Abstract
Thermoregulatory events are associated with activity in the constituents of the spinothalamic tract. Whereas studies have assessed activity within constituents of this pathway, in vivo functional magnetic resonance imaging (fMRI) studies have not determined if neuronal activity in the constituents of the tract is temporally ordered. Ordered activity would be expected in naturally occurring thermal events, such as menopausal hot flashes (HFs), which occur in physiological sequence. The origins of HFs may lie in brainstem structures where neuronal activity may occur earlier than in interoceptive centers, such as the insula and the prefrontal cortex. To study such time ordering, we conducted blood oxygen level-dependent-based fMRI in a group of postmenopausal women to measure neuronal activity in the brainstem, insula, and prefrontal cortex around the onset of an HF (detected using synchronously acquired skin conductance responses). Rise in brainstem activity occurred before the detectable onset of an HF. Activity in the insular and prefrontal trailed that in the brainstem, appearing following the onset of the HF. Additional activations associated with HF's were observed in the anterior cingulate cortex and the basal ganglia. Pre-HF brainstem responses may reflect the functional origins of internal thermoregulatory events. By comparison insular, prefrontal and striatal activity may be associated with the phenomenological correlates of HFs.
Keywords: brainstem, fMRI, hot flash, insula, thermal regulation
Introduction
Thermoreception and thermoregulation are essential evolutionary endowments for mammals, particularly those with homeothermic requirements (Craig 2002). In general, interoceptive systems in the brain are thought to involve the hierarchically organized spinocortical and thalamic tracts. Ascending projections from laminar neurons are the bases for somatosensory reflex arcs in the spinal, medullary, and mesencephalic regions of the nervous system with projections ascending through the layers of this pathway. Ascending activity in this pathway may form the physiological bases for the interoception of pain (Craig 2003; Leone et al. 2006) and in the case of interest in this investigation, the regulation of body temperature (Nakamura and Morrison 2008). In the brain, brainstem and mesencephalic regions are the earliest constituents of this pathway and are considered critical for maintaining homeostasis (Satinoff 1978; Mason 2001). Internally generated thermal events, of which menopausal hot flashes (HFs) are an important instance (Freedman 2005), may result from a pattern of ordered activity in the constituents of the spinothalamic tract; activity in the brainstem may be associated with the origins of the HF, whereas activity in structures such as the insula and the dorsal prefrontal cortex may be associated with the experience of the HF. In a group of symptomatic menopausal women, we used blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to study the time courses of the response of these regions before and after the onset of spontaneous HFs.
Activity in the Spinothalamic Tract: Extant Studies
Recent evidence indicates that activity in brainstem structures such as the dorsal raphe could be driven by nonnoxious cooling of the skin surface, suggesting critical sensitization of the medulla to externally applied thermal stimulation (McAllen et al. 2006). These results are consistent with increased activity in brainstem structures that have been observed in exaggerated responses to pain (hyperalgesia). Punctate stimulation of the leg in healthy controls (to induce secondary hyperalgesia) results in subsequent hyperactivation of midbrain structures like the reticular formation during mechanical stimulation, indicating responsivity to thermal sensitization and neuropathic pain (Zambreanu et al. 2005).
In vivo studies of brain activity in response to thermal stimulation have primarily used exogenous thermal events (nociceptive processing), and studies of endogenous thermal events such as HFs are scarce. However, several studies have assessed the response of subcortical and cortical structures to exogenously applied thermal stimulation. fMRI and positron emission tomography activity associated with exogenous thermal stimulation has been documented in constituents of the interoceptive pathway including brain stem structures such as the dorsal raphe (McAllen et al. 2006), the insula (Craig et al. 2000; Peltz et al. 2011), and dorso-lateral prefrontal cortex (Kong et al. 2006). Graded cooling or heating of the external skin surface results in graded changes in insular regional cerebral blood flow (rCBF) activity indicating responsivity of the insular cortex to exogenous thermal events (Derbyshire et al. 1997; Craig et al. 2000). It has been implied that this activity reflects the integration of afferent signals from the skin surface with internal thermal sensors (Egan et al. 2005). These data are consistent with the idea of the insula as being a central region for modality-independent interoception (Craig 2003; Wiens 2005; Pollatos et al. 2007). Further, delivery of thermal pain to the forearm results in sharply increased fMRI estimated neuronal activity in the dorso-lateral prefrontal cortex and other forebrain structures (Kong et al. 2006). Frontal activity is also modulated by the intensity of thermal pain (Derbyshire et al. 1997; Wiech et al. 2005), indicating that, in addition to the insula, the frontal cortex may be central to the interoceptive experience.
Menopausal Hot Flashes: Unique Endogenous Thermal Events
These imaging studies provide evidence of detectable neural activity in the spinothalamic tract in response to experimentally applied exogenous galvanic stimulation. However, endogenous thermal events, such as menopausal HFs, have physiological origins that are distinct from exogenously delivered heat stimulation (Freedman et al. 2006). HFs involve feelings of intense heat surges, accompanied by sweating and cutaneous vasodilation (Freedman 2005). Whereas the specific neurophysiology of the phenomena are not clearly understood, HF's appear to result from fluctuations in core body temperature within a reduced thermoneutral zone (i.e., the core body temperatures range between the shivering and sweating thresholds), possibly resulting from endocrine changes such as elevated central noradrenergic (NE) activation (Freedman and Krell 1999) and estrogen depletion.
