Abstract
Our previous study shows that conventional protein kinases C (cPKCs) are key signaling mediators that are activated by extracellular inhibitory molecules. Inhibition of cPKC by intrathecal infusion of a cPKC inhibitor, GÖ6976, into the site of dorsal hemisection (DH) induces regeneration of lesioned dorsal column sensory, but not corticospinal tract (CST), axons. Here, we investigated whether a direct cortical delivery of GÖ6976 into the soma of corticospinal neurons promotes regeneration of CST and the recovery of forelimb function in rats with cervical spinal cord injuries. We report that cortical delivery of GÖ6976 reduced injury-induced activation of conventional PKCα and PKCβ1 in CST neurons, promoted regeneration of CST axons through and beyond a cervical DH at C4, formed new synapses on target neurons caudal to the injury, and enhanced forelimb functional recovery in adult rats. When combined with lenti-Chondroitinase ABC treatment, cortical administration of GÖ6976 promoted even greater CST axonal regeneration and recovery of forelimb function. Thus, this study has demonstrated a novel strategy that can promote anatomical regeneration of damaged CST axons and partial recovery of forelimb function. Importantly, such an effect is critically dependent on the efficient blockage of injury-induced PKC activation in the soma of layer V CST neurons.
Keywords: axonal regeneration, cortical plasticity, corticospinal tract, protein kinase C, spinal cord injury
Introduction
Failure of successful axonal regeneration in the central nerve system (CNS) is attributed not only to the intrinsic regenerative incompetence of mature neurons, but also to the environment encountered by these axons (Schwab and Bartholdi 1996; Horner and Gage 2000; Spencer et al. 2003). Ample evidence suggests that the majority of the inhibitory activity in the local CNS environment is associated with components of CNS myelin and molecules in the glial scar at the lesion site (McGee and Strittmatter 2003; Yiu and He 2006). Immobilized CNS myelin proteins have been shown to potently inhibit axon outgrowth of various neurons in vitro (Schwab and Caroni 1988; Savio and Schwab 1989). Antibodies specific to myelin have been used to neutralize the inhibitory effect of myelin and to stimulate regeneration of the corticospinal tract (CST) in vivo (Schnell and Schwab 1990; Bregman et al. 1995). Similarly, the chondroitin sulfate proteoglycan (CSPG) components of the glial scar also play a key role in restricting axon regeneration in the adult CNS; removing of these molecules from lesion sites opens a window of opportunity for axon regeneration and functional recovery (Bradbury et al. 2002; Chau et al. 2004; Garcia-Alias et al. 2009).
The CST is one of the main motor pathways of the spinal cord (Li and Raisman 1994). After transection, CST axonal dieback occurs in an intermittent fashion, assuming a unique temporal and spatial distribution after injury (Seif et al. 2007; Liu et al. 2008).
Protein kinase C (PKC) comprises a family of serine/threonine protein kinases that are widely distributed in a variety of tissues with high concentration in neuronal tissues. On the basis of their structure and substance requirement, PKC isoforms can be divided into 3 classes: the Ca2+ and second messenger diacylglycerol (DAG)-sensitive conventional protein kinases C (cPKCs) (-α, -β, and -γ), the Ca2+-independent but DAG-dependent novel nPKCs (-δ, -ɛ, -η, and -θ), and the Ca2+- and DAG-insensitive atypical aPKCs (-ζ and -λ) (Jaken 1996; Newton 2001). One feature of the PKC catalytic region that is essential to the activity of the kinase is its phosphorylation (Balendran et al. 2000). Our previous study shows that cPKCs are key signaling mediators that are activated by myelin-associated inhibitors and CSPGs (Hasegawa et al. 2004; Sivasankaran et al. 2004). Inhibition of cPKC by local, intrathecal delivery of a PKCα and PKCβ1 selective inhibitor, GÖ6976, induced robust regeneration of lesioned dorsal column axons across and beyond the lesion site in vivo (Sivasankaran et al. 2004). However, the same treatment did not promote axonal regeneration of the CST. We reasoned that the lack of CST regeneration after PKC inhibition at the lesion site might be related to the ineffective site delivery of the cPKC inhibitor. If so, delivery of the cPKC inhibitor, GÖ6976, directly into the cell bodies of layer V motoneurons, from which CST axons originate, may exhibit stronger effect on CST axonal regeneration and recovery of function after spinal cord injury (SCI). Previous studies indicate that SCI itself does not cause death of CST neurons in the motocortex and strategies aimed at preventing cell death of these neurons may be unnecessary (Brock et al. 2010; Nielson et al. 2010).
