Abstract
The relationship between anxious/depressed traits and neuromaturation remains largely unstudied. Characterizing this relationship during healthy neurodevelopment is critical to understanding processes associated with the emergence of child/adolescent onset mood/anxiety disorders. In this study, mixed-effects models were used to determine longitudinal cortical thickness correlates of Child Behavior Checklist (CBCL) and Young Adult Self Report Anxious/Depressed scores in healthy children. Analyses included 341 subjects from 4.9 to 22.3 year-old with repeated MRI at up to 3 time points, at 2-year intervals (586 MRI scans). There was a significant “CBCL Anxious/Depressed by Age” interaction on cortical thickness in the right ventromedial prefrontal cortex (vmPFC), including the medial orbito-frontal, gyrus rectus, and subgenual anterior cingulate areas. Anxious/Depressed scores were negatively associated with thickness at younger ages (<9 years), but positively associated with thickness at older ages (15–22 years), with the shift in polarity occurring around age 12. This was secondary to a slower rate of vmPFC cortical thinning in subjects with higher scores. In young adults (18–22 years), Anxious/Depressed scores were also positively associated with precuneus/posterior cingulate cortical thickness. Potential neurobiological mechanisms underlying this maturation pattern are proposed. These results demonstrate the dynamic impact of age on relations between vmPFC and negative affect in the developing brain.
Keywords: anxiety, brain development, Child Behavior Checklist, depression, magnetic resonance imaging
Introduction
Magnetic resonance imaging (MRI) studies of subjects with major depressive disorder (MDD), or at risk for developing MDD, have greatly improved our understanding of the neurocircuitry involved in internalizing disorders (Peterson et al. 2009; Chen et al. 2010; Price and Drevets 2012). However, one major limitation in the literature is that relations between subclinical anxious/depressive symptoms and healthy brain development have yet to be elucidated. Subclinical depressive and anxious symptoms are frequent in children and teenagers (Bell-Dolan et al. 1990; Pine et al. 1999), and high levels of symptoms in adolescents have been shown to correlate with the risk of adult MDD on longitudinal follow-up (Pine et al. 1999). Understanding how brain structure relates to anxious/depressed problems, over time, in typically developing youths is critical in that it may shed light on neurodevelopmental processes underpinning the emergence of mood and anxiety disorders. In the present study, we investigate cerebral cortical thickness correlates of subclinical anxious/depressed problems across development in a large cohort of healthy youths and young adults. This approach dovetails with the National Institute of Mental Health's Research Domain Criteria project, calling for a shift in research focus from categorical diagnoses towards examination of symptom dimensions (Morris and Cuthbert 2012).
At the level of the cerebral cortex, medial prefrontal cortices (mPFC), in particular the ventromedial prefrontal cortex (vmPFC), have been most consistently associated with major depression, normal sadness, and negative affect, both in morphometric and functional imaging studies (Drevets et al. 1997; Rajkowska et al. 1999; Drevets 2000, 2007; Zald et al. 2002; Coryell et al. 2005; Drevets, Savitz, et al. 2008; van Tol et al. 2010; Hamani et al. 2011; Myers-Schulz and Koenigs 2012). This area of the prefrontal cortex includes the anterior cingulate cortex (ACC), the subgenual anterior cingulate cortex (SgACC), and the medial orbitofrontal cortex (OFC) (Bremner et al. 2002; Drevets 2007; Drevets, Price et al. 2008; Hamani et al. 2011). In general, patients with MDD have been found to exhibit volumetric reductions in the OFC, ACC, and SgACC, as well as increased metabolic activity in the SgACC (Öngür et al. 1998; Mayberg et al. 1999; Drevets, Savitz et al. 2008). The SgACC is also functionally activated in normal sadness reactions and emotional processing tasks in children and adults (Mayberg et al. 1999; Damasio et al. 2000; Phan et al. 2002; Killgore and Yurgelun-Todd 2006). In pediatric populations, Botteron et al. (2002) found reduced left SgACC volume in teenage females with MDD. Morphometric studies of anxiety disorders have reported less consistent results (Richert et al. 2006; Monk 2008; Radua and Mataix-Cols 2009; Kühn et al. 2011). Few studies have looked at neuroanatomical correlates of pediatric depressive and anxious symptoms in nonclinical populations. Boes et al. (2008) found that rostral ACC volume in healthy boys of 7–17 years of age was negatively correlated with depressive symptoms.
Based on converging evidence from both structural and functional neuroimaging research, and nonhuman primate tracer studies, Price and Drevets (2010) have described the medial prefrontal network as central to the mediation of mood disorder symptomatology and negative affect processing (Drevets, Price et al. 2008). The medial prefrontal network includes areas on the ventromedial surface of the frontal cortex, rostral and ventral to the genu, areas along the medial edge of the orbital cortex, and a caudolateral orbital region at the rostral end of the insula (Price and Drevets 2012; Carmichael and Price 1996). This network has extensive connections with both the amygdala and the visceral control areas in the hypothalamus and periaqueductal gray matter (Carmichael and Price 1995). The medial PFC also has connection with the dorsolateral prefrontal cortex, which is considered to be a key brain area in cognitive appraisal of negative affects (Price and Drevets 2012). Finally, there are cortico-cortical connections with the posterior cingulate cortex, bearing resemblance to the default mode network (DMN), a key functional network in self-referential thoughts (Andrews-Hanna et al. 2010). Therefore, medial prefrontal structures are ideally situated to modulate activity in core limbic regions, and thus, regulate affect (Foland-Ross et al. 2010). Indeed, dysfunction in the mPFC network, in conjunction with hyperactive and/or disinhibited amygdala activation, has been posited to underpin both depressive and anxious disorders (Drevets, Price et al. 2008; Monk 2008; Price and Drevets 2010, 2012; Murray et al. 2011), with potentially different roles for posterior vmPFC and perigenual vmPFC (Myers-Schulz and Koenigs 2012).
At the phenotypic level, the Anxious/Depressed syndrome (A/D) of the Child Behavior Checklist (CBCL) is a clinically useful way to assess both depressive and anxious symptoms in children and adolescents (Achenbach and Ruffle 2000). The A/D syndrome, like the 7 other narrow and 2 broad band (internalizing and externalizing behavior) syndromes, resulted from empirically based, factor analytic techniques (Achenbach 1991). The A/D scale has been found to correlate with the diagnosis of adolescent depression (Rey and Morris-Yates 1992), and on longitudinal follow-up, A/D scores were revealed to be more predictive of adolescent and young adult major depression than anxiety disorders (Roza et al. 2003). The A/D syndrome is strongly biologically determined, with up to 61–65% of the variance accounted for by genetic factors (Hudziak et al. 2000). The Young Adult Self Report (YASR) A/D syndrome provides similar information in subjects over 18 years of age, but is based on self report rather than parental ratings (Achenbach 1997).
