Abstract
In recent years, an explosion of neuroimaging studies has examined cognitive reappraisal, an emotion regulation strategy that involves changing the way one thinks about a stimulus in order to change its affective impact. Existing models broadly agree that reappraisal recruits frontal and parietal control regions to modulate emotional responding in the amygdala, but they offer competing visions of how this is accomplished. One view holds that control regions engage ventromedial prefrontal cortex (vmPFC), an area associated with fear extinction, that in turn modulates amygdala responses. An alternative view is that control regions modulate semantic representations in lateral temporal cortex that indirectly influence emotion-related responses in the amygdala. Furthermore, while previous work has emphasized the amygdala, whether reappraisal influences other regions implicated in emotional responding remains unknown. To resolve these questions, we performed a meta-analysis of 48 neuroimaging studies of reappraisal, most involving downregulation of negative affect. Reappraisal consistently 1) activated cognitive control regions and lateral temporal cortex, but not vmPFC, and 2) modulated the bilateral amygdala, but no other brain regions. This suggests that reappraisal involves the use of cognitive control to modulate semantic representations of an emotional stimulus, and these altered representations in turn attenuate activity in the amygdala.
Keywords: affect, fMRI, meta-analysis, reappraisal
Introduction
We humans are nothing if not adaptive. This attribute depends, at least in part, on our ability to regulate responses to life's affective pushes and pulls. Emotion regulation allows us to respond adaptively to these affective events—to keep cool under stress, emerge resilient from tribulations, and resist harmful temptations.
In order to understand how emotions are regulated, it is useful to first consider how they are generated. Theoretically, emotion generation can be understood as a process that unfolds over time. Emotions begin with the individual perceiving a stimulus within a context and attending to its features. Next, the individual appraises a stimulus's emotional significance, and this triggers an affective, physiological and behavioral response (Scherer et al. 2001; Barrett et al. 2007). In this framework, the impact of any given emotion regulation strategy can be understood in terms of the stage of the emotion generation sequence that it impacts (Gross 1998). The best-studied strategy is cognitive reappraisal, which targets the appraisal stage and involves changing one's interpretations or appraisals of affective stimuli. One reason this strategy is so well studied is because reappraisal is highly effective at regulating affect and physiological arousal without the cognitive and physiological costs associated with response-focused strategies (e.g., expressive suppression) (Gross 1998), and with longer-lasting effects than attention-focused strategies (e.g., distraction) (Ochsner and Gross 2005; Kross and Ayduk 2008; Ochsner et al. 2012; Silvers et al. 2013). But it is also well studied because the core elements of reappraisal are central to many forms of therapy, including cognitive behavioral therapy (Beck 2005), dialectical behavioral therapy (Lynch et al. 2007), and psychodynamic therapy (Bateman and Fonagy 2006; Maroda 2010; Have-de Labije and Neborsky 2012), all of which are effective for treating a variety of mood and anxiety disorders. For this reason, recent work has begun applying insights from behavioral and brain imaging research on reappraisal to both characterizing and treating clinical disorders (Denny et al. 2009; Silvers et al. 2013). As such, a clear understanding of the neural systems underlying reappraisal is important for both basic and translational research.
Psychological models of reappraisal suggest that that many of the same cognitive control processes that are used to regulate attention, memory and thoughts in nonemotional contexts are also used in the cognitive regulation of emotion (Ochsner and Gross 2008). If this is the case, we might hypothesize that reappraisal is supported by brain regions supporting domain-general cognitive control processes such as dorsomedial, dorsolateral, and ventrolateral prefrontal cortex (dmPFC, dlPFC, vlPFC) as well as posterior parietal cortex (Duncan and Owen 2000; Miller and Cohen 2001). During reappraisal, we might expect that 1) dlPFC and parietal cortex, regions generally involved in selective attention and working memory, might assist in holding reappraisals in mind, 2) vlPFC, a region strongly implicated in response selection and inhibition, may support the selection of appropriate reappraisals, and 3) dmPFC may be recruited to assist in monitoring and reflecting on the meaning of changing emotional states (Ochsner and Gross 2005, 2008; Ochsner et al. 2012).
But how might these domain-general processes affect activity in regions related to emotional responding? One possibility is that cognitive control regions engage ventromedial prefrontal cortex (vmPFC), which in turn modulates activity in regions related to emotional responding, such as the amygdala (Ochsner and Gross 2005, 2007; Urry et al. 2006; Schiller and Delgado 2010; Diekhof et al. 2011; Etkin et al. 2011). This hypothesis builds on 2 types of data. First, anatomical work suggests that direct connections between lateral PFC regions and the amygdala are relatively sparse in comparison with connections between vmPFC and the amygdala, particularly, in caudal portions of vmPFC (Ghashghaei et al. 2007). Second, animal and human work show that vmPFC is critical for fear extinction and reversal learning (Milad et al. 2007; Finger et al. 2008; Schiller et al. 2008).
Together, these data have motivated the idea that during reappraisal lateral PFC regions affect the amgygdala indirectly via the vmPFC. For example, Diekhof et al. (2011) suggested that vmPFC is not limited to conditioned learning contexts, but also acts as a “domain-general controller of negative affect” in more complex, cognitively-driven regulation contexts such as placebo and reappraisal, supported by structural connectivity between vmPFC and lateral PFC and amygdala. In a similar vein, Schiller and Delgado (2010) posited that vmPFC serves as a general mechanism for reducing learned fear by uniquely encoding a “safety signal” that can be triggered by diverse brain pathways, including high-level cognitive areas in the dlPFC. Finally, in another influential review, Etkin et al. (2011) suggested that vmPFC and adjacent regions might “perform a generic negative emotion inhibitory function that can be recruited by other regions,” such as the domain-general cognitive control regions described above. If these theories are correct, then in studies of reappraisal, we should see reliable activation of vmPFC alongside activation of domain-general cognitive control regions.