Thus, at least 2 important questions of significant ecological relevance are unanswered from work on exogenous thermal events: (1) Are regions in spinothalamic tracts responsive to endogenous thermal events and (2) Is there a detectable ordered temporal sequence of measurable responses in the thermoreceptive pathway to endogenous thermal events? Recent fMRI work has provided evidence of the responsivity of the insula, cingulate, and frontal cortices to the experience of HFs (Freedman et al. 2006), although the antecedents of this activity within the spinothalamic tract were not studied. In the present study, gradient-echo, echo-planar images (EPIs) were collected while spontaneous flash onset was monitored based on a criterion increase in skin conductance level (SCL). Analyses of fMRI data were gated to the onset of the HF. The fMRI response was assessed in 3 equi-temporal windows (Baseline, Pre-Flash, and Flash; 20 s each) across principal regions of interest (the brainstem, insula, dorso-lateral prefrontal cortex, hypothalamus, anterior cingulate, and basal ganglia) to assess potential antecedents and consequents of HFs.
Materials and Methods
Subjects
Twenty healthy symptomatic postmenopausal women (ages 47–58, mean age 51.9 ± 3.06 years) participated in the study. All subjects were free of medication at the time of fMRI assessments and reported frequent HFs (6/day or more). This is at the upper range of HF frequencies, which maximized the probabilities of HFs occurring during fMRI acquisition. The Human Investigation Committee of Wayne State University authorized the study, and informed written consent was obtained from all participants. Participants received monetary compensation for their participation.
Thermal Regulation and Skin Conductance Level
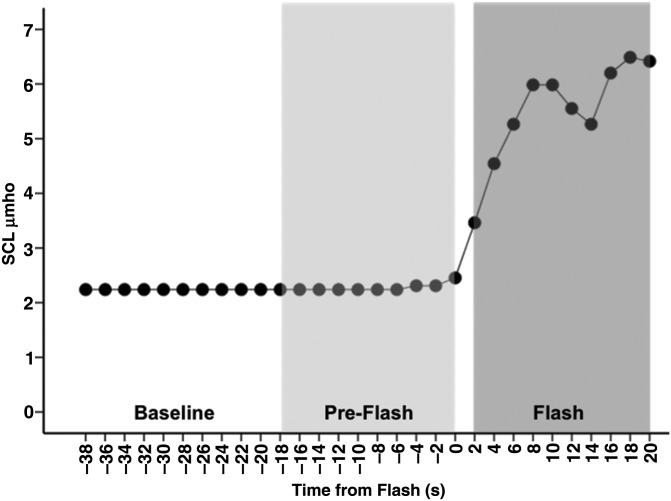
Before being placed into the bore of the magnet, all subjects were covered ventrally and dorsally with two 61 × 152 cm (Cincinnati Sub-Zero Norm-O-Temp, Cincinnati, OH, USA) circulating water pads. Once positioned inside the bore, and prior to the beginning of the first scan, the temperature of the circulating water was raised to 42 °C and maintained throughout, producing a gradual heating stimulus, which we have shown to reliably induce HFs (Freedman et al. 1992). The gradual heating stimulus refers to the maintenance of heat by use of heating pads; the temperature of the pads did not change because it is electronically regulated to remain constant. SCL, an electrical measure of sweating, was recorded from the sternum using 2 Ag/AgCl electrodes, a 0.5-V constant–voltage circuit, and an analog-to-digital converter (Data Translation 9802, Marlborough, MA, USA). This criterion was validated against subject self-reports, both inside and outside the laboratory (Freedman and Woodward 1992; Freedman et al. 1992; de Bakker and Everaerd 1996). The SCL readout was closely monitored in the control room throughout the scanning session. Using previously established criteria (Freedman et al. 2006), flash onset was determined by a 2-μmho/30 s increase in the SCL response (Fig. 1), which corresponds to >90% of HFs that occur.
Figure 1.
Sample SCL shows Baseline, Pre-Flash, and Flash windows. SCL is plotted at 2 s intervals to maintain consistency with subsequent BOLD data (sampled at a frequency of 0.5 Hz, TR = 2 s). Temporal partitions into Flash, Pre-Flash, and Baseline windows (see fMRI Analyses section) are shown for consistency with subsequent depictions of BOLD time series.
Functional MRI
All fMRI was conducted on a Bruker MedSpec 4.0-T system at the Vaitkevicius Imaging Center in the Wayne State University School of Medicine. Gradient-echo EPIs were acquired over multiple scans (5–15 min/scan) for a period of 120 min. EPI parameters were: echo time: 30 ms, repetition time (TR): 2 s, matrix: 64 × 64, 33 slices, field of view: 224 mm, voxel size 3.5 × 3.5 × 4 mm.
fMRI and Statistical Analyses
Functional images were preprocessed using a standard protocol (Friston et al. 1995), including realignment to the first image in the series, correction for susceptibility-by-movement interactions, normalization to a standard EPI template (Montreal Neurological Institute, MNI), and smoothing with an 8-mm full-width at half-maximum isotropic Gaussian kernel. Data were analyzed for a 30-image (60 s) period around flash onset (time = 0 s in Figs 1–4) in 3 equi-temporal windows (Baseline, Pre-Flash, and Flash). In first-level analyses (individual subjects), windows of interest treated as boxcar wave forms were convolved with the canonical hemodynamic response function to produce reference wave forms for contrast assessment within the General Linear Model framework. Subsequent contrasts were used to identify activity associated with each window of interest (Flash or Pre-Flash) relative to the comparison window (Pre-Flash or Baseline). Individual contrast images were submitted to a second-level random-effects analysis (Turner et al. 1998), with time window as repeated measure, to assess group-based activation during the temporal windows of interest.