MRI cortical thickness measurement can identify subtle gray matter variations in the cerebral cortex, and has revealed significant brain–behavior relations in healthy children and pediatric psychiatric disorders (Shaw et al. 2007; Karama et al. 2009; Ducharme et al. 2011). Of note, recent cortical thickness studies on attention and ADHD have demonstrated the importance of taking into account the dynamic impact of age when studying morphometric correlates of specific behaviors (Shaw et al. 2007, 2011, Ducharme et al. 2012). Further supporting this approach, recent evidence suggests that major functional cerebral networks involved in affective processes progressively develop from a structural point of view during childhood and adolescence (Zielinski et al. 2010). To our knowledge, no studies have looked at how anxious/depressed symptoms are related to cortical developmental trajectories over time.
In this study, we investigate the possibility that subclinical anxious/depressed symptoms are related to neurodevelopment within particular prefrontal regions in healthy youths and young adults. Specifically, using a large longitudinal cohort of healthy typically developing youths (from 4.9 to 22.3 years of age), we assess the degree to which anxious/depressed characteristics qualify cerebral cortical thickness development. Based on the above literature, we hypothesized that A/D scores would be associated with cortical development in regions strongly implicated in top-down modulation of core limbic structures—in particular, regions comprising the medial prefrontal network (Price and Drevets 2010, 2012).
Materials and Methods
Sampling and Recruitment
The NIH MRI Study of Normal Brain Development is a multisite project aiming to provide a normative database to characterize healthy brain maturation in relationship to behavior (Evans and Brain Development Cooperative Group 2006). Subjects were recruited at 6 pediatric study centers across the United States of America with a population-based sampling method seeking to minimize biases of selection (Waber et al. 2007). Based on available US Census 2000 data, 431 healthy children from 4 years and 6 months to 18 years and 3 months were recruited (Objective 1) with continuous monitoring in order to assure that the sample was demographically representative of the US population on the basis of variables that included age, gender, race, ethnicity, and socioeconomic status. Informed consent from parents and child assent were obtained for all subjects.
There were extensive exclusion criteria including meeting criteria for current or past Axis I diagnosis established with the Diagnostic Interview Schedule for Children and Adolescents (C-DISC4), except simple phobia, social phobia, adjustment disorder, oppositional defiant disorder (ODD), enuresis, encopresis, and nicotine dependence. Simple phobia, social phobia, adjustment disorder, and ODD were not part of exclusion criteria because, at the time of the study design (2001), there were no convincing data suggesting structural abnormalities in these disorders. Of note, all subjects in this study had CBCL A/D t-scores ≤70 (pathological threshold) at all 3 visits; therefore, this should not represent a confounding factor in this study. Other exclusion criteria included family history of major Axis I disorder, family history of inherited neurological disorder or mental retardation due to nontraumatic events, abnormality on neurological examination, gestational age at birth <37 weeks or >42 weeks, intrauterine exposure to substances known or highly suspected to alter brain structure or function, and so forth. Children with substance misuse disorders other than nicotine were excluded from the study. A more comprehensive list of inclusion/exclusion criteria is available in Evans and Brain Development Cooperative Group (2006).
All subjects underwent extensive cognitive, neuropsychological, and behavioral testing along with up to 3 MRI brain scans at 2-year intervals (Visits 1, 2, and 3). With regard to participants of the present study, the age range at Visit 1 was 4.9–18.3 years; 6.4–20.2 years at Visit 2; and 8.4–22.3 years at Visit 3. Structural MRI and clinical/behavioral data were consolidated and analyzed within a purpose-built database at the Data Coordinating Center of the Montreal Neurological Institute (MNI), McGill University.
Child Behavior Checklist and Young Adult Self Report
The CBCL is an age-appropriate standardized questionnaire completed by parents (Achenbach and Ruffle 2000; Sadock et al. 2009). The CBCL/6–18 that was utilized in this study is divided into 8 subscales, including the A/D scale (Achenbach 1991; Achenbach and Rescorla 2001). It is a reliable measure with high stability over time (8-day test–retest reliability for CBCL A/D is r = 0.82, Cronbach's α = 0.84; 12- and 24-month stabilities of the CBCL A/D scale are r = 0.68 and r = 0.56, respectively), and it has been validated in multiple cultures (Achenbach and Rescorla 2001). There were 10 subjects below 6 years of age who were assessed with the parent rated CBCL/1½–5. Maximum possible A/D scores on the CBCL/6–18 and CBCL/1½–5 are, respectively, 26 and 16. Therefore, A/D scores of the 10 subjects assessed with the CBCL 1½–5 were multiplied by 1.625 to make them comparable to the CBCL/6–18 scale. For subjects over 18 years of age, YASR A/D scores were used for a separate set of analyses since it is a self-reported measure rather than a parent-rated score (Achenbach 1997). The YASR was derived from items on the CBCL and serves as a self-report extension of the CBCL for young adults. Parents rated CBCL A/D scores have been showed to be predictive of YASR A/D scores at 8-year follow-up (Ferdinand and Verhulst 1995).
MRI Protocol
MR data (1.5T) were collected with 1 mm in-plane resolution, 1–2 mm slice thickness, whole-brain coverage and multiple contrasts (T1W, T2W, and PDW) (Evans and Brain Development Cooperative Group 2006). A 3D T1-weighted spoiled gradient recalled echo sequence was selected. Both American College of Radiology and living phantoms were regularly scanned at each site to confirm the intersite reliability of anatomical measurements (Evans and Brain Development Cooperative Group 2006).
Automated Image Processing
Quality controlled native MR images were processed through the CIVET-automated analysis pipeline, described in details in previous manuscripts (Karama et al. 2009; Albaugh et al. 2013). This pipeline includes the CLASP algorithm for generating cortical thickness measurements at 40 962 vertices per hemisphere (Collins et al. 1995; MacDonald et al. 2000; Kim et al. 2005; Lee et al. 2006; Lyttelton et al. 2007). To ensure optimal data, a stringent visual quality control (blinded as to the CBCL/YASR scores of the subjects) of the native cortical thickness images of each subject was implemented to ensure that there were no significant aberrations in cortical thickness estimations for a given measurement (Karama et al. 2009). In total, 586 scans out of 871 were kept for statistical analyses. For the CBCL A/D analysis, there were 300 subjects (165 females, 135 males) of 4.9–18.0-year old with a total of 533 MRI scans (60 subjects with 3 scans, 113 subjects with 2 scans, 127 subjects with 1 scan). For the YASR A/D sample, there were 41 subjects (22 females, 19 males) and a total of 53 MRI scans (12 subjects had 2 data points), with ages ranging from 18.2 to 22.3 years of age. Twenty-eight of the 41 subjects of the YASR sample also had data points with CBCL prior to 18 years of age.