Materials and Methods
Cortical Neuron Culture and Neurite Outgrowth Assay
Rat embryos at embryonic day 16 (E16) were removed from the pregnant Sprague Dawley (SD) rats (Harlan Sprague Dawley, Inc., Indianapolis, IN, USA) under deep anesthesia. Embryos were quickly decapitated and the cerebral cortices were excised. After the careful removal of meninges, the cerebral cortex was cut into small pieces and incubated with 0.1% trypsin plus 0.1 mg/mL DNAse (Gibco/Invitrogen, Carlsbad, CA, USA) for 10 min at 37 °C, and the trypsin was blocked with 10% fetal bovine serum (BioWittacker/Lonza Group Ltd., Basel, Switzerland) for 5 min. Cells were then mechanically dissociated with fine-pipette tips in Hank's Balanced Salt Solution (Gibco/Invitrogen). After filtration with a 70-mm nylon cell strainer (BD Falcon, San Jose, CA, USA), dissociated cells were plated onto poly-l-lysine (0.05 mg/mL, P2636, Sigma-Aldrich, St. Louis, MO, USA) or myelin (50 ng/cm2; M1891, Sigma-Aldrich)-coated coverslips at a density of 100 000 cells/cm2 and cultured in a defined cortical culture medium [2% B27 Supplement, 0.5 mM l-glutamine in Neurobasal Medium, supplemented with 12.5 µM glutamate, 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Gibco/Invitrogen) with or without 100 nM GÖ6976 (365250, EMD chemicals, Gibbstown, NJ, USA)]. Cells were grown at 37 °C, with 5% CO2 for 48 h before fixation with 4% paraformaldehyde (PFA) and staining with a neuron-specific anti-βIII tubulin antibody (Tuj-1, MMS-435P, Covance; Richmond, CA, USA).
Neuron density of each group was calculated by counting the number of βIII tubulin+ Hoechst+ cells in 3 randomly selected fields of each slide under ×20 microscope. Neurite lengths were measured from at least 200 neurons per condition, from duplicate coverslips, and from 3 independent experiments and quantified as described previously (Wang et al. 2002; Sivasankaran et al. 2004). We measured the average length of neurites by measuring the total neurite length of several neighboring neurons and then dividing the neurite length by the same number of neurons to reach the average length of the neurons.
Animals
A total of 68 adult female SD rats (200–220 g, Harlan Sprague Dawley, Inc.) were used in this study. All behavior testing, surgical interventions, and postoperative animal care were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council) and were approved by the Indiana University Institutional Animal Care and Use Committee. Twenty rats were used for unilateral pyramidotomy surgery. Forty eight rats were initially trained for behavior analysis for 2 weeks prior to surgery. In this group, 6 rats were unable to learn the task and were excluded from the experiment. The remaining 42 animals received surgery at the end of the pretraining period and were allowed for recovery before behavioral testing. Analysis of forelimb function using the pellet retrieval test was performed at the sixth and seventh weeks before the animals were traced and sacrificed for histological analysis.
Fluoro-Gold Retrograde Tracing and Unilateral Pyramidotomy
One week before the unilateral pyramidotomy surgery, bilateral layer V corticospinal neurons were retrogradely labeled with Fluoro-Gold (FG; Gibco/Invitrogen) by injecting 1 µL of FG (1% in 0.9% saline) into the ventral portion of the dorsal column (midline, 0.8 mm from the dura surface) of the second thoracic (T2) spinal cord (Suzuki et al. 2004). For the unilateral pyramidotomy, animal was placed in a supine position. A longitudinal incision was made left to the trachea. Blunt dissection was performed to expose the skull base, and a craniotomy in the occipital bone was performed to allow for access to the medullary pyramids. The dura mater overlaying the pyramids was pierced with a 32-gauge hypodermic needle, and the left pyramid was transected with fine iridectomy scissors (Thallmair et al. 1998; Benowitz et al. 1999). The wound was closed in layers with 5.0 Vicryl. Immediately after closure, animals received injections of either the PKC inhibitor GÖ6976 (1 mM) or phosphate buffered saline (PBS) (0.1 M, pH 7.4) into the sensorimotor cortex stereotaxically (0.5 µL/site, 12 sites total) to treat layer V corticospinal neurons (Sivasankaran et al. 2004). The animals were perfused at 1, 4, and 24 h following the injections.