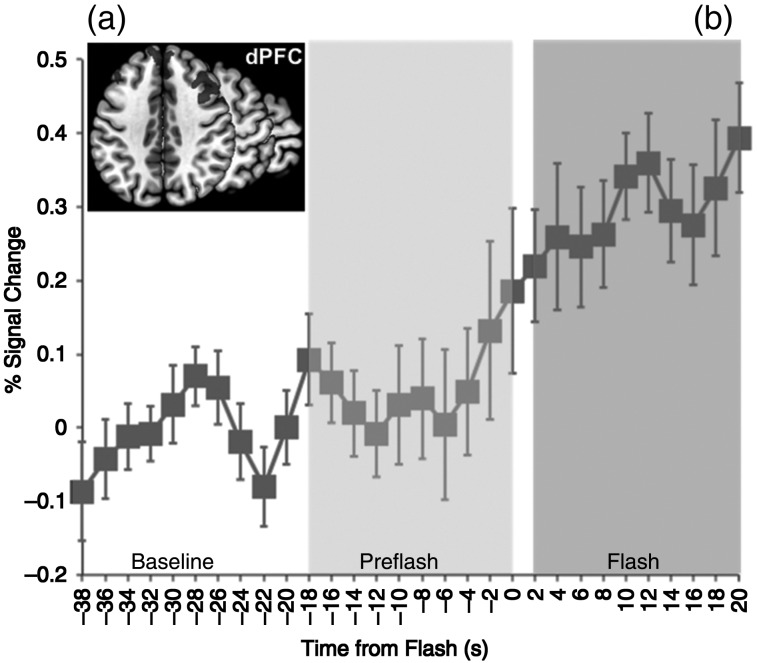
Figure 4.
(a) Activation map in the bilateral prefrontal cortex depicts significant bi-lateral clusters (axial plane, z = 40). The graph depicts the baseline normalized and cross-subject averaged fMRI signal (smoothed over a 2-point neighborhood) through the temporal windows of interest. As with the insula, the prefrontal cortex was not significantly active in the Pre-Flash window. Error bars are ±SEM (calculated over unsmoothed time series).
Statistical analyses and fMRI time-series extraction were conducted in the a priori regions of interest using a combination of anatomical and functionally defined region of interest masks. Significant clusters were identified using minimum cluster-size thresholding (P < 0.05) to identify true positively activated cluster extents in the regions of interest (Ward 2000; Bennett et al. 2009). For reporting peaks, voxel coordinates in MNI space were transformed into Talairach space using a previously established algorithm (Lancaster et al. 2007), and Brodmann areas reported where appropriate (Lancaster et al. 2000). Bilateral anatomical masks in stereotactic space (Tzourio-Mazoyer et al. 2002) were used for both the insula and the dorsal prefrontal cortex (and additional anatomical regions assessed: hypothalamus, anterior cingulate, and basal ganglia).
Several studies have used affine transformation-based stereotactic images to subsequently infer intrabrainstem activation loci based on anatomical landmarks in stereotactic space (Dunckley et al. 2005; Zambreanu et al. 2005), but localization to specific brainstem nuclei is challenging. Therefore, we used a functionally achieved cluster of interest in the brainstem based on activity identified in the Pre-Flash > Baseline contrast (P < 0.05, cluster level) overlaid on a mask of the region (Maldjian et al. 2003).
fMRI signal was extracted from unsmoothed BOLD images to minimize the compromise of spatial resolution (Scouten et al. 2006). For each image, data across voxels within the region of interest were averaged to create a single measure of regional activity at a given time-point. Signal was normalized to the average signal within the baseline temporal window and expressed in typical units (percent signal change).
Results
Activity and fMRI Signal in Brain Stem Structures
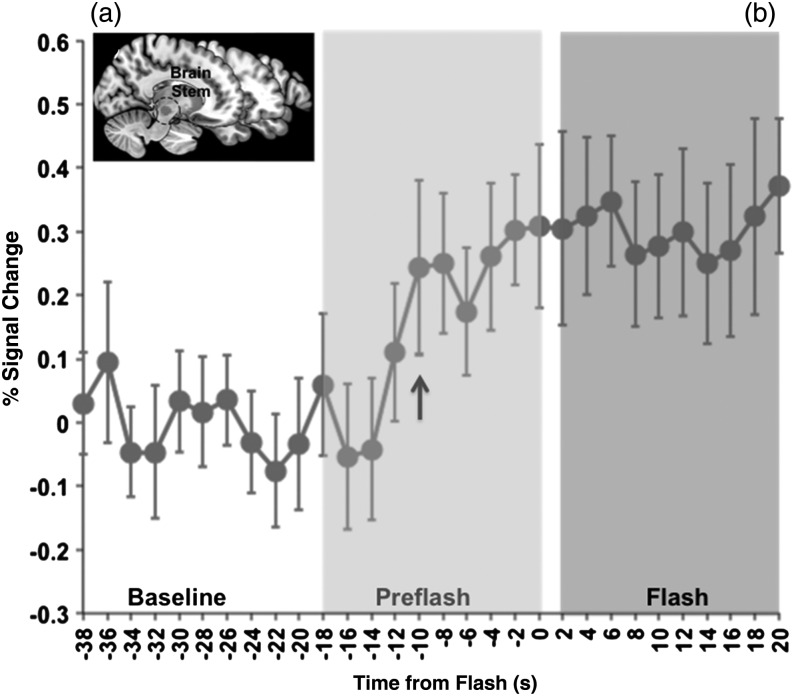
Random-effects analyses of fMRI data demonstrated significant clusters of activity in the brainstem during the Pre-Flash window relative to the Baseline. The bilateral activation peaks (reported in Talairach coordinates) were located in the substantia nigra (t(19) = 2.97, x = 10, y = −16, z = −9; t(19) = 2.76, x = −8, y = −18, z = −8). Additional peaks were also located in the red nucleus (t(19) = 2.03, x = 8, y = −18, z = −6; t(19) = 2.51, x = −8, y = −18, z = −10). Significant clusters are projected on a sagittal slice of the medial brain (Fig. 2a, All activation data and information on largest clusters in the regions of interest are provided in Table 1). These brain stem structures remained active during the subsequent onset of the HF (Flash > Baseline, P < 0.05), and no difference in activity was observed between the Flash and Pre-Flash windows (P > 0.2).