Statistical Analyses
Statistical analyses were implemented using SurfStat (http://www.math.mcgill.ca/keith/surfstat/), a statistical toolbox created for MATLAB (The MathWorks, Inc., Nathan, MA, USA). Each subject's absolute native-space cortical thickness (release version 4.0) was linearly regressed against A/D raw scores at each cortical point in a mixed-effects model, statistically controlling for the effects of age, total brain volume (TBV), gender, and scanner (since different scanners were used at the six sites). Age and TBV were controlled for as continuous variables, while gender and scanner were entered as categorical variables. Mixed-effect models permit the implementation of linear regressions in samples combining subjects with different number of measurements, providing a way in which to analyze unbalanced longitudinal data while maximizing statistical power (i.e., via utilizing all available data) (Diggle 2002; Singer and Willett 2003; Shaw et al. 2011). In each mixed-effects model, subject ID was entered as a random effect in order to account for within-individual dependence. Of note, contrary to previous studies that have identified quadratic and cubic maturation patterns in children (Shaw et al. 2008; Raznahan et al. 2011), cortical thickness maturation follows primarily a first-order linear decline after age 5 in the NIH MRI Study of Normal Brain Development (see Supplementary Fig. 1).
To assess the impact of age on the cortical morphometric correlates of anxious/depressed traits, we tested for an “A/D by age” interaction on cortical thickness, using the following model:
where d1 represents the random effect of subject ID, and e corresponds to residual error.
The 3-way “A/D by age by gender,” and the “A/D by gender” interactions were serially analyzed to determine the impact of gender. Significant interactions were decomposed using a centering method at every year of age (Aiken and West 1991). Handedness, IQ, and socioeconomic status (SES) measured by household income level were also tested in separate analyses as potential confounding variables. To determine the specificity of associations with A/D, results were compared with other CBCL subscales. Although the ACC (Brodmann area (BA) 24, 32), SgACC (BA 25), and OFC (BA 11, 12) were identified as a priori regions of interest (ROI) based on the literature, whole-brain random field theory (RFT) statistical corrections for multiple comparisons were implemented for all analyses (Worsley et al. 2004).
Results
Demographics
Basic demographic variables for both samples (CBCL and YASR) are included in Table 1. Complete socioeconomic demographics of the NIH MRI Study of Normal Brain Development can be found in Evans and Brain Development Cooperative Group (2006). A total of 12 different scanners were used at the 6 sites. The distribution of subjects for each scanner is provided in Supplementary Table 1. There was no difference in mean CBCL A/D score across sites (1-way ANOVA F11,521 = 1.283, P = 0.23).
Table 1.
Demographics of the Child Behavior Checklist (CBCL) and the Young Adult Self Report (YASR) Samples
| CBCL sample, n = 300 (533 scans) | YASR sample, n = 41 (53 scans) |
|---|---|
| Age (years) | |
| 11.9 ± 0.14 (4.9–18.0) | 19.8 ± 0.16 (18.2–22.3) |
| Gender | |
| Males = 230 (43.2%) | Males = 25 (47.2%) |
| Females = 303 (56.8%) | Females = 28 (52.8%) |
| Preferred hand to write | |
| R = 472 (88.6%) | R = 46 (86.8%) |
| L = 58 (10.9%) | L = 7 (13.2%) |
| Unknown = (0.5%) | Unknown = (0.0%) |
| A/D scores | |
| 1.60 ± 0.09 (0–11) | 4.51 ± 0.59 (0–21) |
Average with standard errors of the mean, ranges are between parentheses.
For subjects below 18 years of age, there were no gender differences in average CBCL A/D scores (males 1.50 ± 0.13; females 1.67 ± 0.11, P = 0.29). There was a small magnitude negative correlation between age and A/D scores (Pearson r = −0.09, P = 0.038). For subjects over 18 years of age, females tended to have higher YASR A/D scores (5.43 ± 0.96) relative to males (3.48 ± 0.61), although this trend did not reach statistical significance (P = 0.10).
Child Behavior Checklist A/D
When collapsing across age, cortical thickness was not significantly associated with A/D scores. However, the “age by A/D” interaction was highly significant in the right vmPFC, including the right gyrus rectus (BA 11), medial OFC (BA 11,12), and a portion of the right SgACC (BA 25) (Fig. 1, P ≤ 0.05, RFT corrected). Figure 2 details the interaction from age 5–18 years. Specifically, between 5 and 8 years of age, right vmPFC thickness was negatively associated with A/D scores. The magnitude of this negative association began to diminish at approximately 9 years of age, until a neutral 0 t-score was revealed at ∼12 years of age. The direction of the association then reversed to become a positive trend at age 15, and a significant positive association at age 18.
Figure 1.
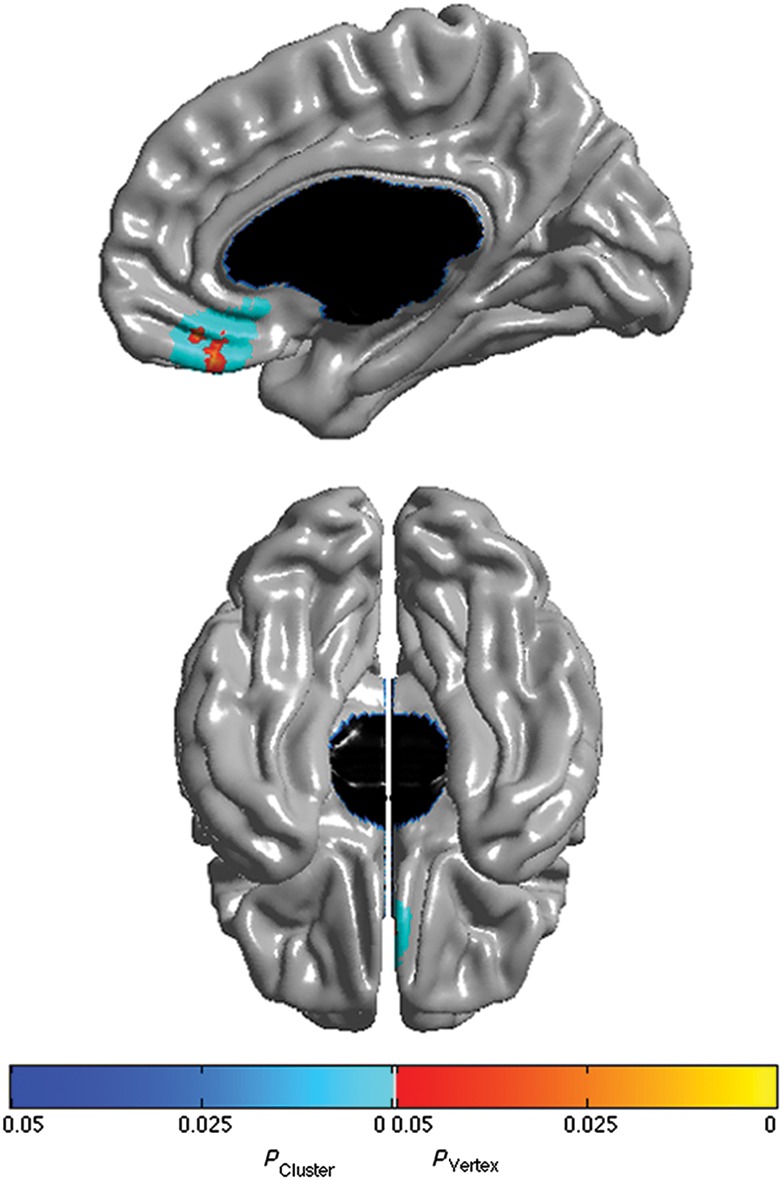
Brain areas where local cortical thickness is associated with the CBCL Anxious/Depressed score by Age interaction when controlling for gender, scanner, and total brain volume (n = 533 scans). Right midsagittal view on top and ventral view at the bottom. The peak is at MNI coordinates x = 2.1, y = 35.6, z = −23.4, which is located in the right gyrus rectus. Brain areas not shown in this figure were not significant. Figure is shown at P ≤ 0.05 with a whole-brain random field theory correction. Blue shades correspond to areas significant at the cluster level and orange shades to areas significant at the vertex level.