A second possibility is that prefrontal and parietal control regions exert their effects via changes in lateral temporal areas associated with semantic and perceptual representations. This view would be consistent with psychological models positing that reappraisal alters semantic and perceptual representations of stimuli in ways that change their emotional significance (Ochsner and Gross 2005, 2007; Ochsner et al. 2012). For example, a participant viewing an image of an accident victim may tell herself, “that's ketchup, not blood,” or “those people are just actors.” If similar transformations of stimulus meaning underlie reappraisal in general, then in studies of reappraisal, we might expect to see consistent activity differences in the lateral temporal cortex regions that encode semantic and perceptual associations.
In contrast to this debate regarding the neural underpinnings of reappraisal, there is greater agreement concerning what brain regions are modulated by reappraisal. Extensive work in animals and humans has implicated the amygdala in the detection, encoding, and organization of responses to arousing, goal-relevant stimuli (Anderson et al. 2003; Phelps and LeDoux 2005; Cunningham and Brosch 2012; Wilson-Mendenhall et al. forthcoming). Given that negative stimuli are commonly perceived as highly arousing, and that the majority of reappraisal studies to date utilize aversive stimuli (of 48 studies in the present meta-analysis, 34 used aversive stimuli, 5 used positive/appetitive stimuli, and 9 used both; see Table 1 for a summary), it is perhaps unsurprising that changes in amygdala activity are among the most consistent findings in the reappraisal literature. However, many other regions are involved in emotion (Kober et al. 2008; Vytal and Hamann 2010; Lindquist et al. 2012). Indeed, individual reappraisal studies sometimes show modulation of other emotion-related regions in association with particular kinds of stimuli and emotions. Such regions include, but are not limited to, the ventral striatum, a structure strongly linked to reward processing (Schultz et al. 1992), the insula, which supports integration of affective and viscerosensory information (Craig 2009; Chang et al. 2012; Zaki et al. 2012), and brainstem regions such as the periaqueductal gray, which is involved in the coordination of behavioral and physiological emotional responses (Buhle et al. 2012).
Table 1.
Reappraisal studies included in meta-analysis
| Study | Contrast type(s) | N | Valence | Stimulus type | Goal | Tactic |
|---|---|---|---|---|---|---|
| Beauregard et al. (2001) | Control | 10 | Pos | Videos | Dec | Dist |
| Domes et al. (2010) | Control, both | 33 | Neg | Photos | Both | Both |
| Eippert et al. (2007) | Control, both, emotion | 24 | Neg | Photos | Both | Both |
| Erk et al. (2010) | Control, emotion | 17 | Neg | Photos | Dec | Dist |
| Goldin et al. (2008) | Control | 17 | Neg | Videos | Dec | Reint |
| Grecucci et al. (2012) | Both | 21 | Neg | Ultimatum offers | Both | Reint |
| Harenski and Hamann (2006) | Control | 10 | Neg | Photos | Dec | Both |
| Hayes et al. (2010) | Control, emotion | 25 | Neg | Photos | Dec | Reint |
| Herwig et al. (2007) | Control (2) | 14 | Both | Anticipation of photos | Dec | Reint |
| Hollmann et al. (2012) | Control | 17 | Pos | Photos | Dec | Reint |
| Ichikawa et al. (2011) | Both, emotion | 17 | Neg | Task errors | Both | Reint |
| Kanske et al. (2011) | Control, emotion | 30 | Both | Photos | Dec | Both |
| Kanske et al. (2012) | Control (2), emotion (2) | 26 | Both | Photos | Dec | Reint |
| Kim and Hamann, (2007) | Both (2), control (2) | 10 | Both | Photos | Both | Reint |
| Kober et al. (2010) | Control, emotion | 21 | Pos | Photos | Dec | Reint |
| Koenigsberg et al. (2010) | Control, emotion | 16 | Neg | Photos | Dec | Dist |
| Krendl et al. (2012) | Control, emotion | 16 | Neg | Photos | Dec | Unclear |
| Kross et al. (2009) | Emotion (2) | 16 | Neg | Memories | Dec | Reint |
| Lang et al. (2012) | Both (2), control (4) | 15 | Neg | Scripts | Both | Dist |
| Leiberg et al. (2012) | Both (2), control, emotion | 24 | Neg | Photos | Both | Dist |
| Levesque et al. (2003) | Control | 20 | Neg | Videos | Dec | Dist |
| Mak et al. (2009) | Control (2), emotion (2) | 12 | Both | Photos | Dec | Unclear |
| McRae et al. (2008) | Control, emotion | 25 | Neg | Photos | Dec | Reint |
| McRae et al. (2010)a | Control, emotion | 18 | Neg | Photos | Dec | Reint |
| McRae, Gross et al. (2012a) | Control, emotion | 38 | Neg | Photos | Dec | Reint |
| McRae, Misra et al. (2012b)a | Control, emotion | 26 | Neg | Photos | Dec | Reint |
| Modinos et al. (2010) | Control, emotion | 18 | Neg | Photos | Dec | Reint |
| New et al. (2009) | Both (2), control (3) | 14 | Neg | Photos | Both | Reint |
| Ochsner et al. (2002) | Control, emotion | 15 | Neg | Photos | Dec | Reint |
| Ochsner et al. (2004) | Both, control, emotion | 24 | Neg | Photos | Both | Both |
| Ochsner et al. (2009) | Both, emotion | 20 | Neg | Photos | Inc | Both |
| Ohira et al. (2006) | Control, emotion | 10 | Both | Photos | Dec | Unclear |
| Opitz et al. (2012) | Control | 31 | Neg | Photos | Both | Reint |
| Perlman et al. (2012) | Control, emotion | 14 | Neg | Photos | Dec | Reint |
| Phan et al. (2005) | Control, emotion | 14 | Neg | Photos | Dec | Reint |
| Pitskel et al. (2011) | Both, control, emotion | 15 | Neg | Photos | Both | Reint |
| Schardt et al. (2010) | Control, emotion | 37 | Neg | Photos | Dec | Dist |
| Schulze et al. (2011) | Both, control, emotion (2) | 16 | Neg | Photos | Both | Both |
| Sokol-Hessner et al. (2012) | Control (2), emotion | 16 | Both | Economic decision-making | Both | Dist |
| Staudinger et al. (2009) | Control | 16 | Pos | Anticipation and receipt of monetary reward | Dec | Dist |
| Staudinger et al. (2011) | Control, emotion | 24 | Pos | Anticipation of monetary reward | Dec | Dist |
| Urry et al. (2006) | Both, control | 17 | Neg | Photos | Both | Reint |
| Urry et al. (2009) | Both, control | 26 | Neg | Photos | Both | Reint |
| Van Reekum et al. (2007) | Both, control, emotion | 29 | Neg | Photos | Both | Reint |
| Vrticka et al. (2011)a | Control, emotion (4) | 19 | Both | Photos | Dec | Reint |
| Wager, Davidson et al. (2008)a | Control, emotion | 30 | Neg | Photos | Dec | Reint |
| Walter et al. (2009) | Control, emotion | 18 | Neg | Photos | Dec | Dist |
| Winecoff et al. (2011) | Control (2), emotion (2) | 42 | Both | Photos | Dec | Dist |
“Control” contrasts indexed activity supporting reappraisal; for example, downregulation of emotional responses to negative stimuli > respond naturally. “Emotion” contrasts indexed only activity that was impacted by regulation; for example, respond naturally to positive stimuli > downregulate. “Both” contrasts indexed both “control” and “emotion” activity; for example, upregulate positive stimuli > respond naturally. Stimulus valence indicates whether positive (pos), negative (neg), or both positive and negative stimuli (both) were used and stimulus type indicates the type of stimulus utilized. Goal indicates whether reappraisal was used to increase (inc), decrease (dec), or increase and decrease (both) affective responses. Tactic indicates whether participants were instructed to reinterpret (reint), distance (distance), use some combination of strategies or their own choice (both), or whether it was unclear what participants were instructed to do (unclear).