Figure 2.
(a) Activation map in the brain stem depicts significant clusters (sagittal plane, y = 12) during the Pre-Flash (relative to baseline) period. The graph depicts the baseline normalized and cross-subject averaged fMRI signal (smoothed over a 2-point neighborhood) through the temporal windows of interest. A noticeable rise in the fMRI response is detected in the brainstem before the onset of the HF (arrow). This rise is evident approximately 10 s before the SCL response (Fig. 1), suggesting that fMRI activity in this region precedes the onset of the flash. Error bars are ±SEM (calculated over unsmoothed time series).
Table 1.
Statistical and spatial information associated with each of the analyses (Figs 2–4 and 6) and conditions/contrasts
| Analyses | Location/BA | Peak (Talairach) | t | kE; Cluster volume |
|---|---|---|---|---|
| Pre-Flash > Baseline | Brain stem (substantia nigra) | (−8, −18, −8) | 2.76 | 130 voxels; 1044 mm3 |
| Brain stem (substantia nigra) | (10, −16, −9) | 2.97 | 117 voxels; 936 mm3 | |
| Flash > Baseline | Insula (BA 13) | (30, 16, −5) | 4.38 | 1032 voxels; 8256 mm3 |
| dPFC (BA 9) | (39, 33, 30) | 4.24 | 232 voxels; 1856 mm3 | |
| Anterior cingulate (BA 32) | (−16, 12, 36) | 4.06 | 1219 voxels; 9752 mm3 | |
| Basal ganglia (Putamen) | (23, 1, 16) | 3.38 | 766 voxels; 6128 mm3 |
Note: For each set of analyses, we provide the locations of significance peaks, cluster sizes under the peak cluster (kE), and cluster volume in the significant cluster. For the Flash > Baseline contrast, information is provided for the largest bilateral clusters in each of the regions of interest. dPFC, dorsal prefrontal cortex.
Bilaterally averaged fMRI time series (Fig. 2b) were extracted from bilateral regions from this cluster of interest. The bilaterally derived regions were symmetrical [volumes of 1044 and 936 mm3 (see Table 1) for the left and right hemispheres, respectively]. As seen, a detectable upward shift in the fMRI response at t = −10 s was maintained throughout the temporal window and through the subsequent experience of the HF.
Activity and BOLD in the Insula
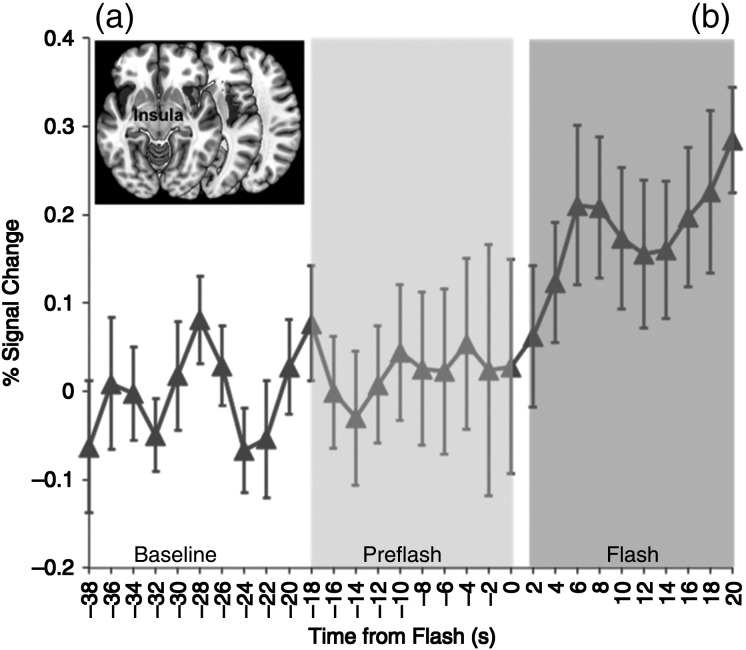
Increased activity was observed bilaterally in the insula during the Flash relative to the Pre-Flash window. Bilateral activation peaks were observed (t(19) = 4.38, x = 30, y = 16, z = −5; t(19) = 4.11, x = −38, y = −38, z = 20). Bilateral activity is visualized on 3 successive axial slices (Fig. 3a). No significant activation was observed in the Pre-Flash period (Pre-Flash > Baseline, P > 0.1).
Figure 3.
(a) Activation map in the bilateral insula depicts significant bi-lateral clusters (axial plane, z = −12) during the HF (relative to baseline) period. The graph depicts the normalized (relative to baseline) and averaged (across subjects) fMRI signal (smoothed over a 2-point neighborhood) through the temporal windows of interest. Note that the insula was not active in the Pre-Flash window, and that the rise in the fMRI response follows the onset of the flash by approximately 6 s. Error bars are ±SEM (calculated over unsmoothed time series).
Bilaterally averaged fMRI time series across the entire insula (Fig. 3b) showed significant increases in neuronal activity at approximately t = +6 s, remaining tonic through the subsequent duration of the window.