Figure 2.
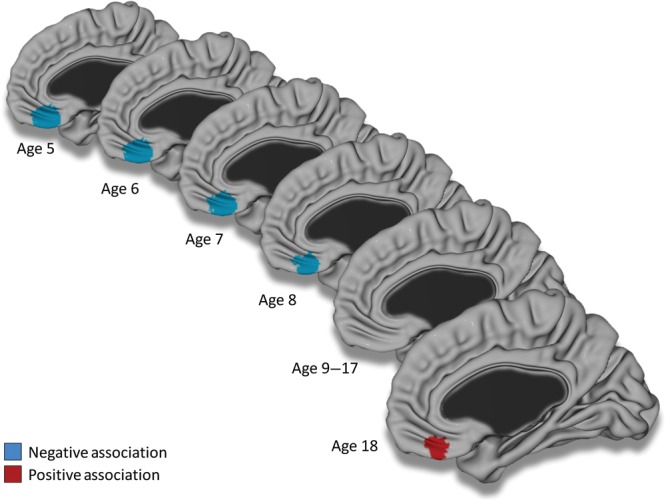
Associations between CBCL Anxious/Depressed scores and cortical thickness centered from age 5 to 18 years old. The brain is shown in the right midsagittal view with the main association in the right ventromedial prefrontal cortex. Figure is shown at P ≤ 0.05 with a whole-brain random field theory correction. Note that a negative trend was present at age 9, and positive trends were present from age 15 to 17. Controlled for gender, total brain volume, and scanner.
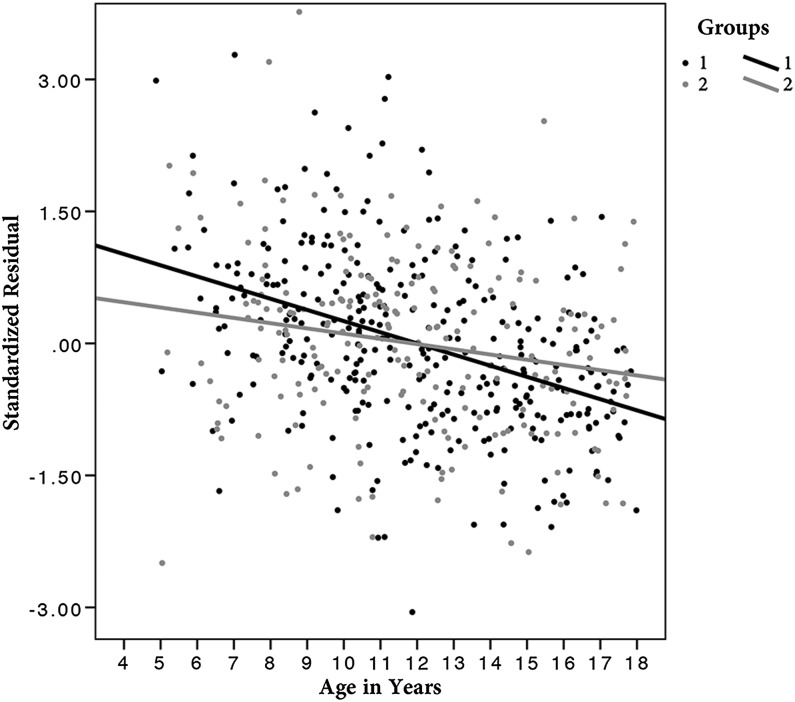
To further illustrate this interaction, subjects were divided in a group with lower scores (A/D 0 or 1) and another with higher scores (A/D > 1). As shown on the scatterplot in Figure 3, subjects with higher scores had thinner right vmPFC (mean thickness of the significant area) at baseline, but evidenced thicker cortices later in teenage years due to a slower rate of cortical thinning.
Figure 3.
Scatterplot of the right ventromedial prefrontal cortex (vmPFC) mean cortical thickness against age in years (for visualization purpose). Values on the y-axis are the standardized residuals of the linear regression between right vmPFC and total brain volume, gender, and scanner to account for these variables. Subjects were divided in 2 groups based on Child Behavior Checklist Anxious/Depressed scores (A/D). Group 1 in black is composed of children with lower scores (A/D = 0 or 1), and group 2 in gray includes subjects with higher scores (A/D > 1).
There were no “A/D by gender” or three-way “A/D by age by gender” interactions on cortical thickness. When controlling for handedness, results remained significant in the right gyrus rectus and the right medial OFC, with a trend of association in the right SgACC (see Supplementary Fig. 2). However, the “Handedness by Age by A/D” interaction was not significant, precluding any formal conclusion on the impact of handedness on the aforementioned developmental pattern. Controlling for IQ and SES had no impact on the results. To verify the specificity of our findings for CBCL A/D, other CBCL subscales were tested. There were no associations between cortical thickness and the Somatic Symptoms score, and previous studies on CBCL externalizing scales in the same sample have revealed specific cortical thickness associations in different distributions than the A/D scale (Ducharme et al. 2011 2012).
Given the slightly different scoring between CBCL 6/18 and CBCL 1½/5 that we have adjusted for, results were verified after excluding subjects assessed with the CBCL 1½/5. Results remained significant—but slightly reduced in spatial extent, confirming the initial finding. Of note, the distribution of the mean cortical thickness in the identified region of interest (mean of the significant area) had a normal distribution at each level A/D scores (confirmed by the Shapiro–Wilk test), which confirmed the validity of using a linear regression model despite some apparent positive skewness in the A/D scores distribution.
Young Adult Self-Report A/D
In subjects over 18 years of age, robust associations were revealed between YASR A/D scores and cortical thickness in regions belonging to, or sharing close anatomical connections with, the mPFC. In particular, cortical thickness in the right vmPFC (medial OFC and portions of the gyrus rectus and SgACC) and right precuneus/posterior cingulate (BA 23,31) was positively associated with YASR A/D raw scores (Fig. 4, P ≤ 0.05, RFT corrected). Outside of the extended medial prefrontal network, a positive association between YASR A/D raw score and cortical thickness was revealed in the right lingual gyrus (BA 18,19). These results were independent of age (i.e., no interaction with age), however, the age range for subjects with YASR data was small.
Figure 4.
