observed spectral responses differently for the 3 considered regions over the whole 2 s period. The model explained ∼95% of the original spectral variance (Control: 93.4% ± 4, mean ± SD; PD-OFF: 95.1% ± 2.7%; PD-ON: 96.3% ± 1.9). On the group level, there were no significant differences in time–frequency spectra between the healthy controls, PD patients in the OFF state and PD patients in the ON state in any of the regions, indicating that the observed differences in effective connectivity were not confounded by differences in power.
Figure 3.
Results of Bayesian model selection for random effects. Model 1, which considers coupling both within and across frequencies in extrinsic and intrinsic (self)-connections, explains the data best in all groups.
Figure 4.
Spectral responses. (A) EEG raw data during the motor task. Data of a 2-s epoch is shown for a representative patient. (B) Time–frequency transformation of EEG data. The observed spectra (averaged over trials) of the same patient as in 4A are shown in the upper panel, the spectra predicted by model 1 (the best model) are shown in the lower panel. It can be clearly seen that model 1 provided a good fit explaining ∼95% variance of the data (Control group: 93.4%; PD-OFF 95.1%; PD-ON 96.3%).
Task-Induced Changes in Effective Connectivity
An overview of connections showing significant coupling during the task is given in Table 2.
Table 2.
Task-induced effective connectivity
| Control |
PD-OFF |
PD-ON |
||||
|---|---|---|---|---|---|---|
| Connection | Frequency (peak activation) | P-value (FWE-corr.) | Frequency (peak activation) | P-value (FWE-corr.) | Frequency (peak activation) | P-value (FWE-corr.) |
| lPM → M1 | γ-γ (35–36 Hz) | 0.003 | γ-γ (39–35 Hz) | 0.01 | γ-γ (32–32 Hz) | 0.021 |
| lPM → SMA | γ-γ (35–33 Hz) | 0.018 | – | – | γ-γ (35–31 Hz) | <0.001 |
| β-β (17–18 Hz) | 0.024 | |||||
| M1 → lPM | – | – | – | – | β-β (16–26 Hz) | 0.002 |
| θ-β (5–27 Hz) | 0.002 | |||||
Connections expressing significant coupling are shown for the control group (Control), PD patients OFF medication (PD-OFF) and PD patients ON medication (PD-ON). P-values are familiywise error (FWE) corrected at the cluster level.
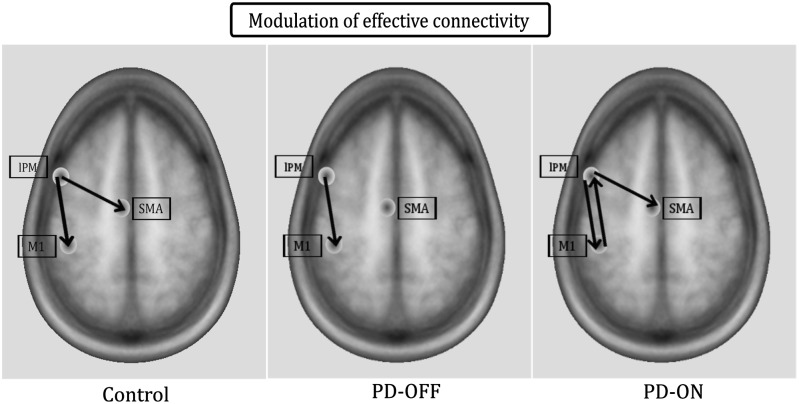
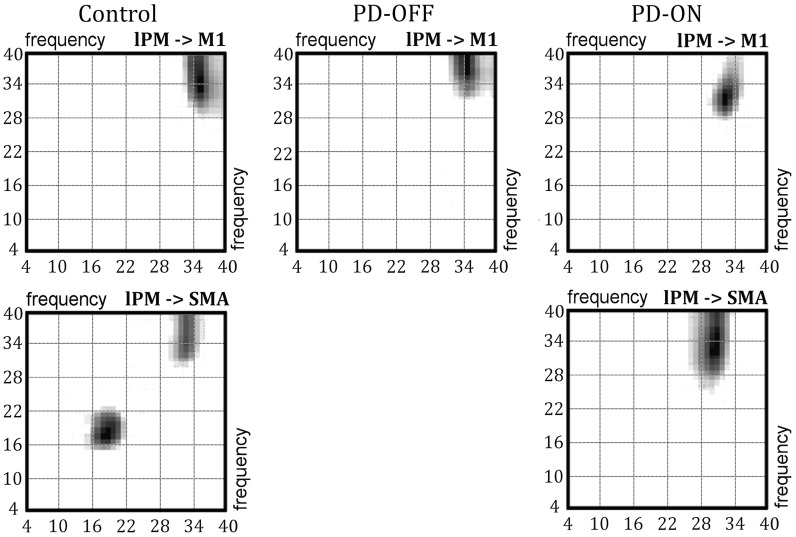
In the control group, task-induced changes in effective connectivity occurred in connections from lPM to both SMA and M1 (Fig. 5 left). Increased task-related coupling from lPM to M1 was prominent in the γ-band (γ-γ coupling), while coupling from lPM to SMA was modulated in the γ- and β-band (Fig. 6 left). No other neural connection within the tested DCM showed a task-related modulation in oscillatory coupling in healthy controls.