Activity and BOLD in the Prefrontal Cortex
Significantly increased activity was observed bilaterally in the prefrontal cortex during the flash relative to the Pre-Flash window. Bilateral activation peaks were observed (t(19) = 4.24, x = 39, y = 33, z = 30; t(19) = 3.86, x = −44, y = 27, z = 30). Bilateral activity is visualized on successive axial slices (Fig. 4a). As with the insula, no significant activation was observed in the Pre-Flash period (Pre-Flash > Baseline, P > 0.1).
Bilaterally averaged fMRI time series (Fig. 4b) across the entire prefrontal cortex show increases in neuronal activity at approximately t = −2 s sustained through the subsequent duration of the HF.
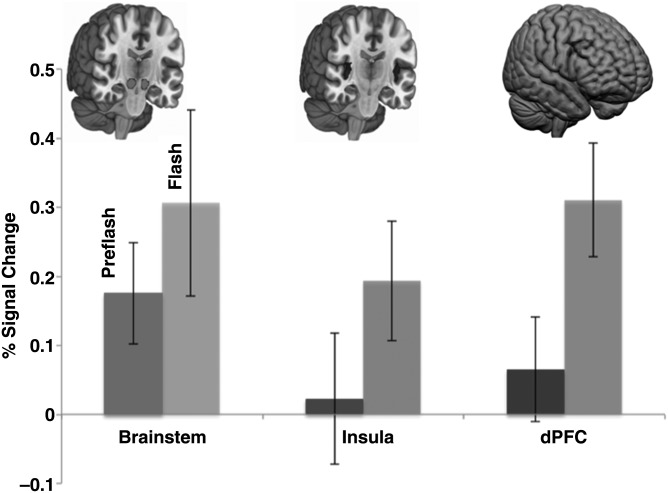
To directly compare activity in the Flash and Pre-Flash windows within the 3 regions of interest, as well as Pre-Flash activity across the 3 regions of interest, for each subject signal change was collapsed within each window and expressed as a single average. Average fMRI signal from the Pre-Flash and Flash windows are depicted in Figure 5 (with significantly activated clusters depicted for each of the regions of interest above corresponding bars). As seen, significantly greater activity in the Flash relative to the Pre-Flash window was observed in the insula and prefrontal cortex, but not the brainstem. Interwindow differences assessed using paired sample t-tests confirmed differences in this temporal sequencing across the regions. Specifically, mean activity in the brainstem during the Pre-Flash and the Flash windows was not significantly different (t(19) = 1.2, P > 0.2). By comparison, mean activity in the Flash window exceeded that in the Pre-Flash window in both the insula (t(19) = 2.47, P < 0.05) and the prefrontal cortex (t(19) = 3.48, P < 0.002). Moderate-to-large effect sizes (Cohen's d) were observed for both comparisons (0.44 and 0.72, respectively). These results are consistent with the activation maps (Figs 2–4) in suggesting a specific sequencing of events in spinothalamic regions.
Figure 5.
Averaged activity in the Pre-Flash and Flash windows across the 3 regions of interest. Images depict volume-rendered activations in the brainstem, the insula, and the dorsal prefrontal cortex successively. Data are shown collapsed across image within the Pre-Flash and Flash windows for each subject. Relative to the Pre-Flash window, significantly increased activity in the Flash window is seen in the insula and prefrontal cortex, but not in the brainstem. Error bars are ±SEM.
We also directly compared interregional activity in the Pre-Flash window to assess whether mean brainstem activity was significantly greater than that observed in both the insula and prefrontal cortex. Both comparisons were significant, with brainstem activity in the Pre-Flash window exceeding both the insula (t(19) = 1.82, P < 0.05, Cohen's d = 0.43) and the prefrontal cortex (t(19) = 1.79, P < 0.05, Cohen's d = 0.37).
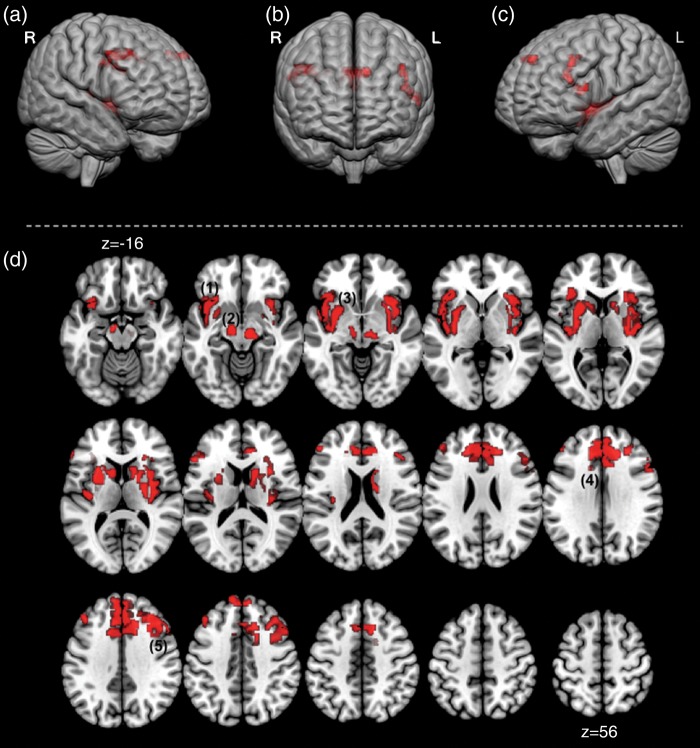
We also opened analyses to other critical regulatory and forebrain regions including (1) the hypothalamus implicated in thermal regulation of internal body temperature (Lee et al. 1985; Craig 2002; Clapham 2012), 2) the basal ganglia, a key site for dopaminergic projections from the substantia nigra implicated in thermal homeostasis (Lee et al. 1985), and 3) the anterior cingulate that has been implicated in the experience of noxious thermal stimulation to the hand (Davis et al. 1998a, 1998b; Kwan et al. 2000). These results are depicted in Figure 6. Figures 6a through c show 3 anterior views of the cortical surface with significant clusters projected. Dorsal prefrontal clusters are visible (Fig. 6a,c), as are clusters in the dorsal anterior cingulate (Fig. 6b). Figure 6d depicts a mosaic of axial views (z = −16 and superior), with significant clusters overlaid showing comprehensive activations during the flash interval that additionally included the dorsal anterior cingulate, the caudate nucleus, and the putamen. No significant clusters were observed in the hypothalamus. Table 1 shows activation cluster volumes with loci peaks for all analyses performed.