aIndicates that coordinates were obtained via personal communication with authors.
To resolve these questions, we performed the largest meta-analysis to date of neuroimaging studies of cognitive reappraisal. This analysis included 116 contrasts from 48 studies of healthy individuals, allowing us to identify brain regions that consistently support reappraisal (show increases in activity during reappraisal) as well as those that that are modulated by reappraisal (show decreases in activity during reappraisal when the goal is to downregulate affect).
Methods
Procedure
To identify neuroimaging studies of reappraisal, we 1) performed literature searches in PubMed and Google Scholar, 2) examined the references of relevant papers, and 3) contacted numerous researchers with a history of publishing on this topic. Inclusion was based on the following criteria: 1) Participants were free from psychiatric diagnosis (nicotine dependence was not considered exclusionary); 2) peak coordinates were reported in either Talairach (Talairach and Tournoux 1988) or Montreal Neurological Institute (MNI) space; 3) analyses compared conditions using a subtraction methodology. We did not include coordinates from between-group comparisons (e.g., men vs. women), functional connectivity analyses (e.g., psychophysiological interactions), or correlations (e.g., with individual difference measures like age). Given that the primary focus of the analysis was to examine reappraisal of emotion versus naturalistic emotional responses, we did not include contrasts comparing reappraisal with different goals (e.g., using reappraisal to increase vs. decrease emotional responses) or reappraisal of different types of stimuli (e.g., reappraisal of positive vs. negative stimuli) excluded. Finally, we did not include any contrasts that sought to examine manipulations of attention (i.e., distraction) or the adoption of a general mindset (i.e., relaxation).
We searched the literature for studies published between 2001 and 2012, spanning the duration from when the first neuroimaging study of reappraisal was published to when the present paper was submitted. We identified 1268 peaks from 116 contrasts in 48 studies that met our criteria (see Table 1). Each contrast was coded according to the type of regulation-related neural activity we expected it to index. For example, a typical contrast may compare a condition wherein participants used reappraisal to downregulate emotional responses to negative stimuli to a condition wherein participants responded naturally (passive viewing) to negative stimuli. In such a contrast, brain regions showing greater activity would be interpreted as supporting reappraisal. However, if a brain region showed greater activation when responding naturally than during reappraisal, it would be interpreted as a region that is modulated by reappraisal. These codes were applied independently of one another, so it was possible for a given contrast to be coded as both supporting reappraisal and being modulated by reappraisal. An example would be a contrast in which reappraising a positive image as more positive (i.e., upregulating) was compared with simply viewing a positive image.
The meta-analysis technique we used nests peaks within contrasts rather than within studies (for more details, see below). As a result, if a study reported multiple contrasts, each of which isolated quite similar processes (e.g., by comparing reappraising a picture as less negative to both simply viewing a negative picture and to performing a simple cognitive task, e.g., Harenski and Hamann 2006), then including both of these contrasts would oversample highly dependent data. In these rare situations, we included only the contrast deemed most methodologically canonical, which was defined as the one that was closest to a comparison between reappraising a stimulus and responding naturally to it.
Analyses
First, coordinates reported in Talaraich space were converted to MNI space using the Tal2MNI algorithm (Matthew Brett; http://www.imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m). Next, we assessed the spatial density of reported peak coordinates using the Multilevel Kernel Density Analysis (MKDA) approach developed by Wager and colleagues (Wager et al. 2007; Kober et al. 2008; Kober and Wager 2010). This approach nests peaks within contrasts and thus controls for the total number of peaks a given contrast reports. This is important to prevent a single contrast from unduly influencing the overall results, and it overcomes artifactual differences in peak counts that might result from analysis choices such as voxel size or smoothing kernel.
Next, an 8-mm Gaussian smoothing kernel was created around each peak such that voxels closest to the peak were given a value of 1 with those further away receiving values closer to 0. This allowed us to create graded contrast-indicator maps (CIMs) for each peak reported. These CIMs were then combined to form a single nested map for each contrast, thus ensuring each contrast could contribute no more than a value of 1 for a given voxel when overall proportion statistics were calculated. These combined CIMs were then weighted by the square root of the sample size of the contrast, so that data based on many participants would exert greater influence on the meta-analyses than data based on just a few participants. The 8-mm kernel used in the present analyses is slightly smaller than what has been used in other MKDA meta-analyses (typically 10–15 mm), making this approach somewhat more conservative. When identical analyses were performed using a 10-mm kernel, all peaks identified using the 8-mm kernel were found to be included within the clusters identified using the 10-mm kernel. Yet, the clusters identified using the 10-mm kernel contained more voxels. One additional cluster was identified in the 10-mm kernel results that was located in left middle frontal gyrus (−27 51 30). This cluster did not meet FWE correction for the 8-mm kernel analysis (k = 38) and thus was not reported in the present results.