Figure 5.
Spatial distribution of task-induced effective connectivity. The frequencies in which the connections are modulated are listed in Table 2.
Figure 6.
Frequency–frequency matrices. The matrices (4–40 Hz) of connections from lPM to M1 (first row) and from lPM to SMA (second row) are illustrated. The left column shows matrices from the healthy control group, the middle column shows matrices from PD patients OFF medication, and the right column shows matrices from PD patients ON medication.
In the OFF medication state, PD patients showed significant coupling within the γ-band from lPM to M1, but there was no significant coupling from lPM to SMA (Figs 5 and 6 middle). After levodopa intake, PD patients expressed significant γ-γ coupling from lPM to both M1 and SMA, similar to healthy controls (Figs 5 and 6 right). Additionally, significant coupling emerged after dopamine replacement in the feedback connection from M1 to lPM, which was expressed as β-β as well as θ-β coupling (Table 2). The β-β coupling from M1 to lPM in the ON state was significantly stronger than in the control group (peak: 13–26 Hz, P = 0.01).
Correlation Between Disease Severity and Effective Connectivity
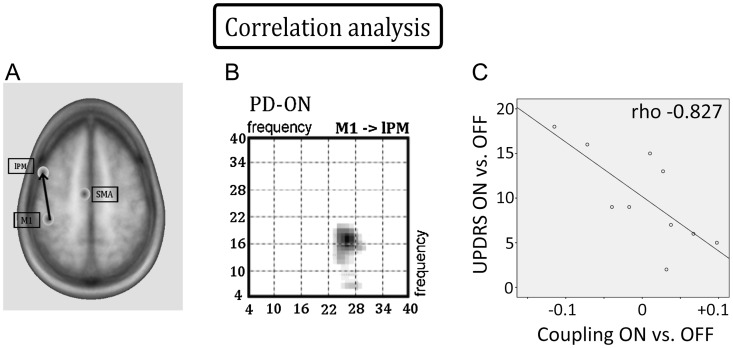
There was a significant correlation between levodopa-induced change (ON vs. OFF) of coupling from M1 to lPM and levodopa-induced benefit in motor function. This negative correlation was found for cross-frequency θ-β coupling in the feedback connection from M1 to lPM (ρ = −0.827, P < 0.01). Individual improvement in motor function was associated with an increased negative θ-β coupling from M1 to lPM. The stronger the suppressive effect of θ-activity in M1 on β-activity in lPM, the more pronounced was the individual improvement in motor function (Fig. 7). There were no significant correlations between UPDRS motor scores and effective connectivity in any of the other considered connections, neither for levodopa-induced changes of connectivity (ON vs. OFF) nor for connectivity within the OFF or ON state (P > 0.5).
Figure 7.