Figure 6.
Forebrain activation in the Flash window is depicted in complimentary renditions. (a–c) depicts significant clusters projected onto the cortical surface (depicted from an anterior view). Dorsal prefrontal clusters are visible (a,c) as are clusters in the dorsal anterior cingulate (b). In addition, (d) depicts a mosaic of axial views (z = −16 and superior). Significant clusters overlaid showing comprehensive activations during the Flash interval. Depicted clusters are (1) the bilateral insula, (2) the brain stem, (3) the basal ganglia, (4) the anterior cingulate cortex, and (5) the dorsal prefrontal cortex. These results in the Flash window depict a network of forebrain regions joining the brainstem (that remains active) during the experience of the HF. These regions have previously been shown to be active in empirical fMRI investigations of noxious heat stimulation and/or are thought to be associated with neurochemical correlates (e.g. dopaminergic) of thermal regulation. This overlap in previous studies of heat pain present an interesting extension of fMRI studies that has been previously undocumented.
Discussion
The present study provides evidence of time-ordered activity within key constituents of the spinothalamic tract for endogenously generated thermal events. Rises in fMRI activity in the brainstem were observed before the onset of the HF (Fig. 2c). Activity in the insula (Fig. 3c) and prefrontal cortex (Fig. 4c) significantly trailed that in the brain stem, appearing linked to the onset of the HF (insula) or marginally preceded the onset of HF (prefrontal cortex). Other regions including the anterior cingulate and the basal ganglia were also active during the HF, suggesting a network of forebrain cortical and striatal regions activated in response to this event.
The differences in the average signal change in these temporal windows were significant in the insula and the dorso-lateral prefrontal cortex, but not in the brain stem (Fig. 5). These results suggest a differential temporal pattern of the fMRI response during the experience of menopausal HFs. Whereas the quantitative bases of these results appear similar to previous studies of nociceptive or exogenously generated thermal stimulation, the physiological origins of HFs are most likely distinct.
Recent evidence suggests that the application of thermal stimulation results in responses in several regions we assessed, including the brain stem (particularly regions closer to the spinal cord (Kubina et al. 2010; Summers et al. 2010). Thus, application of noxious thermal stimulation (as opposed to innocuous stimulation) to the face or the hand generates a measurable difference in the fMRI response in regions of the brainstem and the spinal cord. These responses to cutaneous delivery of noxious heat pain have been explained as reflecting the ascending architecture of somatosensory fibers carrying afferent signals from the extremities of the body through the spinal tract and into forward mid- and forebrain regions (Willis and Coggeshall 2005). However, endogenous thermal events such as HFs have distinct components and neurophysiological and neurochemical antecedents that are absent in exogenous events such as galvanic skin stimulation. Specifically, activity in medullary and mesencephalic regions may signal the onset of the HF, whereas cortical regions such as the insula and the prefrontal cortex may be responsive to the experience of the HF. By their nature, exogenous thermal events are more closely related to the interoceptive bases of thermal experiences, whereas HFs may have distinct neurochemical bases. These bases, however, have proven challenging to identify. Furthermore, their putative relationship to the fMRI response remains a matter of speculation.
HFs: Neurochemical Correlates
The neurochemical mechanisms underlying HFs are not completely understood. Our studies suggest plasma 3-methoxy-4-hydroxyphenylglycol (MHPG), the main metabolite of the NE pathway was significantly higher in symptomatic versus asymptomatic postmenopausal women during resting conditions and increased significantly further during HFs (Freedman and Woodward 1991). Clinical studies have shown that clonidine, an α2-adrenergic agonist that reduces brain NE, significantly reduces HF frequency (Clayden et al. 1974; Laufer et al. 1982). The role of serotonin has also been considered in that HF's have been hypothesized to result from complex interactions between reduced estrogen and serotonin (Berendsen 2000). Indeed, some studies have suggested that treatment with selective serotonin reuptake inhibitors (SSRIs) can ameliorate HF symptoms (Santoro et al. 2004), although recent studies (including our own) looking at 5-hydroxytryptamine treatment of HF's have indicated that the outcomes of employing SSRIs and SNRIs are highly variable (Nelson 2004; Freedman 2010; Freedman et al. 2011).