C4 Dorsal Hemisection and Treatment Groups
Adult female SD rats were anesthetized and subjected to laminectomy to expose the dorsum of the spinal cord at the third and fourth cervical (C3–4) vertebral levels. The dura was open transversely, and a dorsal hemisection (DH) was performed at C4 to a depth of 1.6 mm from the dorsal surface of the spinal cord using a VibraKnife lesioning device (Louisville Injury System Apparatus, LISA, Louisville, KY, USA) (Sivasankaran et al. 2004). The lesion was advanced to a level beyond the central canal to ensure that the entire bilateral dorsal funiculi, including the CST, were transected (Sivasankaran et al. 2004). After C4 DH, animals were randomly assigned into 7 groups (n = 6/group): (1) DH only; (2) DH + dimethyl sulfoxide (DMSO) cortical injection (0.1% DMSO in 0.1 M PBS); (3) DH + lenti-ChABC (Chondroitinase ABC) lesion site injection (1.96 × 106 TU/µL; 3 injection sites: at the lesion epicenter, 0.5 mm rostral, and 0.5 mm caudal to the lesion epicenter, with 2 injection depths: 1.2 and 0.6 mm from the dorsal surface, each at 0.5 µL); (4) DH + GÖ6976 cortical injection (1 mM in 0.1% DMSO in 0.1 M PBS); (5) DH + GÖ6976 cortical injection (same as Group 4) + lenti-ChABC lesion site injection (same as Group 3); (6) DH + GÖ6976 intrathecal infusion (20 µM, 200 µL) via Alzet osmotic pump (Model 2002, Alza Corporation, Palo Alto, CA, USA); and (7) DH + GÖ6976 intraperitoneal injection (2.50 mg/kg body weight, 50 µM stock solution in 0.1% DMSO) 1 time daily. All intracortical and intraspinal injections were made immediately after the DH. During the first week postinjury, animals were given daily subcutaneous injections of the analgesic buprenorphine hydrochloride (Reckitt and Colman, UK). Behavior tests of pellet retrieval were assessed at sixth and seventh weeks after surgery and treatment.
Immunocytochemistry
After unilateral pyramidotomy and proper treatment, rats were anesthetized with 60 mg/kg pentobarbital and perfused transcardially with 100 mL saline followed by 400 mL 4% PFA in 0.01 M PBS (pH 7.4). The brains were removed and postfixed in the same perfusion fixative overnight at 4 °C, and cryoprotected in 30% sucrose buffer for 1 week. Brains were sectioned transversely at 20 µm on a cryostat, mounted onto gelatin-subbed slides, and stored at –70 °C. The frozen slides were air dried at room temperature (RT) for 10 min and washed with PBS for 10 min. They were also blocked with 10% donkey serum in Tris-buffered saline (TBS) containing 0.3% Triton X-100 (TBST) for 1 h at RT, and the primary antibody was applied in the same blocking solution overnight at 4 °C. The following primary antibodies were used: polyclonal rabbit anti-phospho-PKCα (1:400; 07-871, Millipore, Temecula, CA, USA) and polyclonal rabbit anti-phospho-PKCβ1 (1:400; 07-872, Millipore). The slides were washed in PBS 3 times and incubated with rhodamine-conjugated donkey antirabbit IgG (711-025-152, Jackson ImmunoResearch Lab, West Grove, PA, USA; all 1:200) for 1 h at 37 °C. The slides were washed 3 times with PBS and coverslipped with Gel/Mount aqueous mounting media. The images were taken with an Olympus BX60 microscope, and the relative mean fluorescence intensity was calculated by comparing the fluorescence intensity of p-PKCα or p-PKCβ1 in FG-labeled cell soma with that of the staining background using the ImageJ software. In all experiments, primary antibody omission controls were used to confirm the specificity of the immunofluorescence labeling.