Correlation analysis. (A) The feedback connection from M1 to lPM expressed significant coupling in PD patients ON but not OFF medication. (B) Frequency–frequency matrix of the connection from M1 to lPM in the ON state showing significant β-β and θ-β couplings. C: Negative correlation between θ-β coupling from M1 to lPM and motor impairment (ρ = −0.827, P < 0.01). Negative coupling values indicate that θ-activity in M1 suppresses β-activity in lPM, while positive values indicate that θ-activity in M1 enhances β-activity in lPM. The stronger β-activity in lPM was suppressed, the more pronounced was the improvement in motor function.
Discussion
In this pharmacological EEG study, we used DCM of task-induced responses to model oscillatory cortico-cortical coupling during fast repetitive finger movements in patients with PD. Our study sheds significant new light on the impact of PD on motor-related cortical oscillatory coupling. Connectivity analysis revealed a functional disconnection of mesial premotor cortex with reduced γ-γ coupling from lPM to SMA. This mesial to lateral coupling in the premotor cortex was restored by dopamine replacement along with strengthening β-β as well as θ-β coupling in the feedback connection from M1 to lPM. Critically, the beneficial effect of levodopa on motor function was closely related to enhanced cross-frequency θ-β coupling from M1 to lPM.
Connectivity Pattern in Healthy Elderly Controls
In healthy elderly individuals, fast repetitive finger movements induced a change in effective connectivity in cortico-cortical connections from left lPM to M1 and SMA, presumably reflecting feed-forward computations that facilitate the regular and fast repetition of the finger movements. The connections from lPM to M1 and SMA were predominantly modulated within the γ-band, but also within lower frequency bands. The increase in γ coupling from lPM to SMA and from lPM to M1 during fast repetitive finger movements replicates the coupling pattern that we found in young healthy participants performing the same task (Herz et al. 2012). Together, the findings show a stable association between premotor-to-motor γ coupling and fast repetitive finger movements across a wide age range. This is in good accordance with previous studies, which consistently reported γ-band activity in participants performing phasic movements (Sheer et al. 1966; Pfurtscheller et al. 1997, 2003; Miller et al. 2007). We defined coordinates of the modeled sources based on a study using fMRI (Haslinger et al. 2001), which has a much higher spatial resolution than EEG studies. As there is little localizing information in electromagnetic signals, slight changes of source localization would not very much alter the data features (Chen et al. 2010). However, one has to bear in mind that EEG and fMRI recordings measure different aspects of neural activation. Scheeringa et al. (2011) have demonstrated that the blood-oxygen-level-dependent signal measured in fMRI studies correlates differently with distinct frequency bands measured in electrophysiological recordings. This incongruency between fMRI and EEG studies has to be considered when interpreting the results of the current study where EEG analysis was informed by findings from fMRI studies. Another critical point is whether the “correct” number of sources was included in the model. Importantly, including more sources in the model would not affect the conclusion about effective connectivity drawn in this study, because effective connectivity can be polysynaptic and could therefore be mediated by unmodeled sources. The main concern would be that choosing too few sources would leave variance in the data features unexplained. This, however, is unlikely given the high proportion of variance explained by our DCM (on average 95%).
Abnormal Oscillatory Coupling Between Premotor and Motor Cortex in PD
Healthy individuals and patients with PD shared motor network properties with respect to the spatial distribution of oscillatory coupling. During fast repetitive finger movements, PD patients irrespective of medication state as well as healthy controls showed γ-γ coupling from lPM to M1. However, DCM analysis revealed impaired cortico-cortical coupling as PD patients in the OFF state lacked the physiological γ-γ coupling from lPM to SMA that was present in healthy participants. In healthy individuals, γ-activity is associated with the preparation and execution of phasic movements, whereas β-activity in the motor system is thought to be related to the maintenance of a stable sensorimotor state (Engel and Fries 2010). In PD patients, decreased activity in the γ-band and increased activity in the β-band during movement execution has been assigned an antikinetic effect (Brown and Marsden 1998; Schnitzler and Gross 2005). Application of levodopa can restore γ-activity in cortical areas, which may be induced through efferent connections from the STN (Williams et al. 2002; Litvak et al. 2012). However, β-activity cannot be viewed as pathological per se. Isometric contractions are related to cortico-muscular (Salenius and Hari 2003; Schoffelen et al. 2008) and cortico-cortical coupling (Herz et al. 2012) in the β-band. A recent study identified modulation of β-activity during encoding of incremental force generation (Florin et al. 2013). Thus, modulation of neural activity in different frequency bands is state dependent and has to be interpreted with caution depending on the specific task.