Statistical inferences were made by comparing the proportion of contrasts showing activation in a given voxel to an empirical null distribution derived by Monte-Carlo random sampling of spatially scrambled peaks. The data were resampled to a voxel size of 3 mm, and 5000 simulations were conducted for each analysis. Results were masked using a gray matter mask created through segmentation of the MNI-T1 template (the “Colin” brain) supplied in SPM5 (49 653 3-mm isotropic voxels). All maps were thresholded using a familywise error correction rate of P < 0.05, as implemented in NeuroElf (http://neuroelf.net/). All analyses and visualizations were implemented in NeuroElf.
Results
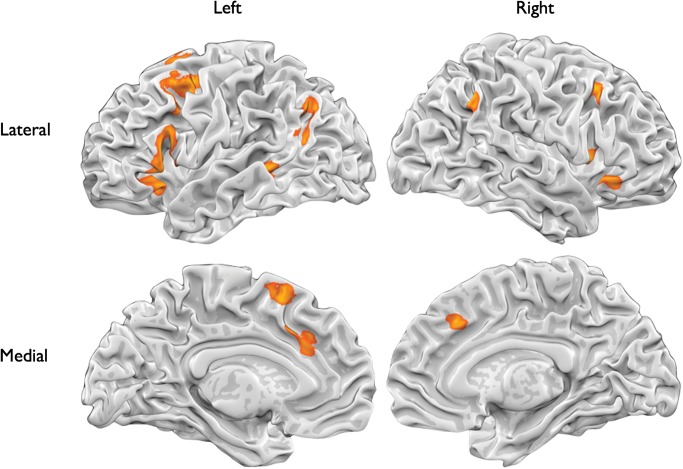
To address our first question regarding what brain regions support reappraisal, we performed an analysis of contrasts comparing reappraisal to a baseline condition (e.g., “responding naturally” to an emotional stimulus). As expected, this revealed extensive recruitment of regions commonly observed in cognitive control tasks including posterior dmPFC and bilateral dlPFC, vlPFC, and posterior parietal cortex (Table 2; Fig. 1).
Table 2.
Peak voxel and corresponding maximum z-values for brain regions supporting reappraisal (reappraise > emotional baseline)
| |
MNI coordinates |
|||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | z | x | y | z |
| Middle frontal gyrus | Right | 175 | 3.72 | 60 | 24 | 3 |
| Middle frontal gyrus | Right | – | 3.72 | 48 | 24 | 9 |
| Middle frontal gyrus | Right | – | 3.72 | 48 | 15 | 6 |
| Inferior frontal gyrus | Right | 101 | 3.72 | 51 | 15 | 48 |
| Inferior frontal gyrus | Right | – | 3.72 | 51 | 6 | 48 |
| Inferior frontal gyrus | Right | – | 3.72 | 42 | 21 | 45 |
| Inferior frontal gyrus | Right | – | 3.72 | 42 | 30 | 39 |
| Medial frontal gyrus | Right | 309 | 3.72 | 9 | 30 | 39 |
| Medial frontal gyrus | Midline | – | 3.72 | 0 | 15 | 63 |
| Medial frontal gyrus | Midline | – | 3.72 | 0 | 6 | 63 |
| Medial frontal gyrus | Midline | – | 3.72 | 0 | −9 | 63 |
| Medial frontal gyrus | Midline | – | 3.72 | 0 | 18 | 42 |
| Anterior cingulate gyrus | Left | – | 3.72 | −3 | 24 | 30 |
| Superior frontal gyrus | Left | – | 3.72 | −9 | 12 | 69 |
| Middle frontal gyrus | Left | 517 | 3.72 | −33 | 3 | 54 |
| Middle frontal gyrus | Left | – | 3.72 | −36 | 15 | 57 |
| Anterior insula | Left | – | 3.72 | −36 | 21 | −3 |
| Anterior insula | Left | – | 3.72 | −42 | 18 | 9 |
| Inferior frontal gyrus | Left | – | 3.72 | −42 | 45 | −6 |
| Inferior frontal gyrus | Left | – | 3.72 | −51 | 12 | 21 |
| Inferior frontal gyrus | Left | – | 3.72 | −51 | 21 | 9 |
| Superior parietal lobule | Right | 77 | 3.72 | 63 | −51 | 39 |
| Superior parietal lobule | Right | – | 3.72 | 60 | −60 | 30 |
| Superior parietal lobule | Right | – | 3.72 | 51 | −60 | 42 |
| Inferior parietal lobule | Right | – | 3.16 | 60 | −45 | 27 |
| Superior parietal lobule | Left | 126 | 3.72 | −42 | −66 | 42 |
| Parietooccipital sulcus | Left | – | 3.72 | −45 | −69 | 18 |
| Middle temporal gyrus | Left | – | 3.72 | −51 | −60 | 27 |
| Superior occipital lobe | Left | – | 3.72 | −54 | −72 | 27 |
| Superior temporal gyrus | Left | – | 3.09 | −63 | −51 | 21 |
| Middle temporal gyrus | Left | 125 | 3.72 | −51 | −39 | 3 |
| Middle temporal gyrus | Left | – | 3.35 | −57 | −24 | -12 |
Local maxima are denoted with “–.”
Figure 1.
Results of the meta-analysis of brain regions supporting reappraisal (reappraise > emotional baseline).