Anterograde Tracing and Quantification of the CST
Six microliter of biotinylated-dextran amine (BDA, 10 000 MW, 10% in 0.1 M PBS; Molecular Probes, Eugene, OR, USA) was injected into each of 12 sites (0.5 μL each site) in both hemisphere of the rat forelimb sensorimotor cortex as we described previously (Sivasankaran et al. 2004). Two weeks later, animals were perfused and 20-µm thick longitude sections were cut from 10-mm length tissue blocks between C3 and C5. BDA-labeled CST axons were visualized by using avidin–biotin peroxidase incubation followed by biotinyl tyramide and Extra-Avdin-TRIFC (SAT700, PerkinElmer, Waltham, MA, USA; Liu et al. 2008). Lesion gaps were identified in the same section by immunolabeling for glial fibrillary acidic protein (GFAP; 1:400; AB5804, Chemicon, Temecula, CA, USA). Quantification of BDA-labeled CST axons was performed with the experimenter blind to the treatment groups as described previously (Liu et al. 2008). Briefly, 2 sagittal sections of the spinal cord spaced 200 μm apart were chosen. The first section was chosen through the midline where the central canal was visualized. The second section is 200 μm away from the midline on the injured side. The number of BDA-positive CST axons is presented as CST axon index at −1, −0.5, −0.25, −0.1, 0, 0.5, 1.0, and 3.0 mm positions, relative to the lesion center, which is indicated as “0.” Axon index is a ratio of the BDA+ axon number at a specific position over the axon number 3 mm rostral to the lesion (100% for the 3 mm position; Cafferty et al. 2007; Liu et al. 2008).
Pellet Retrieval Assessment
Pellet retrieval was performed in a double-blind manner. Rats reached into 1 cm shoulder-high feeding tubes to grasp chocolate-flavored dustless precision purified food pellets (45 mg, Bio-Serv, Inc., Frenchtown, NJ, USA). Injured animals (n = 42) were pretrained for 2 weeks before injury and were tested at sixth and seventh weeks postinjury. Rats were diet restricted to promote acquisition of the pellet retrieval behavior (Titsworth et al. 2007). For training, animals were intermittently fasted on alternate nights for 18 h and allowed ad libitum feeding on weekends. For testing, animals were fasted for 18 h before testing. Food was removed from the cages at 5 PM prior to randomized testing between 8 and 11 AM the next morning. All rats were given two 5 min training sessions a day for 10 days. Once a rat met training criteria, training was reduced to 1 session per day. The training criteria were defined as at least 20 pellets retrieved from the feeding tube within 5 min with at least 70% of pellets successfully eaten. On the last day of the training period, baseline data were collected. At the end of each acquisition trial, an observer blinded to the treatment groups calculated the percentage of pellets retrieved by dividing the difference between the number of pellets retrieved from the feeding tube and the number remaining on the floor of the feeding chamber by the number of pellets retrieved.
Lesion Completeness
Criteria to assess the completeness of CST transection included the disruption of GFAP labeling at all dorsal levels through the lesion site in all serial sagittal sections examined by GFAP and phase contrast analysis.
Statistical Analysis
Data were compared between two groups using a Student's t test. In the case of unequal variances, the nonparametric Mann–Whitney rank sum test was used. Data with more than two groups were analyzed using parametric ANOVA (one-way or two-way) of the appropriate design, followed by restricted analyses or Bonferroni's post hoc pairwise comparisons whenever a main effect or interaction attained statistical significance. Data are presented as mean ± SD.