Our results extend previous work by showing that PD patients are capable of expressing γ-activity in cortical premotor areas during phasic finger movements even in the OFF state. However, γ-activity in the lPM is not efficiently coupled with the mesial premotor cortex in the absence of levodopa. Critically, dopamine replacement reinstated a normal amount of γ-γ coupling from lPM to SMA. It has been shown that distinct cortico-cortical and cortico-subcortical connections express their functional coupling at specific frequencies (Silberstein et al. 2005; Hirschmann et al. 2011; Litvak et al. 2011a). Here, we show that in PD, the expression of altered frequency-specific coupling in cortico-cortical pathways is state dependent and can be modulated by dopamine replacement. The patients in this study were tested in a “relative OFF state” after 12 h withdrawal of dopaminergic medication. This approach is common in neuroimaging studies of PD, but it does not allow studying a “real OFF state,” because long-term adaptation in neural networks has probably been induced by chronic treatment. Future electrophysiological studies in de novo PD patients need to assess the effect of dopamine replacement on “drug naïve” neural networks in PD.
Several neuroimaging studies have provided evidence for abnormal cortical activity and connectivity in PD. In the OFF state, PD patients consistently showed hypoactivation of SMA both at rest (Skidmore et al. 2013) and during movements (Sabatini et al. 2000; Haslinger et al. 2001; Buhmann et al. 2003; Wu et al. 2010). Additionally, increased activation of lPM has repeatedly been reported in the OFF state (Sabatini et al. 2000; Haslinger et al. 2001; Rowe et al. 2010; Wu et al. 2010). In a recent fMRI study, DCM revealed that the selection of actions enhanced coupling between prefrontal cortex (PFC) and the rostral SMA in medicated PD patients (Rowe et al. 2010). The same PD patients showed increased coupling between PFC and lPM but not rostral SMA after medication withdrawal (Rowe et al. 2010). Although we did not model prefrontal sources in the current model and the fMRI study by Rowe et al. used a more cognitively demanding action selection task, the switch from a more lateral information flow (via a PFC-lPM connection) in the OFF state to a more latero-medial information flow (via a PFC-rostral SMA connection) in the ON state was similar to the finding that γ-γ coupling from lPM to SMA was only observed in the ON state. Extending the finding by Rowe et al. (2010), we show that dysfunctional cortico-cortical communication is expressed as aberrant oscillatory coupling in PD, comprising a functional lateral to medial disconnection in the premotor cortex. The alterations in coupling patterns are likely to be task dependent. Here, we chose a highly automated motor task, which required the fast release of sequential agonist-antagonist movements (i.e., fast self-paced extension-flexion movements). This task was not demanding in terms of cognitive control of actions, but was highly challenging in terms of motor execution. This enabled us to focus our analysis on a core premotor-motor network. Future studies need to examine to what extent PFC can modulate the altered communication between premotor and motor areas, for instance, by using motor tasks requiring a higher amount of attention.
It is important to note that we cannot draw conclusion about the causality between absence of γ-γ coupling from lPM to SMA and impaired motor function. As PD patients OFF medication performed the motor task more slowly than healthy participants and PD patients ON medication, the observed change in effective connectivity might be due to differences in performance. This question can be addressed by applying motor tasks that are more rigorously controlled for equal task performance, even though withdrawal of medication in PD patients will always lead to at least subtle changes in motor performance. Another shortcoming of this study is that PD patients always performed the motor task in the OFF state followed by a second recording in the ON state, while healthy subjects only performed the task once. We chose a simple motor task that is highly overlearned and automatic and additionally included a 5-min training session before recordings to make sure that all participants were able to perform the task adequately. This makes learning effects underlying the observed differences in effective connectivity highly unlikely. However, we cannot discount possible unspecific effects of task order.