With regards to our second question of whether implementation of reappraisal involves recruitment of vmPFC or recruitment of temporal regions known to support semantic and perceptual representations, 2 observations were made. First, we failed to observe any clusters in vmPFC. Second, a sizeable cluster was observed in left posterior temporal cortex. In this initial analysis, we included all contrasts aimed at identifying brain regions that support reappraisal. As described in the Materials and Methods section, this included both contrasts in which participants decreased their affective responses, and those in which they increased their affective responses. However, increasing one's affective response may upregulate activity in both regions that support reappraisal and in regions that are modulated by reappraisal and therefore may complicate interpretation of results. Therefore, we conducted a second meta-analysis that included only contrasts that compared downregulation of affect to a baseline condition, excluding any contrasts that examined neural responses supporting upregulation of affect. The results of this analysis were nearly identical, suggesting consistent prefrontal, parietal, and temporal regions are consistently recruited by reappraisal, regardless of whether one is reappraising to increase or decrease affective responses. Interested readers can examine these results at http://www.scan.psych.columbia.edu/supplementary/Buhle_Silvers_FigS1_SourceOnly.html. Finally, to directly test whether any subset of studies reported peaks in vmPFC, we conducted an ROI analysis using a 15-mm radius sphere centered on a peak in vmPFC reported by Diekhof and coworkers in their meta-analysis of reappraisal studies (2011; 6, 40, −22). We found that only 1 of the 838 coordinates included in this analysis fell within this sphere (Krendl et al. 2012).
To address our third question about whether the amygdala or other regions are modulated by reappraisal, we performed an analysis of brain regions that responded more strongly during a baseline condition than during reappraisal, excluding any contrasts that indexed activity related to upregulation of affect. This analysis sought to identify regions where activation decreased in the presence of reappraisal. The analysis revealed bilateral amygdala clusters, with the left cluster extending into the ventral striatum and pallidum (Table 3; Fig. 2). No clusters were observed anywhere else in the brain.
Table 3.
Peak voxel and corresponding maximum z-values for brain regions modulated by reappraisal (emotional baseline > reappraise)
| |
MNI coordinates |
|||||
|---|---|---|---|---|---|---|
| Region | Side | Extent | z | x | y | z |
| Amygdala | Right | 126 | 3.72 | 30 | −3 | −15 |
| Extended amygdala | Right | – | 3.72 | 27 | 6 | −12 |
| Amygdala | Left | 111 | 3.72 | −18 | −3 | −15 |
Local maxima are denoted with “–.”
Figure 2.
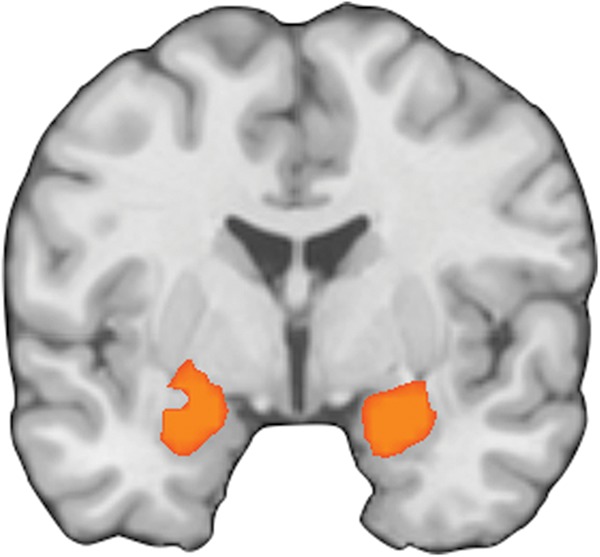
Results of the meta-analysis of brain regions modulated by reappraisal (y = −3; emotional baseline > reappraise).
Discussion
In the present meta-analysis, we found that the implementation of reappraisal consistently activated cognitive control regions, including dmPFC, dlPFC, vlPFC, and posterior parietal lobe. These results are in line with the predictions of psychological models of reappraisal, which emphasize the role of domain-general cognitive control processes in the cognitive regulation of emotion (Ochsner and Gross 2008). Specifically, the present results are consistent with the hypotheses that dlPFC may support manipulation of appraisals in working memory (Wager and Smith 2003), vlPFC may support selection and inhibition of appraisals (Robbins 2007; Simmonds et al. 2008), and dmPFC may support semantic and self-reflective processes relevant to elaborating the affective meaning of stimuli or perceiving one's affective state (Crosson et al. 2002; Cato et al. 2004; Amodio and Frith 2006; Olsson and Ochsner 2008; Binder et al. 2009). These results also are broadly consistent with the results of earlier meta-analyses that examined smaller samples of studies (Kalisch 2009; Diekhof et al. 2011).
However, we found that the implementation of reappraisal in the current set of studies did not consistently recruit vmPFC. This null finding contradicts the hypothesis that vmPFC provides a necessary link by which frontal and parietal regions modulate emotion-related activity in the amygdala (Ochsner and Gross 2005, 2007; Schiller and Delgado 2010; Diekhof et al. 2011; Etkin et al. 2011). This result may appear somewhat surprising, given the rich neuroscience literature demonstrating the involvement of vmPFC in fear extinction, reversal learning, and regulation of social behavior (Bechara et al. 2005; Quirk and Beer 2006; Quirk et al. 2006; Milad et al. 2007; Wager et al. 2007; Finger et al. 2008). One possibility is that vmPFC activation is not observable in contrasts comparing reappraisal to responding naturally to emotional stimuli because it is engaged by both. This could be because vmPFC is engaged during reappraisal as well as on baseline trials, either because it is important for relatively passive or nongoal-oriented forms of emotion regulation, such as extinction (Schiller and Delgado 2010; Diekhof et al. 2011), that might arise spontaneously on baseline trials. Alternatively, it could be that vmPFC is involved in processes related to emotion generation, such as self-reflection (Denny et al. 2012). Indeed, whether emotion generation and regulation necessarily rely upon distinct neural mechanisms remains an open question, given that partially overlapping prefrontal regions have been shown in prior work to support emotion generation, perception, experience, and regulation (Kober et al. 2008; Fusar-Poli et al. 2009; Ochsner et al. 2009; Vytal and Hamann 2010; Gross and Barrett 2011; Lindquist et al. 2012). To further investigate this issue, future work might seek to examine neural responses during reappraisal using experimental and analytic methods that do not rely on subtraction logic between emotion generation and regulation conditions.