Finally, dopamine is a key neurotransmitter implicated in thermal regulation (Balthazar et al. 2010), particularly through dopaminergic projections from brain stem structures such as the substantia nigra to other subcortical regions including the basal ganglia (Lee et al. 1985). By way of convergence patients with Parkinson's disease, characterized by the substantia nigra related hypodopaminergia, are characterized by thermoregulatory anomalies including excessive sweating (Swinn et al. 2003; Hirayama 2006; Chaudhuri and Schapira 2009) and fall in nocturnal core body temperature (Pierangeli et al. 2001). However, the contribution of dopaminergic mechanisms in HFs is unclear (Sturdee 2008), and there is no evidence of a relationship between Parkinson's disease and HFs. Thus, the thermoregulatory mechanisms that are associated with sweating variations in Parkinson's may be fundamentally different from mechanisms associated with the experience of HFs (some common dopaminergic origins notwithstanding).
Whereas the neurochemical correlates of our results cannot be definitively identified, the functional relevance of our results is intriguing. Changes in brainstem areas may precede the measured onset of the HF and the response of interoceptive regions of the cortex, rendering plausible the idea that activity in deep brain stem nuclei is responsive to gradual increases in core body temperature. Whereas we have no independent (other than fMRI related) confirmation of these speculations, our previous studies are suggestive. Rises in core body temperature (measured using ingested radiotelemetry pills) are detected approximately 10–14 s before the rise in the externally measured SCL (Freedman et al. 1995), suggesting that the onset of cascading thermal events occur and can be measured before expression of a full blown HF. We interpret our fMRI data to suggest that the response of brainstem structures is more proximate to the physiological origins of the HF, whereas the response of cortical structures may be more associated with the phenomenological experience of the HF. Thus, our data (distinct from exogenous thermal stimulation) provide evidence of the origins of, and interoceptive responses to, internal thermal events.
Limitations
Several limitations of our studies must be explicitly acknowledged. Precise localization of our activity within the brainstem is rendered difficult both by the detailed cellular organization of brainstem nuclei, as well as by limitations in fMRI imaging methodology. Even with improved methods of cytoarchitectonic mapping (Amunts and Zilles 2001), voxel sizes in the acquired image exceed the dimensions of most brain stem nuclei rendering precise localization of activity exceedingly difficult. Differentiating fMRI-measured activity into separate nuclei is theoretically possible using stereotactic masks (Maldjian et al. 2003), and these masks were used for statistical purposes (Fig. 2). For instance, the dorsal raphe nuclei are central to thermal regulation (Morrison and Nakamura 2011), and a priori evidence would suggest that this nucleus is the seat of early activity in response to rises in core body temperature. The medial aspects of our brainstem clusters can be mapped to coordinates that have been labeled as the dorsal raphe in other fMRI studies (Hui et al. 2005). However, the absence of empirically viable anatomical masks limits the spatial conclusions that can be drawn. Moreover, our reliance on using statistical (as opposed to anatomical) criteria toward identifying brainstem activations may create a potential bias toward achieving significance in the Pre-Flash condition. We acknowledge this limitation and note that detailed parcellation of activity within brain stem nuclei must wait until more advanced in vivo imaging techniques are widely available (Harel et al. 2006).
We also do not have a clear explanation for why we did not detect measurable fMRI responses in other important thermoreceptive regions such as the hypothalamus. The hypothalamus is heavily implicated in thermoregulation based both on its sensitivity to estrogen (Weiss et al. 2004), and its hypothesized role in integrating thermal afferents. Imaging studies have detected measurable changes in rCBF in the hypothalamus in response to cutaneous heating or cooling (Egan et al. 2005), but studies have been mixed and the insula (and not the hypothalamus) appears to be more responsive to heat stimulation (Davis et al. 1998a, 1998b). The hypothalamus is a relatively small structure and fMRI demonstrations of its sensitivity to thermal stimulation have been notably sparse in contrast to the abundance of physiological studies.
These methodological limitations notwithstanding the current results provide novel contributions to the understanding of thermal processes measured in vivo. They indicate that fMRI is sensitive not only to exogenous thermal stimulation as previously demonstrated, but also to the temporal sequencing of endogenous thermal events such as spontaneous HFs. Moreover, it appears that interoception of HFs activates several frontal-striatal regions, indicating the possibility of a frontal network that underlies the experience of internal thermal events. Studying precise network interactions underlying these regions may further illuminate our understanding of the experience of HFs. For example, both dynamic causal modeling (Stephan et al. 2010) and seed-based connectivity analyses, such as psycho-physiological interaction, (Friston et al. 1997) may expand on activation-based analyses and address issues of functional integration of signals between regions (Friston 2005). As we have recently shown, these methods provide significant new insights to the schizophrenia diatheses (Bakshi et al. 2011; Diwadkar 2012; Diwadkar et al. 2012). In future iterations of our work, we aim to expand the aims of our current paper (limited to identifying activation-related markers of HFs), with network analyses, to investigate brainstem, striatal, and cortical interactions during the experience of HF.
We have also noted that the neurochemical interpretation of these fMRI results is difficult to arrive at. Nevertheless, fMRI studies of HFs may provide an objective in vivo neurobiological marker of these important thermoregulatory phenomena and may potentially be useful in identifying the neural response to disparate treatments that have been applied to HFs. More generally, we suggest that the conjoint measurement of peripheral physiological markers of internal interoceptive events in conjunction with in vivo fMRI may open new vistas in the methodological application of fMRI in understanding the neural correlates of internal physical states.
Funding
This study was supported by National Institutes of Health grants Merit award R37-AG05233 (R.R.F.) and MH68680 (V.A.D.), and the Joseph A. Young Fund.
Notes
Conflict of Interest: None declared.