Results
PKC Inhibitor Attenuated Myelin Inhibition of Primary Cortical Neurons
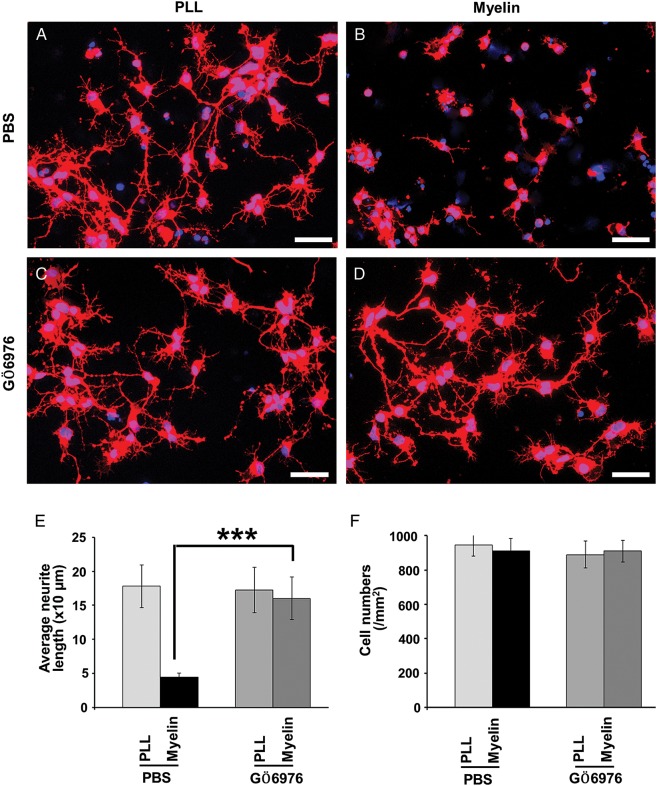
We first examined whether the PKC inhibitor, GÖ6976, had an effect on neurite outgrowth of cortical neurons plated on myelin substrate. When platted on a nonmyelin (poly-l-lysine) substrate for 2 days, cortical neurons elicited ramified neurites (Fig. 1A). The neurite outgrowth from these neurons was dramatically inhibited when they were platted on a myelin substrate (Fig. 1B; t = 7.15, P = 0.002). Treating cortical neurons with GÖ6976 significantly promoted their neurite outgrowth on the myelin substrate (Fig. 1D,E; t = 5.45, P = 0.006). The GÖ6976, however, did not elicit any detectable growth-promoting effects on cortical neurons in the group coated with poly-l-lysine (Fig. 1C; t = 0.65, P = 0.56). The effect of GÖ6976 on neurite outgrowth of cortical neurons was comparable with that on cerebellar granule neurons (CGNs; Sivasankaran et al. 2004). The neuron densities were identical among the 4 groups (Fig. 1F; two-way ANOVA, F = 0.40, P = 0.76).
Figure 1.
GÖ6976 blocks myelin inhibition in E16 rat cortical neurons. (A–D) E16 cortical neurons were cultured on PLL (A and C) or myelin (B and D) substrate for 48 h in the absence (A and B) or presence (C and D) of GÖ6976 (100 nM). The average neurite lengths from at least 200 neurons on each condition (E) and neuronal density (F) were plotted and analyzed using Student's t test and two-way ANOVA, respectively. With the same neuronal densities in four groups (F = 0.40, P = 0.76), the neurite length is significantly high in the GÖ6976-treated group than the control group on the myelin substrate (n = 3; ***P < 0.01).
Cortical Delivery of GÖ6976 Inhibited Phosphorylated Expression of PKCα and PKCβ1
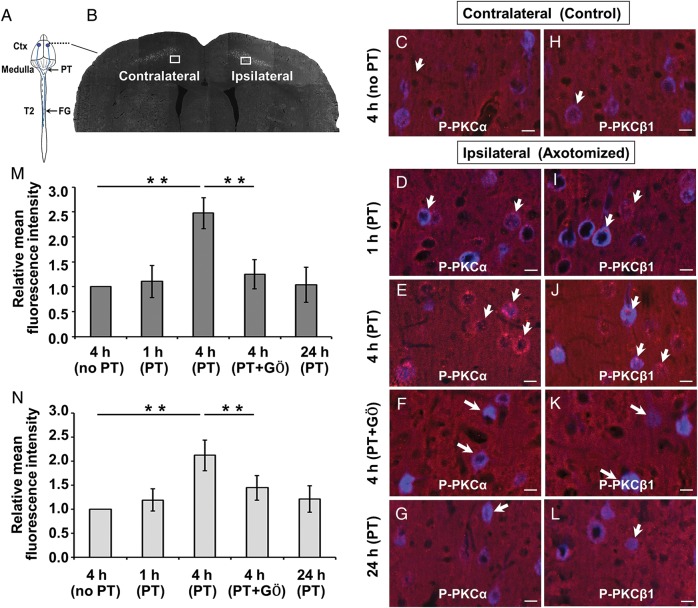
We next examined the expression of phosphorylated PKCα (p-PKCα) and PKCβ1 (p-PKCβ1), as indication of cPKC activation, in corticospinal neurons after their descending pathway, the CST, was transected unilaterally at the medullar pyramid (pyramidotomy). We further examined whether cortical delivery of the cPKC inhibitor, GÖ6976, could effectively block the activated cPKC. To visualize the layer V corticospinal neurons, a retrograde tracer FG was injected into the dorsal column of the T2 spinal cord prior to the pyramidotomy, so that the dye could be retrogradely transported to these neurons. Compared with contralateral FG-labeled motor neurons (Fig. 2C,H), the expression of p-PKCα and p-PKCβ1 in the ipsilateral layer V motor neurons were increased at 1 h (Fig. 2D,I), peaked at 4 h (Fig. 2E,M, t = 9.04, P = 0.003; Fig. 2J,N, t = 6.81, P = 0.006), and returned to the baseline level at 24 h (Fig. 2G,L) following unilateral medullary pyramidotomy. Cortical delivery of GÖ6976 efficiently inhibited the injury-induced PKCα (Fig. 2F,M; t = 5.44, P = 0.002) and PKCβ1 (Fig. 2K,N; t = 3.28, P = 0.009) phosphorylation in corticospinal neurons.