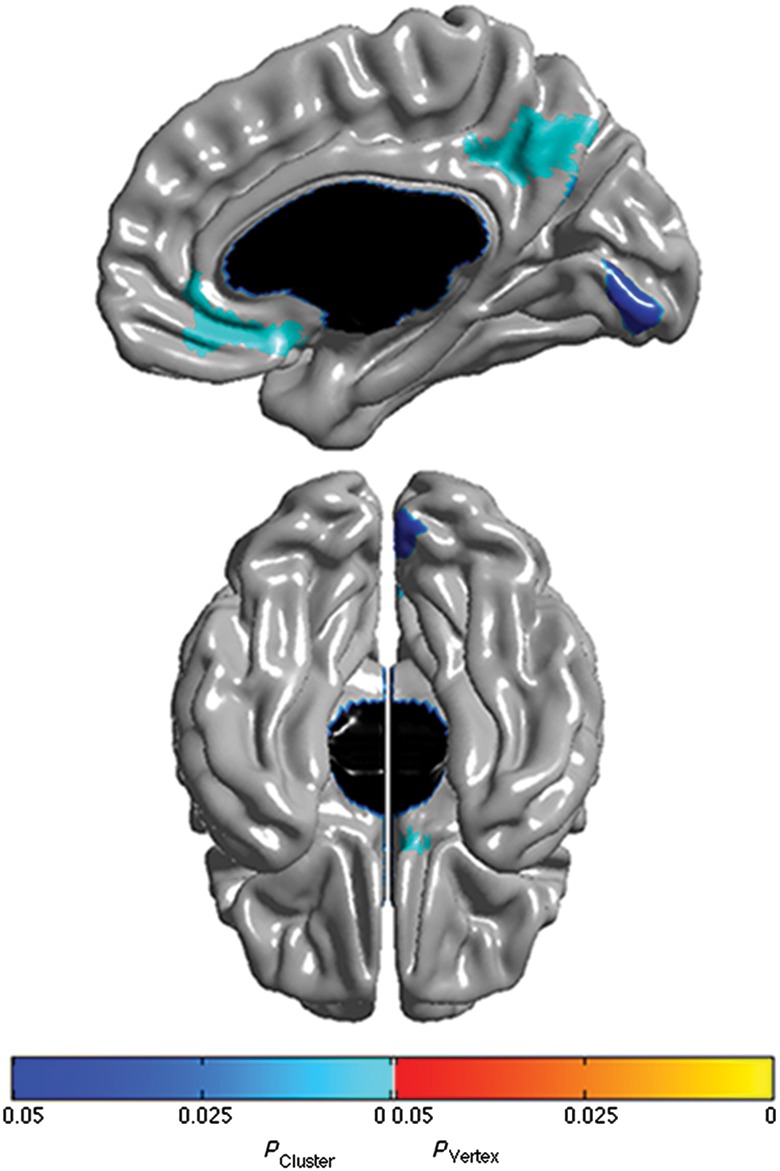
Brain areas where local cortical thickness is associated with YASR Anxious/Depressed raw score when controlling for gender, scanner, and total brain volume (n = 53 scans). Right midsagittal view on top and ventral view at the bottom. Brain areas not shown in this figure were not significant. Figure is shown at P ≤ 0.05 with a whole-brain random field theory correction. Blue shades correspond to areas significant at the cluster level and orange shades to areas significant at the vertex level.
When controlling for subjects' handedness, results remained significant in the right vmPFC and right precuneus/posterior cingulate region (see Supplementary Fig. 3). Further, controlling for handedness revealed an additional significant finding in the ventral aspect of the right inferior frontal gyrus. When controlling for IQ, again results remained significant in the right vmPFC and right precuneus/posterior cingulate region, and an additional significant finding was revealed in right frontopolar cortex (see Supplementary Fig. 4).
Discussion
This study revealed a strong interaction between age and CBCL A/D scores on cortical thickness in the right vmPFC, including the medial OFC, gyrus rectus, and SgACC. Of note, the right vmPFC was the only cortical area in which thickness was significantly associated with the “A/D by age” interaction. Post hoc analyses revealed that anxious/depressed symptoms were associated with thinner right vmPFC at younger ages (5–8 years of age) and thicker right vmPFC during adolescence (after 15 years of age). This later positive association between the right vmPFC cortical thickness and anxious/depressed symptoms was replicated in healthy young adults, using a self-report measure (as opposed to parent report). YASR A/D scores were also significantly associated with cortical thickness in the right precuneus/posterior cingulate region, as well as the right lingual gyrus. The vmPFC has been repeatedly associated with negative affect, both in structural and functional imaging studies (Mayberg et al. 1999; Drevets, Savitz et al. 2008; Hamani et al. 2011; Murray et al. 2011), but these results are the first to show a developmental relation between vmPFC morphology and anxious/depressed symptoms in typically developing youths. In terms of the rightward lateralization, our results are in line with multiple other studies (Peterson et al. 2009; Schwartz et al. 2010; Kühn et al. 2011; Light et al. 2011) however lateralization has not been a consistent finding in the literature (Botteron et al. 2002; Boes et al. 2008).
Probing of the “A/D by age” interaction indicated that the relation between vmPFC thickness and A/D was negative early on in development; however, this relation reversed and became positive later in adolescence. Our analyses suggest that this reversal was due to a slower rate of cortical thinning within the right vmPFC among subjects with higher CBCL A/D scores. Several hypotheses could potentially explain this maturation pattern. Cortical thickness of the vmPFC has been found in adults to be inversely correlated with ipsilateral amygdala activation during an emotional processing task (Foland-Ross et al. 2010). Thus, there is some evidence that thickness in this portion of the cerebral cortex is related to hemodynamic activity within limbic structures. In addition, Milad et al. (2005) have demonstrated that thickness of the right vmPFC is positively associated with fear extinction in humans. Taken together, these results suggest that thinner right vmPFC could be associated with a decreased capacity to regulate negative affective states that are mediated, at least in part, by activity in limbic and subcortical structures.
This would be compatible with our findings in younger children, but would not explain the developmental shift from a negative to a positive association around 12 years of age. A recent fMRI study of healthy children and young adults has provided interesting insights into the development of the amygdala–mPFC connectivity (Gee et al. 2013). The functional connectivity between the amygdala and the mPFC (in an area slightly dorsal but anatomically very close to our results) was found to be positive from 4 to 10 years of age, before shifting to negative connectivity after age 10. This process was associated with a gradual reduction in amygdala reactivity to fearful faces with aging. In younger subjects with positive connectivity, amygdala reactivity was associated with higher mPFC activity. Of note, some subjects maintained a positive connectivity pattern after age 10, and positive connectivity was associated with higher levels of anxiety (Gee et al. 2013). Our finding of slowed right vmPFC cortical thinning leading to a shift from a negative to a positive association between thickness and A/D could parallel the persistence of positive amygdala–mPFC connectivity. One potential mechanism to explain changes in thickness could be that higher vmPFC neuronal activity in young children with higher levels of negative affect leads to a relative increase in right vmPFC thickness over time. Although definite evidence that increased neuronal activity leads to increase cortical thickness is lacking at the present time, several experiments have indirectly supported this possibility. In healthy children, longitudinal changes in verbal and nonverbal IQ have been shown to be positively associated with cortical thickness in brain areas shown to be more active during related neuropsychological tasks (Ramsden et al. 2011). In addition, multiple studies have shown that specific athletic and musical training (indirectly implying increased area-specific neuronal activity) can lead to increased gray matter detectable with imaging techniques, both in adults (Draganski et al. 2004; Boyke et al. 2008; Bermudez et al. 2009; Bezzola et al. 2011) and children (Hyde et al. 2009a, 2009b). Using a rodent model, Lerch et al. (2011) have confirmed that training can lead to rapid changes in morphology that are detectable using MRI, chiefly secondary to the remodeling of neuronal processes. A major caveat is that neuronal activity in the vmPFC was not directly measured with our study design; and therefore, this remains to be prospectively tested with multimodal imaging strategies in the future.