That said, although an early meta-analysis of 13 reappraisal studies did not find any consistent activity in vmPFC (Kalisch 2009), a larger, more recent meta-analysis of 25 studies by Diekhof et al. (2011) did report consistent engagement of vmPFC. There are at least 4 possibilities for the differences in results between the present meta-analysis and those of Diekhof and coworkers. First, the study of Diekhof and coworkers included far fewer studies and thus would be more prone to false positives than the present meta-analysis. A second possibility is that vmPFC recruitment is only observed in a subset of studies and such studies were more heavily sampled in the Diekhof meta-analysis than the present one. For example, it may be that vmPFC is not critical for emotion regulation per se, but is recruited in paradigms that rely more heavily on holding conceptual information in mind (Roy et al. 2012). This includes paradigms such as those utilized by Delgado et al. (2008) that involve imagining a calming stimulus like the ocean during exposure to affective stimuli. Indeed, Diekhof and coworkers only observed vmPFC activity in 3 of the 25 studies included. Yet, when we conducted an ROI analysis to examine this possibility, we found that only 1 of the 838 coordinates included in our meta-analytic dataset fell within this sphere (Krendl et al. 2012), rendering this explanation unlikely. A third possibility is that vmPFC recruitment is not observed in main effect contrasts of reappraisal, but may relate to individual differences. Support for this comes from several studies have found that connectivity between vmPFC and subcortical structures differs between individuals as a function of psychiatric status (Johnstone et al. 2007; Erk et al. 2010), genetics (Schardt et al. 2010), and behavioral measures of reappraisal success (Wager, Davidson, et al. 2008). Importantly, 1 of the 3 vmPFC peaks observed in the meta-analysis by Diekhof and coworkers) came from a study that utilized a between-subjects correlational analysis rather than a main effect contrast (Urry et al. 2006), and another involved comparisons of vmPFC connectivity between healthy controls and a patient population (Johnstone et al. 2007). In the present analysis, we endeavored to include only analyses that best represented the main effect of reappraisal in healthy individuals, as described in detail in the Materials and Methods section. Therefore, unlike the Diekhof meta-analysis, we did not include 1) contrasts comparing patients and healthy controls, or 2) correlational analyses or functional connectivity. We believe this approach enhances the interpretability of our results and also effectively combats the growing multiple comparisons problem in neuroimaging, in which greater analytic flexibility increases the likelihood of finding regions compatible with one's hypotheses (Carp 2012, 2012).
The lack of involvement of vmPFC in reappraisal suggests that while reappraisal may lead to a similar outcome as extinction (i.e., cue-related emotional activity is reduced), there are likely notable differences in the psychological and neural processes that lead to this outcome. In fear extinction, activity in regions related to emotional responding is reduced as a result of contextual learning processes that may operate outside awareness. In contrast, psychological models of reappraisal posit that it is the reconstruction of semantic and perceptual representations that in turn alters a stimulus's emotional import (Ochsner and Gross 2005, 2007). In line with this interpretation, we observed recruitment of left posterior temporal regions commonly implicated in interpreting actions, reflecting on intentions and extracting semantic meaning (Vigneau et al. 2006; Van Overwalle and Baetens 2009; Rapp et al. 2012). This finding could be interpreted in 2 ways. On the one hand, reappraisal could involve constructing an alternative internally represented version of a perceptual stimulus to which participants respond. For example, one imagines that a sick person will be well—and emotional responding is driven by that imagined stimulus. This interpretation seems plausible given prior work showing that reappraisal is effective even when individuals maintain fixation on emotionally relevant aspects of photographic stimuli (Urry 2010). On the other hand, temporal lobe activity could reflect differential attention to emotion-relevant features that are essential for triggering one's emotions and constructing a reinterpretation of them (Bebko et al. 2011). Future work could attempt to disentangle these possibilities.
Finally, we found strong evidence that reappraisal modulates activity in bilateral amygdala, but not other regions related to emotional responding, such as anterior insula, periaqueductal gray, hypothalamus, thalamus, orbital frontal cortex, temporal pole, or rostral and subgenual anterior cingulate cortex. It is not surprising that amygdala is modulated by reappraisal, given its importance for the detection and encoding of affectively salient stimuli (Wager, Barrett et al. 2008; Lindquist et al. 2012). Furthermore, prior work shows that amygdala responses track affective intensity, especially for aversive stimuli like those photos used widely in reappraisal studies (Canli et al. 2000; Williams et al. 2001; Anderson et al. 2003; Cunningham et al. 2004; Ochsner et al. 2004, 2009; Phan et al. 2004). However, it is somewhat surprising that no other regions were reliably modulated by reappraisal. Current models of emotion suggest that emotional responses are reflected in activity across a network of regions (Kober et al. 2008), and in changing one's emotional response one might expect reappraisal to affect this network as a whole.
There are several possible explanations for the amygdala-specific reductions we observed. One possibility is that this reflects the widespread use of aversive stimuli in reappraisal studies and the particular sensitivity of the amygdala in detecting threat (Phelps and LeDoux 2005). Given the role of the ventral striatum and pallidum in craving and the formation of appetitive appraisals, it could be the case that reappraisal modulates activity in the ventral striatum and pallidum for positive stimuli and activity in the amygdala for aversive stimuli. This might explain why the left amygdala cluster observed in this analysis extended somewhat into the ventral striatum and pallidum—perhaps the studies using positive stimuli contributed to one part of that cluster while studies using negative stimuli contributed to another part of that cluster. However, this may be an overly simplistic characterization of these structures' roles in emotional generation and regulation. Although the ventral striatum is involved in reward, prior research has shown that the ventral striatum responds both to positive and negative stimuli (Roitman et al. 2005) and that stimulation of this region can result in both approach and avoidance (Reynolds and Berridge 2001, 2002, 2003). Thus, it may be that the ventral striatum is involved in coordinating behavioral responses to emotion more broadly and that reappraisal is most likely to modulate activity in this region when an emotion activates a behavioral response component. In either case, a direct comparison between the neural bases of reappraisal for positive and negative stimuli in the present analysis was not possible because the relatively scant number (14) of studies using positive stimuli would have rendered such a comparison underpowered.