Figure 2.
Cortical delivery of GÖ6976 effectively blocks the activation of PKCα and PKCβ1 in corticospinal neurons following pyramidotomy. (A) Schematic drawing of the experimental design showing the sites of FG injection, pyramidotomy, and observation (Ctx). (B) A coronal section shows FG retrogradely labeled CST neurons on both the contralateral (control) and ipsilateral (axotomy) sides to the pyramidotomy. Boxed areas indicate where p-PKCα (C–G) and p-PKCβ1 (H–L) immunoreactivity (IR) were examined. Compared with the contralateral CST motoneurons (C and H), p-PKCα and p-PKCβ1 IR (red, short arrows) increased at 1 h (D and I), peaked at 4 h (E and J), and returned to the normal level at 24 h (G and L) in the FG-labeled corticospinal neuronal soma (blue, long arrows) after ipsilateral pyramidotomy. Cortical delivery of GÖ6976 significantly blocked the expression of p-PKCα and p-PKCβ1 in CST motoneurons (F and K, arrows). (M and N) Quantification of the relative mean fluorescence intensity of p-PKCα (M) and p-PKCβ1 (N) at different time points between different groups. (**P < 0.01). Scale bars = 15 µm.
Gö6976 Promoted CST Axonal Regeneration Only When it Was Delivered into the Motor Cortex
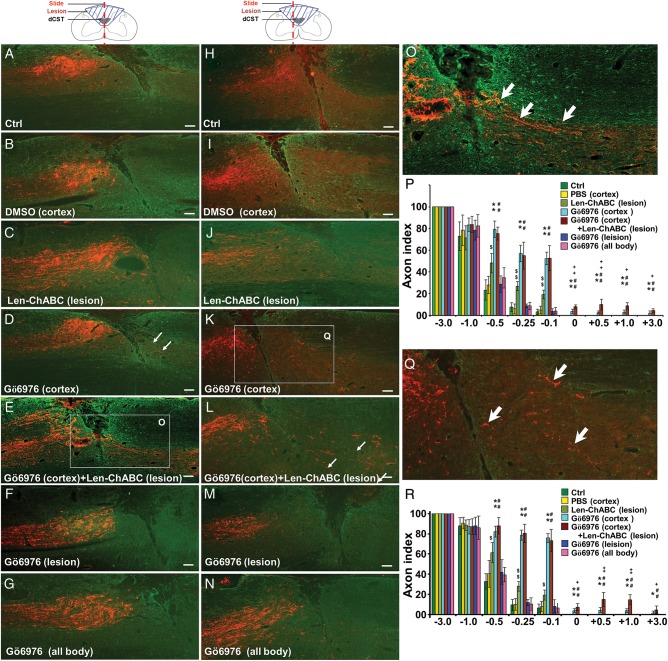
To further test whether cortical delivery of GÖ6976 to inhibit phosphorylated expression of cPKC in layer V corticospinal neurons promote CST axonal regeneration through and beyond the injury, an anterograde tracer BDA was injected into the sensorimotor cortex of lesioned animals at 7 weeks after the C4 DH and various treatments. BDA and GFAP double immunostaining in sagittal (Fig. 3A–G) and parasagittal (Fig. 3H–N) sections were performed to correlate CST axons to the lesion border. We found that, compared with control (Fig. 3A,H) and DMSO (Fig. 3B,I) groups, lenti-ChABC injection into the lesion site promoted CST axonal growth toward the lesion border but not into the lesion gap (Fig. 3C,J). When GÖ6976 was delivered into the motor cortex, either alone (Fig. 3D,K) or in combination with the delivery of lenti-ChABC into the lesion site (Fig. 3E,L), many BDA-labeled CST axons regenerated through and beyond the lesion gap and reentered into both the white and gray matters of the host spinal cord. Such CST axonal regeneration, however, was not found in the other 5 groups with (1) no treatment (Fig. 3A,H), (2) DMSO injection into the motor cortex (Fig. 3B,I), (3) lenti-ChABC injection into the lesion site (Fig. 3C,J), (4) Gö6976 intrathecal infusion into the lesion site (Fig. 3F,M), and (5) intraperitoneal injection of Gö6976 (Fig. 