While dopaminergic medication reinstated physiological γ-γ coupling from lPM to SMA, it did not normalize the task-related cortical coupling pattern. Healthy subjects expressed both γ-γ and β-β coupling from lPM to M1, but PD patients ON medication only showed significant γ-γ coupling from lPM to M1 during the task. This is in line with previous studies showing that cortical activity is modified but not normalized by levodopa intake (Haslinger et al. 2001, Palmer et al. 2010, Tropini et al. 2011). This is particularly interesting, because there was no difference in task performance between PD patients ON medication and healthy controls in the current study. These findings suggest that clinical improvement induced by dopamine replacement is not mediated by restoration of physiological neural networks, but that improved motor function might be related to abnormal neural activation patterns. This notion is further supported by the finding that feedback coupling from M1 to lPM was only expressed in PD patients ON medication, but not in healthy participants.
Enhanced Feedback Coupling from Primary Motor Cortex to Lateral Premotor Cortex
In the current study, levodopa increased within- and cross-frequency coupling from M1 to lPM, which was closely associated with levodopa-induced improvement in motor function. This finding suggests that levodopa modulates feedback connections in PD patients performing fast repetitive finger movements.
Although the minimum distance between cortical sources was 4 cm to account for the low spatial resolution of EEG, the oscillatory activity recorded over M1 is likely to contain activity from adjacent parts of dorsal premotor and somatosensory cortex, which are located in close proximity to the primary motor hand area (Geyer et al. 1996; Geyer et al. 2000; Srinivasan et al. 2006). The increase in feedback connections from M1 after application of levodopa might therefore also convey the “efference copy” signals of the executed movements, which constitute a central role in the control of movements (Sperry 1950; Ghasia et al. 2008). The basal ganglia with the direct and indirect pathway provide a dual system for center (excitatory)-surround (inhibitory) mechanism to focus its effect on selected cortical neurons (Mink 1996). It has been argued that this center-surround mechanism is used to focus the output to a specific group of muscles required for performing a specific task (Mink 2003). This operation is made possible through opening the sensory channel for the expected sensory feedback afferents during movement. Thus, one of the important functions of basal ganglia seems to be the gating of sensory input for motor control. Even though levodopa leads to an alleviation of motor impairment in PD, it does not restore physiological motor function. Hence, PD patients might rely stronger on feedback and efferent copy signals than healthy individuals. Sensory feedback originating from receptors in skin, muscle, and joints plays a pivotal role during the execution of movements (Lemon and Porter 1976; Riddle and Baker 2005; Baker 2007; Patino et al. 2008). In the current study, we found a strong inverse correlation between cross-frequency θ-β coupling from M1 to lPM and individual motor improvement after levodopa intake. The more β-activity in lPM was suppressed by θ-activity in M1, the stronger was the individual benefit in motor function. Activity in the θ-band has been linked to a range of different cognitive functions, for example, working memory (Jensen and Tesche 2002) or spatial navigation (Caplan et al. 2001), which demand integration of information from various brain regions. In the current study, activity in the θ-band also corresponded roughly to the repetition frequency of the fast finger movements at ∼4 Hz. Interestingly, recent studies have shown that θ-oscillations are involved in sensorimotor integration in humans (Caplan et al. 2003; Cruikshank et al. 2012). Thus, even though previous studies have focused mainly on activity in higher frequency bands during movement, θ-oscillations might play a central role in the human motor system. Oscillations in the β-band, on the other hand, are thought to have an antikinetic effect during phasic movements (Brown and Marsden 1998). We therefore hypothesize that negative feedback coupling between the θ- and β-band observed in this study might indicate improved integration of the afferent feedback with the efference copy signal leading to a suppression of antikinetic β-oscillations in the premotor cortex. An improved integration of sensory feedback might thus constitute a dopamine-dependent compensatory mechanism in patients with PD.
Funding
This work was supported by the German Research Foundation [Clinical Research Group 219 to L.T.]; the Koeln Fortune Program/Faculty of Medicine, University of Cologne [179/2007 to D.M.H.]; the Danish Medical Research Council [FSS 09-072163 to D.M.H.]; and Lundbeckfonden [Grant of Excellence “ContAct” R59 A5399 to H.R.S.]. Funding to pay the Open Access publication charges for this article was provided by the German Research Foundation (Clinical Research Group 219).