A second possibility for why reappraisal modulated amygdala by, but not other brain regions, is that the overwhelming majority of the studies comprising the present meta-analysis utilized visual stimuli as a means for eliciting emotion (36 utilized photographs and 3 utilized videos). Given the dense connectivity between the amygdala and the visual system, it may be that such stimuli are more likely to elicit amygdala responses than are nonvisual ones (Price 2003; Phelps and LeDoux 2005; LeDoux 2007; Pessoa and Adolphs 2010).
A third possibility is that other emotion-related regions are at least sometimes modulated by reappraisal, but these reductions have not been identified because of a lack of sensitivity of most commonly used analysis techniques. Indeed, the robust results observed in the amygdala reflect in part the selective targeting of this region with region of interest (ROI) analyses. Of the 33 studies that contributed to the analysis of brain regions modulated by reappraisal, only 9 used an ROI analysis. However, a higher percentage of studies with ROI methods yielded peaks in the left amygdala (7 of 9 ROI studies vs. 8 of 24 whole-brain studies) than did those relying exclusively on whole-brain analyses, χ2(1, N = 33) = 5.215, P < 0.05, and a similar trend was observed for the right amygdala (7 of 9 ROI studies vs. 10 of 24 whole-brain studies), χ2(1, N = 33) = 3.417, P = .07. Thus, we encourage more widespread use of ROI analyses in other brain regions thought to support emotion generation (e.g., ventral striatum, insula, vmPFC) in future reappraisal research.
Limitations
While the present analysis represents the largest and most complete meta-analysis of reappraisal studies to date, it is not without limitations. First, in order to clearly characterize the neural bases of reappraisal in healthy individuals, our meta-analysis relied solely on main effect contrasts comparing reappraisal to a baseline condition in healthy individuals. This is the most commonly reported type of contrast in reappraisal manuscripts, and it offers the most straightforward way to identify the neural regions that support reappraisal. Yet, by relying heavily on the subtraction approach for identifying brain regions related to reappraisal, we may sometimes overlook brain regions that are commonly involved in both reappraisal and in either emotion generation or spontaneous (i.e., not driven by an external goal) emotion regulation. Future work will need to employ novel paradigms in order to address this possibility.
A second limitation of the present meta-analysis is that it is largely comprised of contrasts examining downregulation of negative affect induced by viewing aversive photographic images (As seen in Table 1, 34 of the 48 studies included this type of contrast). This homogeneity in research practices makes it difficult to formally test what brain regions support reappraisal regardless of methodological practices and which vary as a function of variables such as stimulus valence (positive or negative), stimulus type (pictures, videos, memories, etc.), tactic (reinterpretation or distancing), and reappraisal goal (increase or decrease affective response). For example, one might hypothesize that reappraisal of negative pictures would elicit different patterns of cognitive control than reappraisal in the domain of decision-making. Yet, testing this hypothesis is virtually impossible at present given that only 4 studies have examined reappraisal in the domain of decision-making and within those 4 studies, 2 examined reappraisal at the time of decision-making (Grecucci, Giorgetta, Van't Wout et al. 2012; Sokol-Hessner et al. 2012), another examined reappraisal during anticipation of monetary reward (Staudinger et al. 2011), and another examined reappraisal during both anticipation and receipt of monetary reward (Staudinger et al. 2009). In order to use meta-analysis to compare reappraisal across different types of emotional situations, we must adopt a more diverse approach to studying reappraisal. The use of more diverse stimuli is also needed to enhance connections between basic research on reappraisal and therapeutic practices that involve elements of reappraisal (Grecucci, Giorgetta, Bonini et al. 2012; Grecucci et al. 2013). For example, in order to better characterize clinical disorders and customize treatment, it may prove particularly useful to ask patients to reappraise stimuli specific to their symptomology. More studies have attempted to do this recently with work examining reappraisal of phobogenic stimuli in phobic populations (Hermann et al. 2009) or social stimuli in populations characterized by unstable social relationships, such as borderline personality disorder (Koenigsberg et al. 2009).
Conclusion
In line with existing models, the implementation of reappraisal consistently activated domain-general cognitive control regions, including dmPFC, dlPFC, vlPFC, and posterior parietal lobe (Ochsner and Gross 2005, 2008; Kalisch 2009; Schiller and Delgado 2010; Diekhof et al. 2011; Ochsner et al. 2012). However, in contrast to several prominent theories, the present results indicate that reappraisal does not rely on vmPFC-mediated emotional control (Ochsner and Gross 2005, 2007; Schiller and Delgado 2010; Diekhof et al. 2011; Etkin et al. 2011), but instead may act though the lateral temporal cortex, an area associated with semantic and perceptual representation. Lastly, reappraisal was consistently associated with bilateral amygdala modulation, but no change was observed in other regions related to emotional responding, or anywhere else in the brain. While these results affirm that the amygdala is modulated by reappraisal of aversive stimuli, more sensitive analyses and the use of a wider range of affective stimuli may be necessary to assess whether additional regions involved in emotional responding regions are similarly impacted by reappraisal.
Funding
This work was supported by: the National Institutes of Health (grant numbers MH094056, awarded to J.A.S.; R01 MH076137, R01 HD069178 and R01 DA022541 awarded to K.N.O.; R01 MH076136, RC1 DA028608 and R01 DA027794, awarded to T.D.W.) and the National Science Foundation (grant number 0631637, awarded to K.N.O).