Of note, Schwartz et al. (2010) reported increased right vmPFC thickness in 18-year-old subjects that had a high reactive temperament during infancy, independent of subjects' current symptoms. This finding is seemingly compatible with the aforementioned hypothesis. However, in a small study of healthy adults (19–47 years of age), a negative association was found between self-reported trait anxiety and right medial OFC thickness (Kühn et al. 2011). Taking our results and these 2 studies together (Schwartz et al. 2010; Kühn et al. 2011), this suggests that there could be a delay in maturation of the right vmPFC in subjects with elevated anxious/depressed problems, leading to thicker cortex in late teenage/early adulthood, but ultimately thinner cortex at the end of brain development. Indeed, it has been shown that normal developmental cortical thinning occurs up to the late 20 s/early 30s (Shaw et al. 2008). At a functional level, this might be recapitulated by a delay in developing a negative amygdala–mPFC connectivity (Gee et al. 2013). Such possibilities underscore the importance of taking into account dynamic changes in the developing brain.
A few additional factors could be involved in this maturational pattern. It is important to consider that changes in cortical thickness in children are not solely associated with cortical gray matter changes, but may also be related to white matter development and myelination patterns (Paus et al. 2008; Miller et al. 2012). The slower rate of cortical thinning among subjects with higher CBCL A/D scores may therefore be underpinned by delayed maturation of white matter pathways implicated in affect regulation and emotional behavior (e.g., the uncinate fasciculus). Thus, the relation between A/D and right vmPFC thickness could be mediated by maturational changes (e.g., myelination) of vmPFC projections to core limbic structures such as the amygdala. In addition, given the temporal overlap between the change in the direction of the association and pubertal development, in conjunction with changes in depression prevalence after puberty, neuroendocrine factors constitute another potential mechanism influencing vmPFC development over time. Indeed, androgen-related cortical maturation has also been shown to vary across the age span in previous examinations of the same sample, and our recent analyses suggest endocrine-related changes in structural connectivity between the vmPFC and amygdala/hippocampus throughout adolescence (Nguyen et al. 2013).
Our analysis of the YASR scores in subjects over 18 years of age confirmed a positive association between A/D score and cortical thickness in the right vmPFC. Interestingly, positive associations between cortical thickness and A/D scores were also found in the right precuneus/posterior cingulate region. Although the right precuneus/posterior cingulate region was not significant in our analysis of younger children, this apparent inconsistency may be explained, in part, by the maturation of particular brain networks during the course of normal human development (Zielinski et al. 2010). More specifically, the DMN, which contains significant overlap with the medial prefrontal network, becomes significantly more integrated with increasing age during late childhood and adolescence (Fair et al. 2008). This increasing functional integration of the DMN with age involves increased functional connectivity between the vmPFC and the precuneus/posterior cingulate in a region very similar to our findings here for adolescents (Fair et al. 2008). Although there has been some debate regarding the validity of developmental studies of the DMN (Power et al. 2012; Van Dijk et al. 2012), it has been reported that increased coordinated activation of anterior and posterior DMN regions during adolescence is associated with increased fractional anisotropy within white matter tracts (e.g., the cingulum bundle) connecting these regions (Gordon et al. 2011). We hypothesize that the lack of precuneus/posterior cingulate findings in younger children may be the result of the DMN not being fully developed.
This apparent topographical overlap with the DMN is noteworthy. In particular, our findings in the right vmPFC and right precuneus seem to dovetail with recent evidence of increased activity and functional connectivity within the DMN among depressive patients. Given this topographical overlap, the prospect of anxious/depressed symptoms in healthy subjects (e.g., rumination, negative self evaluations, etc.) being mediated by heightened activity within the DMN warrants further investigation (Raichle et al. 2001; Sheline et al. 2009; Berman et al. 2011; Gaffrey et al. 2010).
Lastly, although speculative since this study excluded subjects with clinical mood disorders, these results raise a few interesting hypotheses on the link between cortical development and MDD. Previous research has revealed that the overall rates of clinical depression increase between the ages of 15 and 18 years, suggesting that developmental changes during this age range directly influence disease vulnerability (Hankin et al. 1998; Paus et al. 2008). It is noteworthy that, in the present study of healthy youths, the positive association between right vmPFC and anxious/depressed symptoms emerged around 15 years of age. Reduced gray matter volume of the SgACC has been found to be associated with MDD, including in patients with depression onset prior to 18 years of age (Botteron et al. 2002; Drevets, Savitz et al. 2008; van Tol et al. 2010), while CBCL A/D scores were associated with thicker right vmPFC after 15 years of age in this study. Although this could appear contradictory, the difference may stem from the fact that our sample included only healthy subjects. Despite the tendency in some subjects to have more mood and anxiety symptoms, these children did not develop a clinically significant disorder. Therefore, the relative increase in thickness for older teens and young adults might be a form of biological compensation to mitigate negative emotional experiences associated with an overly active limbic system, potentially preventing the onset of mood and anxiety disorders. In line with this hypothesis, healthy siblings of individuals with MDD have been found to possess thicker right mPFC and posterior cingulate cortex (Peterson et al. 2009). Thus, we hypothesize that the absence of vmPFC cortical thickness compensation might be a risk factor for the development of MDD in adolescence and young adulthood, although this should be specifically tested prospectively. Indeed, the functional topography of the vmPFC in the context of mood and anxiety disorders continues to be debated (Myers-Schulz and Koenigs 2012).
There are several weaknesses to mention in the current investigation. It is not possible to determine the exact source of cortical thickness changes with the current state of MRI technology. Changes in size/density of neurons, interneurons/glial cells, unmyelinated neuronal processes, and extension of white matter fibers as they myelinate in the cortical layer are all possibilities (Paus et al. 2008; Zatorre et al. 2012). In addition, the restriction to structural imaging did not allow direct testing of hypothetical links between brain activity and the identified cortical maturation pattern. The use of a mixed-effects model allowed the inclusion of subjects with a different number of measurements to maximize the age range and statistical power. Therefore, the pattern of slower thinning in the right vmPFC was identified by modeling trajectories from different subjects instead of comparing single subjects at different points over time. Although studying healthy children in isolation is informative in terms of association between normal cortical development and behavior, extrapolations to the potential physiology of clinical disorders remain speculative. None of the subjects enrolled in the study had a CBCL A/D t-score >70 during the 4-year follow-up; consequently, it was not possible to directly test the impact of clinical mood disorders on vmPFC thickness maturation. The use of 2 different scales for subjects below and above 18 years of age did not allow us to pool all subjects together, and could have been a confounding factor. However, the fact that older adolescents tested with the CBCL and young adults assessed with the YASR both had positive associations between A/D scores and vmPFC thickness decreases the possibility of statistical artifacts stemming from the use of different scales. Despite these limitations, this is the largest neuroanatomical study of subclinical anxious/depressive symptoms in a healthy representative cohort of children across a large age range. All subjects included in the analysis met rigorous quality control criteria and whole-brain multiple comparison corrections were applied for all analyses.