Notes
Conflict of Interest: None declared.
References
- Aoki T, Francis PR, Kinoshita H. Differences in the abilities of individual fingers during the performance of fast, repetitive tapping movements. Exp Brain Res. 2003;152:270–280. doi: 10.1007/s00221-003-1552-z. [DOI] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol. 2007;17:649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351:1801–1804. doi: 10.1016/S0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI—cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Henson RN, Stephan KE, Kilner JM, Friston KJ. Forward and backward connections in the brain: a DCM study of functional asymmetries. Neuroimage. 2009;45:453–462. doi: 10.1016/j.neuroimage.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kiebel SJ, Friston KJ. Dynamic causal modelling of induced responses. Neuroimage. 2008;41:1293–1312. doi: 10.1016/j.neuroimage.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kilner JM, Friston KJ, Kiebel SJ, Jolly RK, Ward NS. Nonlinear coupling in the human motor system. J Neurosci. 2010;30:8393–8399. doi: 10.1523/JNEUROSCI.1194-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank LC, Singhal A, Hueppelsheuser M, Caplan JB. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. J Neurophysiol. 2012;107:65–77. doi: 10.1152/jn.00893.2010. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Reyns N, Blond S, Guieu JD, Destee A, Defebvre L. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson's disease. Brain. 2004;127:408–419. doi: 10.1093/brain/awh053. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL UPDRS Development Committee. MacMillan Healthcare Information. 1987. Unified Parkinson's disease rating scale. Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson's disease. Vol. 2, pp. 153–163. Florham Park, NJ: Macmillan Health Care Information. [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Florin E, Dafsari HS, Reck C, Barbe MT, Pauls KA, Maarouf M, Sturm V, Fink GR, Timmermann L. Modulation of local field potential power of the subthalamic nucleus during isometric force generation in patients with Parkinson's disease. Neuroscience. 2013;240:106–116. doi: 10.1016/j.neuroscience.2013.02.043. [DOI] [PubMed] [Google Scholar]
- Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage. 2008;42:936–944. doi: 10.1016/j.neuroimage.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Uenishi N, Nagamine T, Kunieda T, Hallett M, Shibasaki H. Cortical activation during fast repetitive finger movements in humans: steady-state movement-related magnetic fields and their cortical generators. Electroencephalogr Clin Neurophysiol. 1998;109:444–453. doi: 10.1016/S0924-980X(98)00045-9. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Ghasia FF, Meng H, Angelaki DE. Neural correlates of forward and inverse models for eye movements: evidence from three-dimensional kinematics. J Neurosci. 2008;28:5082–5087. doi: 10.1523/JNEUROSCI.0513-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A. The neural basis of intermittent motor control in humans. Proc Natl Acad Sci USA. 2002;99:2299–2302. doi: 10.1073/pnas.032682099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Herz DM, Christensen MS, Reck C, Florin E, Barbe MT, Stahlhut C, Pauls KA, Tittgemeyer M, Siebner HR, Timmermann L. Task-specific modulation of effective connectivity during two simple unimanual motor tasks: a 122-channel EEG study. Neuroimage. 2012;59:3187–3193. doi: 10.1016/j.neuroimage.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson's disease. Neuroimage. 2011;55:1159–1168. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol. 2004;91:978–993. doi: 10.1152/jn.00651.2003. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo E, Thobois S, Sharott A, Polo G, Mertens P, Pogosyan A, Brown P. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci. 2008;28:3008–3016. doi: 10.1523/JNEUROSCI.5295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998b;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998a;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976;194:313–339. doi: 10.1098/rspb.1976.0082. [DOI] [PubMed] [Google Scholar]
- Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, et al. Movement-related changes in local and long-range synchronization in Parkinson's disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci. 2012;32:10541–10553. doi: 10.1523/JNEUROSCI.0767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011a;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, Kilner J, Barnes G, Oostenveld R, Daunizeau J, Flandin G, et al. EEG and MEG data analysis in SPM8. Comput Intell Neurosci. 2011b;2011:852961. doi: 10.1155/2011/852961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson's disease. Brain. 2001;124:378–388. doi: 10.1093/brain/124.2.378. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/S0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Lee PW, Wang ZJ, Au WL, Mckeown MJ. theta, beta But not alpha-band EEG connectivity has implications for dual task performance in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:393–397. doi: 10.1016/j.parkreldis.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Patino L, Omlor W, Chakarov V, Hepp-Reymond MC, Kristeva R. Absence of gamma-range corticomuscular coherence during dynamic force in a deafferented patient. J Neurophysiol. 2008;99:1906–1916. doi: 10.1152/jn.00390.2007. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol. 2003;114:1226–1236. doi: 10.1016/S1388-2457(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int J Psychophysiol. 1997;26:121–135. doi: 10.1016/S0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/S0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Muller K, Aschersleben G, Schnitzler A. The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage. 2005;24:646–655. doi: 10.1016/j.neuroimage.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, Delong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol. 2005;566:625–639. doi: 10.1113/jphysiol.2005.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R. Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage. 2002a;17:988–998. doi: 10.1006/nimg.2002.1156. [DOI] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain. 2002b;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes LE, Barker RA, Owen AM. Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment? Neuroimage. 2010;52:1015–1026. doi: 10.1016/j.neuroimage.2009.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Siebner HR. The motor system and its disorders. Neuroimage. 2012;61:464–477. doi: 10.1016/j.neuroimage.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Salenius S, Hari R. Synchronous cortical oscillatory activity during motor action. Curr Opin Neurobiol. 2003;13:678–684. doi: 10.1016/j.conb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Schaul N. The fundamental neural mechanisms of electroencephalography. Electroencephalogr Clin Neurophysiol. 1998;106:101–107. doi: 10.1016/S0013-4694(97)00111-9. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Imaging the human motor system's beta-band synchronization during isometric contraction. Neuroimage. 2008;41:437–447. doi: 10.1016/j.neuroimage.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Pogosyan AH, Brown P. Cortico-cortical coupling patterns during dual task performance. Exp Brain Res. 2004;157:79–84. doi: 10.1007/s00221-003-1822-9. [DOI] [PubMed] [Google Scholar]
- Sheer DE, Grandstaff NW, Benignus VA. Behavior and 40-c-sec. electrical activity in the brain. Psychol Rep. 1966;19:1333–1334. doi: 10.2466/pr0.1966.19.3f.1333. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Limmer C, Peinemann A, Bartenstein P, Drzezga A, Conrad B. Brain correlates of fast and slow handwriting in humans: a PET-performance correlation analysis. Eur J Neurosci. 2001;75:249–261. doi: 10.1046/j.0953-816x.2001.01694.x. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Pogosyan A, Kuhn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain. 2005;128:1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- Skidmore FM, Yang M, Baxter L, Von Deneen KM, Collingwood J, He G, White K, Korenkevych D, Savenkov A, Heilman KM, et al. Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage. 2013;240:106–116. doi: 10.1016/j.neuroimage.2011.06.056. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Nunez PL. Source analysis of EEG oscillations using high-resolution EEG and MEG. Prog Brain Res. 2006;159:29–42. doi: 10.1016/S0079-6123(06)59003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl AJ, Martin WW, Mckeown MJ, Sossi V. Advances in imaging in Parkinson's disease. Lancet Neurol. 2011;10:987–1001. doi: 10.1016/S1474-4422(11)70214-9. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Fink GR. Pathological network activity in Parkinson's disease: from neural activity and connectivity to causality? Brain. 2011;134:332–334. doi: 10.1093/brain/awq381. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Florin E, Reck C. Pathological cerebral oscillatory activity in Parkinson's disease: a critical review on methods, data and hypotheses. Expert Rev Med Devices. 2007;4:651–661. doi: 10.1586/17434440.4.5.651. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tropini G, Chiang J, Wang ZJ, Ty E, Mckeown MJ. Altered directional connectivity in Parkinson's disease during performance of a visually guided task. Neuroimage. 2011;56:2144–2156. doi: 10.1016/j.neuroimage.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain. 2010;133:2394–2409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]