Notes
Author contributions: Design: J.T.B.; J.A.S.; T.D.W.; R.L; C.O; H.K.; K.N.O.; Data collection: J.T.B.; J.A.S.; R.L; C.O; H.K.; Analysis: J.T.B.; J.A.S.; J.W.; T.D.W; Writing: J.T.B.; J.A.S.; K.N.O. Conflict of Interest: None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu Rev Psychol. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Mentalization-based treatment for borderline personality disorder: a practical guide. Oxford: Oxford University Press; 2006. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko GM, Franconeri SL, Ochsner KN, Chiao JY. Look before you regulate: differential perceptual strategies underlying expressive suppression and cognitive reappraisal. Emotion. 2011;11:732–742. doi: 10.1037/a0024009. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes BL, Kross E, Atlas LY, McRae K, Wager TD. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss038. http://scan.oxfordjournals.org/content/early/2012/04/17/scan.nss038.long . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front Neurosci. 2012;6:149. doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage. 2012;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, et al. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 2004;16:167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2012;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crosson B, Cato MA, Sadek JR, Gokcay D, Bauer RM, Fischler IS, Maron L, Gopinath K, Auerbach EJ, Browd SR, et al. Semantic monitoring of words with emotional connotation during fMRI: contribution of anterior left frontal cortex. J Int Neuropsychol Soc. 2002;8:607–622. doi: 10.1017/s1355617702801394. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci. 2012;21:54–59. [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16:1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Silvers JA, Ochsner KN. How we heal what we don't want to feel: the functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: a transdiagnostic approach to etiology and treatment. New York: Guilford Press; 2009. pp. 59–87. [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Mitchell DG, Jones M, Blair RJ. Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage. 2008;43:748–755. doi: 10.1016/j.neuroimage.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Bonini N, Sanfey AG. Living emotions, avoiding emotions: behavioral investigation of the regulation of socially driven emotions. Front Psychol. 2012;3:616. doi: 10.3389/fpsyg.2012.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Van't Wout M, Bonini N, Sanfey AG. Reappraising the ultimatum: an fMRI study of emotion regulation and decision making. Cereb Cortex. 2012;23:399–410. doi: 10.1093/cercor/bhs028. [DOI] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Van't Wout M, Bonini N, Sanfey AG. Reappraising the ultimatum: an fMRI study of emotion regulation and decision making. Cereb Cortex. 2013;23:399–410. doi: 10.1093/cercor/bhs028. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Barrett LF. Emotion generation and emotion regulation: one or two depends on your point of view. Emot Rev. 2011;3:8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Have-de Labije Jt, Neborsky RJ. Mastering intensive short-term dynamic psychotherapy: a roadmap to the unconscious. London: Karnac; 2012. [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, Labar KS. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Schafer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, Bruhl A, Kottlow M, Schreiter-Gasser U, Abler B, Jancke L, Rufer M. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37:652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Ichikawa N, Siegle GJ, Jones NP, Kamishima K, Thompson WK, Gross JJ, Ohira H. Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2011;11:354–371. doi: 10.3758/s13415-011-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:1–23. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Wager TD. Meta-analyses of neuroimaging data. Wiley Interdiscip Rev Cogn Sci. 2010;1:293–300. doi: 10.1002/wcs.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, Tecuta L, Guerreri S, Goodman M, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Kensinger EA, Ambady N. How does the brain regulate negative bias to stigma? Soc Cogn Affect Neurosci. 2012;7:715–726. doi: 10.1093/scan/nsr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Facilitating adaptive emotional analysis: distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Pers Soc Psychol Bull. 2008;34:924–938. doi: 10.1177/0146167208315938. [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65:361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Kotchoubey B, Frick C, Spitzer C, Grabe HJ, Barnow S. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2012;59:1727–1734. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Eippert F, Veit R, Anders S. Intentional social distance regulation alters affective responses towards victims of violence: an fMRI study. Hum Brain Mapp. 2012;33:2464–2476. doi: 10.1002/hbm.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TR, Trost WT, Salsman N, Linehan MM. Dialectical behavior therapy for borderline personality disorder. Annu Rev Clin Psychol. 2007;3:181–205. doi: 10.1146/annurev.clinpsy.2.022305.095229. [DOI] [PubMed] [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM. Neural correlates of regulation of positive and negative emotions: an fmri study. Neurosci Lett. 2009;457:101–106. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Maroda KJ. Psychodynamic techniques: working with emotion in the therapeutic relationship. New York: Guilford Press; 2010. [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012a;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc Cogn Affect Neurosci. 2012b;7:253–262. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JDE, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Process Intergr Relat. 2008;11:145–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc Cogn Affect Neurosci. 2010;5:369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, Thompson RH, editors. The handbook of emotion regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiol Aging. 2012;33:645–655. doi: 10.1016/j.neurobiolaging.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell-Sills L, et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Mutschler DE, Erb M. Where in the brain is nonliteral language? A coordinate-based meta-analysis of functional magnetic resonance imaging studies. Neuroimage. 2012;63:600–610. doi: 10.1016/j.neuroimage.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt DM, Erk S, Nusser C, Nothen MM, Cichon S, Rietschel M, Treutlein J, Goschke T, Walter H. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2010;53:943–951. doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Scherer KR, Schorr A, Johnstone Te. Appraisal processes in emotion: theory, methods, research. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Domes G, Kruger A, Berger C, Fleischer M, Prehn K, Schmahl C, Grossmann A, Hauenstein K, Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN. The neuroscience of emotion regulation: basic mechanisms and their role in development, aging and psychopathology. In: Ochsner KN, Kosslyn SM, editors. The handbook of cognitive neuroscience. New York: Oxford University Press; 2013. [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P, Camerer CF, Phelps EA. Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc Cogn Affect Neurosci. 2012;8:341–350. doi: 10.1093/scan/nss002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain; 3-dimensional proportional system: an approach to cerebral imaging. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Urry HL. Seeing, thinking, and feeling: emotion-regulating effects of gaze-directed cognitive reappraisal. Emotion. 2010;10:125–135. doi: 10.1037/a0017434. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vrticka P, Sander D, Vuilleumier P. Effects of emotion regulation strategy on brain responses to the valence and social content of visual scenes. Neuropsychologia. 2011;49:1067–1082. doi: 10.1016/j.neuropsychologia.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci. 2010;22:2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, Joseph J, Davidson M, Mize J. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3rd ed. New York: Guilford; 2008. pp. 249–271. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS ONE. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall C, Barrett LF, Barsalou LF. Psychol Sci. Neural evidence that human emotions share core affective properties. forthcoming. http://pss.sagepub.com/content/early/2013/04/19/0956797612464242.long . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Soc Cogn Affect Neurosci. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]