References
- Amunts K, Zilles K. Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am. 2001;11:151–169. vii. [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. 2011;45:1067–1076. doi: 10.1016/j.jpsychires.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Balthazar CH, Leite LH, Ribeiro RM, Soares DD, Coimbra CC. Effects of blockade of central dopamine D1 and D2 receptors on thermoregulation, metabolic rate and running performance. Pharmacol Rep. 2010;62:54–61. doi: 10.1016/s1734-1140(10)70242-5. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000;36:155–164. doi: 10.1016/S0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Clapham JC. Central control of thermogenesis. Neuropharmacol. 2012;63:111–123. doi: 10.1016/j.neuropharm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Clayden JR, Bell JW, Pollard P. Menopausal flushing: double-blind trial of a non-hormonal medication. Br Med J. 1974;1:409–412. doi: 10.1136/bmj.1.5905.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Event-related fMRI of pain: entering a new era in imaging pain. Neuroreport. 1998a;9:3019–3023. doi: 10.1097/00001756-199809140-00018. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998b;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- de Bakker IP, Everaerd W. Measurement of menopausal hot flushes: validation and cross-validation. Maturitas. 1996;25:87–98. doi: 10.1016/0378-5122(96)01046-8. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA. Adolescent risk pathways toward schizophrenia: sustained attention and the brain. Curr Topics Med Chem. 2012;12:2339–2347. doi: 10.2174/156802612805289962. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, Eickhoff SB. Disordered cortico-limbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by fMRI and dynamic causal modeling. Arch Gen Psychiatry. 2012;69:231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley MJ, Lancaster J, Denton D, Fox PT. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci USA. 2005;102:5262–5267. doi: 10.1073/pnas.0409753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Sem Reprod Med. 2005;23:117–125. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas. 2010;65:383–385. doi: 10.1016/j.maturitas.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, Benton MD, Genik RJ, II, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–678. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/S0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Kruger ML, Tancer ME. Escitalopram treatment of menopausal hot flashes. Menopause. 2011;18:893–896. doi: 10.1097/gme.0b013e31820ccae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metabol. 1995;80:2354–2358. doi: 10.1210/jc.80.8.2354. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol. 1992;167:436–439. doi: 10.1016/S0002-9378(11)91425-2. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Woodward S. Elevated α2-adrenergic responsiveness in menopausal hot flushes: pharmacologic and biochemical studies. In: Lomax P, Schönbaum E, editors. The Pathophysiolocial basis of clinical disorders basel. Switzerland: Karger; 1991. pp. 6–9. [Google Scholar]
- Freedman RR, Woodward S, Norton DA. Laboratory and ambulatory monitoring of menopausal hot flashes: comparison of symptomatic and asymptomatic women. J Psychophysiol. 1992;6:162–166. [Google Scholar]
- Friston KJ. Models of brain function in neuroimaging. Ann Rev Psychol. 2005;56:57–87. doi: 10.1146/annurev.psych.56.091103.070311. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsely KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- Harel N, Ugurbil K, Uludag K, Yacoub E. Frontiers of brain mapping using MRI. J Magn Reson Imaging. 2006;23:945–957. doi: 10.1002/jmri.20576. [DOI] [PubMed] [Google Scholar]
- Hirayama M. Sweating dysfunctions in Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII42–VII47. doi: 10.1007/s00415-006-7010-7. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage. 2005;27:479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubina B, Ristic D, Weber J, Stracke CP, Forster C, Ellrich J. Bilateral brainstem activation by thermal stimulation of the face in healthy volunteers. J Neurol. 2010;257:271–280. doi: 10.1007/s00415-009-5307-z. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85:359–374. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer LR, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol. 1982;60:583–586. [PubMed] [Google Scholar]
- Lee TF, Mora F, Myers RD. Dopamine and thermoregulation: an evaluation with special reference to dopaminergic pathways. Neurosci Biobehav Rev. 1985;9:589–598. doi: 10.1016/0149-7634(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Leone M, Proietti Cecchini A, Mea E, Tullo V, Curone M, Bussone G. Neuroimaging and pain: a window on the autonomic nervous system. Neurol Sci. 2006;27(Suppl 2):S134–137. doi: 10.1007/s10072-006-0588-9. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Ann Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF. Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc Natl Acad Sci USA. 2006;103:809–813. doi: 10.1073/pnas.0509862103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610–1620. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. NeuroImage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Pierangeli G, Provini F, Maltoni P, Barletta G, Contin M, Lugaresi E, Montagna P, Cortelli P. Nocturnal body core temperature falls in Parkinson's disease but not in Multiple-System Atrophy. Mov Disord. 2001;16:226–232. doi: 10.1002/mds.1039. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Santoro NF, Clarkson TB, Freedman RR. Treatment of menopause-associated vasomotor symptoms: Position statement of the North American Menopause Society. Menopause. 2004;11:11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- Scouten A, Papademetris X, Constable RT. Spatial resolution, signal-to-noise ratio, and smoothing in multi-subject functional MRI studies. NeuroImage. 2006;30:787–793. doi: 10.1016/j.neuroimage.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. NeuroImage. 2010;49:3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdee DW. The menopausal hot flush—anything new? Maturitas. 2008;60:42–49. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Summers PE, Ferraro D, Duzzi D, Lui F, Iannetti GD, Porro CA. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. NeuroImage. 2010;50:1408–1415. doi: 10.1016/j.neuroimage.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson's disease. Mov Disord. 2003;18:1459–1463. doi: 10.1002/mds.10586. [DOI] [PubMed] [Google Scholar]
- Turner R, Howseman A, Rees GE, Josephs O, Friston K. Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res. 1998;123:5–12. doi: 10.1007/s002210050538. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Milwaukee, WI: Medical College of Wisconsin; 2000. [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. NeuroImage. 2005;27:59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Curr Opin Neurobiol. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. New York: Kluwer; 2005. [Google Scholar]
- Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]