In conclusion, this study of healthy youths and young adults supports the putative role of the right vmPFC in the regulation and/or experience of anxious and depressive affects throughout development. Importantly, the demonstration of slower cortical thinning leading to a reversal in the polarity of cortical thickness associations during the development of this region demonstrates the crucial influence of age on this brain–behavior relation, and provides an important developmental context for studies of children and adolescents with mood and anxiety disorders. In conjunction with recent data on attention problems in healthy children and in ADHD (Shaw et al. 2011; Ducharme et al. 2012), this study highlights the importance of considering dynamic changes associated with age in morphometric studies of other behavior and pediatric psychiatric disorders as well. These results lay the foundation for integrative research looking at the impact of genetic, hormonal, cognitive, and other factors on associations between brain maturation and anxious/depressed symptoms. Future clinical studies could prospectively follow right vmPFC cortical thickness in children and adolescents, and analyze the impact of this measure on the development of MDD and anxiety disorders. This could provide important information to further specify the neurobiology of these pathologies, and get us closer to the ultimate goal of identifying neuroimaging risk factors of MDD in children to better target preventive and treatment interventions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This project has been funded in whole or in part with Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319, and -2320). S.D. and T.-V.N. have received financial support from the Canadian Institutes of Health Research with a Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarship. S.D. is currently supported by a Fellowship Award from the “Fonds de Recherche du Québec – Santé” and the American Psychiatric Association Lilly Psychiatric Research Fellowship Award. M.D.A. receives funding from the Child and Adolescent Psychology Training and Research Foundation. S.K. is supported by the “Fonds de Recherche du Québec – Santé.”
Disclaimer
The views herein do not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, the U.S. Department of Health and Human Services, or any other agency of the United States Government.
Brain Development Cooperative Group
Key personnel from the six pediatric study centers are as follows: Children's Hospital Medical Center of Cincinnati, Principal Investigator William S. Ball, M.D., Investigators Anna Weber Byars, Ph.D., Mark Schapiro, M.D., Wendy Bommer, R.N., April Carr, B.S., April German, B.A., Scott Dunn, R.T.; Children's Hospital Boston, Principal Investigator Michael J. Rivkin, M.D., Investigators Deborah Waber, Ph.D., Robert Mulkern, Ph.D., Sridhar Vajapeyam, Ph.D., Abigail Chiverton, B.A., Peter Davis, B.S., Julie Koo, B.S., Jacki Marmor, M.A., Christine Mrakotsky, Ph.D., M.A., Richard Robertson, M.D., Gloria McAnulty, Ph.D; University of Texas Health Science Center at Houston, Principal Investigators Michael E. Brandt, Ph.D., Jack M. Fletcher, Ph.D., Larry A. Kramer, M.D., Investigators Grace Yang, M.Ed., Cara McCormack, B.S., Kathleen M. Hebert, M.A., Hilda Volero, M.D.; Washington University in St. Louis, Principal Investigators Kelly Botteron, M.D., Robert C. McKinstry, M.D., Ph.D., Investigators William Warren, Tomoyuki Nishino, M.S., C. Robert Almli, Ph.D., Richard Todd, Ph.D., M.D., John Constantino, M.D.; University of California Los Angeles, Principal Investigator James T. McCracken, M.D., Investigators Jennifer Levitt, M.D., Jeffrey Alger, Ph.D., Joseph O'Neil, Ph.D., Arthur Toga, Ph.D., Robert Asarnow, Ph.D., David Fadale, B.A., Laura Heinichen, B.A., Cedric Ireland B.A.; Children's Hospital of Philadelphia, Principal Investigators Dah-Jyuu Wang, Ph.D. and Edward Moss, Ph.D., Investigators Robert A. Zimmerman, M.D., and Research Staff Brooke Bintliff, B.S., Ruth Bradford, Janice Newman, M.B.A. The Principal Investigator of the data coordinating center at McGill University is Alan C. Evans, Ph.D., Investigators Rozalia Arnaoutelis, B.S., G. Bruce Pike, Ph.D., D. Louis Collins, Ph.D., Gabriel Leonard, Ph.D., Tomas Paus, M.D., Alex Zijdenbos, Ph.D., and Research Staff Samir Das, B.S., Vladimir Fonov, Ph.D., Luke Fu, B.S., Jonathan Harlap, Ilana Leppert, B.E., Denise Milovan, M.A., Dario Vins, B.C., and at Georgetown University, Thomas Zeffiro, M.D., Ph.D. and John Van Meter, Ph.D. Ph.D. Investigators at the Neurostatistics Laboratory, Harvard University/McLean Hospital, Nicholas Lange, Sc.D., and Michael P. Froimowitz, M.S., work with data coordinating center staff and all other team members on biostatistical study design and data analyses. The Principal Investigator of the Clinical Coordinating Center at Washington University is Kelly Botteron, M.D., Investigators C. Robert Almli Ph.D., Cheryl Rainey, B.S., Stan Henderson M.S., Tomoyuki Nishino, M.S., William Warren, Jennifer L. Edwards M.SW., Diane Dubois R.N., Karla Smith, Tish Singer and Aaron A. Wilber, M.S. The Principal Investigator of the Diffusion Tensor Processing Center at the National Institutes of Health is Carlo Pierpaoli, MD, Ph.D., Investigators Peter J. Basser, Ph.D., Lin-Ching Chang, Sc.D., Chen Guan Koay, Ph.D. and Lindsay Walker, M.S. The Principal Collaborators at the National Institutes of Health are Lisa Freund, Ph.D. (NICHD), Judith Rumsey, Ph.D. (NIMH), Lauren Baskir, Ph.D. (NIMH), Laurence Stanford, PhD. (NIDA), Karen Sirocco, Ph.D. (NIDA) and from NINDS, Katrina Gwinn-Hardy, M.D., and Giovanna Spinella, M.D. The Principal Investigator of the Spectroscopy Processing Center at the University of California Los Angeles is James T. McCracken, M.D., Investigators Jeffry R. Alger, Ph.D., Jennifer Levitt, M.D., Joseph O'Neill, Ph.D.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Achenbach TM. Manual for the Child behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach T. Manual for the young adult self-report and young adult behavior checklist. Burlington, VT: University of Vermont. Department of Psychiatry; 1997. [Google Scholar]
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families; 2001. [Google Scholar]
- Achenbach T, Ruffle T. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Aiken L, West S. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. NeuroImage. 2013;71:42–49. doi: 10.1016/j.neuroimage.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Dolan D, Last C, Strauss C. Symptoms of anxiety disorders in normal children. J Am Acad Child Adolesc Psychiatry. 1990;29:759–765. doi: 10.1097/00004583-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Berman M, Peltier S, Nee D, Kross E, Deldin P, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb Cortex. 2009;19:1583–1596. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- Bezzola L, Mérillat S, Gaser C, Jäncke L. Training-induced neural plasticity in golf novices. J Neurosci. 2011;31:12444–12448. doi: 10.1523/JNEUROSCI.1996-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes A, McCormick L, Coryell W, Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol Psychiatry. 2008;63:391–397. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron K, Raichle M, Drevets W, Heath A, Todd R. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J. Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Stalb L, Charney D. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael S, Price J. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chen M, Hamilton J, Gotlib I. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Holmes C, Peters T, Evans A. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen N. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162:1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- Damasio A, Grabowski T, Bechara A, Damasio H, Ponto L, Parvizi J, Hichwa R. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Diggle P. Analysis of longitudinal data. Oxford, New York: Oxford University Press; 2002. [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Drevets W. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets W. Orbitofrontal cortex function and structure in depression. Ann NY Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Drevets W, Price J, Furey M. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W, Price J, Simpson J, Todd R, Reich T, Vannier M, Raichle M. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets W, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Hudziak J, Botteron K, Albaugh M, Nguyen T, Karama S, Evans A. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 2012;51:18–27. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Hudziak J, Botteron K, Ganjavi H, Lepage C, Collins D, Albaugh M, Evans A, Karama S Brain Development Cooperative Group. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with Child Behavior Checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A Brain Development Cooperative Group. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fair D, Cohen A, Dosenbach N, Church J, Miezin F, Barch D, Raichle M, Petersen S, Schlagger B. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand R, Verhulst F. Psychopathology from adolescence into young adulthood: an 8-year follow-up study. Am J Psychiatry. 1995;152:1586–1594. doi: 10.1176/ajp.152.11.1586. [DOI] [PubMed] [Google Scholar]
- Foland-Ross L, Altshuler L, Bookheimer S, Lieberman M, Townsend J, Penfold C, Moody T, Ahlf K, Shen J, Madsen S, et al. Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. J Neurosci. 2010;30:16673–16678. doi: 10.1523/JNEUROSCI.4578-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M, Luby J, Repvoš G, Belden A, Botteron K, Luking K, Barch D. Subgenual cingulate connectivity in children wih a history of preschool depression. Neuroreport. 2010;21:1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E, Lee P, Maisog J, Foss-Feig J, Billington M, Vanmeter J, Vaidya C. Strength of default mode resting-state connectivity relates to white matter integrity in children. Dev Sci. 2011;14:739–751. doi: 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano A. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hankin B, Abramson L, Moffitt T, Silva P, McGee R, Angell K. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hudziak J, Rudgier L, Neale M, Heath A, Todd R. A twin study of inattentive, aggressive and anxious/depressed behaviors. J Am Acad Child Adolesc Psychiatry. 2000;39:469–476. doi: 10.1097/00004583-200004000-00016. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. The effects of musical training on structural brain development. Ann N Y Acad Sci. 2009a;1169:182–186. doi: 10.1111/j.1749-6632.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. Musical training shapes structural brain development. J. Neurosci. 2009b;29:3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier R, Deary I, Lyttelton O, Lepage C, Evans A. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W, Yurgelun-Todd D. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- Kim J, Singh V, MacDonald D, Lee J, Kim S, Evans A. Automated 3D extraction and evaluation of the outer cortical surface using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kühn S, Schubert F, Gallinat J. Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord. 2011;134:315–319. doi: 10.1016/j.jad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee J, Kim J, Kim I, Evans A, Kim S. A novel quantitative cross-validation of different cortical surface reconstructions algorithms using MRI phantom. NeuroImage. 2006;31:572–584. doi: 10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, Henkelman RM, Josselyn SA, Sled JG. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. NeuroImage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Light SN, Heller AS, Johnstone T, Kolden GG, Peterson MJ, Kalin NH, Davidson RJ. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol Psychiatry. 2011;70:962–968. doi: 10.1016/j.biopsych.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans A. Automated 3D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;13:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mayberg H, Liotti M, Brannan S, McGinnins S, Mahurin R, Jerabek P, Silva J, Tekell J, Martin C, Lancaster J, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Milad M, Quinn B, Pitman R, Orr S, Fischl B, Rauch S. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Šestan N, Wildman DE, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Morris S, Cuthbert B. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Wise S, Drevets W. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-V, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Drevets W, Price J. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Warner V, Bansal R, Zhu H, Hao X, Burkin K, Adams P, Wickramaratne P, Weissman M. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA. 2009;106:6272–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pine D, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Power J, Barnes K, Snyder A, Schlaggar B, Petersen S. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Drevets W. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo J, Wei J, Dilley G, Pittman S, Meltzer H, Overholser J, Roth B, Stockmeier C. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1089. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Richardson FM, Josse G, Thomas MS, Ellis C, Shakeshaft C, Seghier ML, Price CJ. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479:113–116. doi: 10.1038/nature10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace G, Greenstein D, Clasen L, Gogtay N, Giedd J. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey J, Morris-Yates A. Diagnostic accuracy in adolescents of several depression rating scales extracted from a general purpose behavior checklist. J Affect Disord. 1992;26:7–16. doi: 10.1016/0165-0327(92)90029-6. [DOI] [PubMed] [Google Scholar]
- Richert K, Carrion V, Karchemskiy A, Reiss A. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety. 2006;23:17–25. doi: 10.1002/da.20131. [DOI] [PubMed] [Google Scholar]
- Roza S, Hofstra M, van der Ende J, Verhulst F. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence and young adulthood. Am J Psychiatry. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Sadock B, Sadock V, Ruiz P. Kaplan & Sadocks's comprehensive textbook of psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- Schwartz C, Kunwar P, Greve D, Moran L, Viner J, Covino J, Kagan J, Stewart S, Snidman N, Vangel M, et al. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Arch Gen Psychiatry. 2010;67:78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport J. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani N, Lerch J, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport J, et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y, Barch D, Price J, Rundle M, Vaishnavi S, Snyder A, Mintun M, Wang S, Coalson R, Raichle M. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford, New York: Oxford University Press; 2003. [Google Scholar]
- Van Dijk K, Sabuncu M, Buckner R. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M, van der Wee N, van den Heuvel A, Nielen M, Demenescu L, Aleman A, Renken R, van Buchem M, Zitman F, Veltman D. Regional brain volume in depression and anxiety. Arch Gen Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Waber D, De Moor C, Forbes P, Almli C, Botteron K, Leonard G, Milovan D, Paus T, Rumsey J, Group BDC. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Worsley K, Taylor J, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. NeuroImage. 2004;23:189–195. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Zald D, Mattson D, Pardo J. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci USA. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.