3G,N). Compared with the delivery of GÖ6976 alone into the motor cortex and the delivery of lenti-ChABC alone into the lesion site, the combinational treatment with cortical delivery of GÖ6976 and intraspinal injection of lenti-ChABC significantly enhanced CST axonal regeneration, as is shown in quantitative analysis in both the sagittal sections (Fig. 3P) and parasagittal sections (200 µm away from the midline, Fig. 3R). These results not only confirmed our previous finding that intrathecal delivery of GÖ6976 at the lesion site failed to promote CST axonal regeneration through and beyond the injury (Sivasankaran et al. 2004), but also indicated the importance of delivery site (cortical vs. lesion site or systemic) of a cPKC inhibitor on the success of CST axonal regeneration. Moreover, our study also provides an example of a successful combinatorial therapy with complementary strategies aimed at increasing intrinsic regenerative capability at the cell soma (the function of GÖ6976) and inhibiting local inhibitory environment at the site of axonal growth (the function of ChABC).
Figure 3.
GÖ6976 promotes CST axonal regeneration across and beyond a lesion gap only when it was delivered into the motor cortex. Representative sagittal (A–G) and parasagittal (H–N, 200 μm lateral to the sagittal) sections of the rat spinal cords of 6 treatment groups at 7 weeks after C4 DH. CST axonal regeneration across and beyond a lesion gap was found only when GÖ6976 was delivered into the motor cortex (D, K, and Q). Greater CST axonal regeneration was found when cortical GÖ6976 delivery was combined with lesion site delivery of lenti-ChABC (E, L, and O). In all other 5 groups, including when GÖ6976 was delivered intrathecally (F and M) or intraperitoneally (G and N), CST axons failed to regenerate into the caudal cords, though among which lenti-ChABC alone (C and J) promote the CST growth toward lesion border compared with other 4 groups. (P and R) Statistical comparison of axon index at sagittal (P) and parasagittal (R) sections among 7 treatment groups at different distances from the lesion site (n = 6/group; error bars = SD). “*” and “#” denote significant differences (* and #, P < 0.05; ** and ##, P < 0.01) between cortical GÖ6976-delivered groups (GÖ6976 alone or in combination with lenti-ChABC) and the other 5 groups. “$” denotes significant differences ($, P < 0.05; $$, P < 0.01) between lenti-ChABC alone and the other 6 groups. “+” denotes significant differences (+, P < 0.05; ++, P < 0.01) between the group that received cortical GÖ6976 delivery alone and the combined treatment with cortically delivery of GÖ6976 and lesion site delivery of lenti-ChABC. Scale bars in A–L = 100 µm, in M and O = 200 µm.
Regenerating CST Axons Formed Synapses in Target Neurons
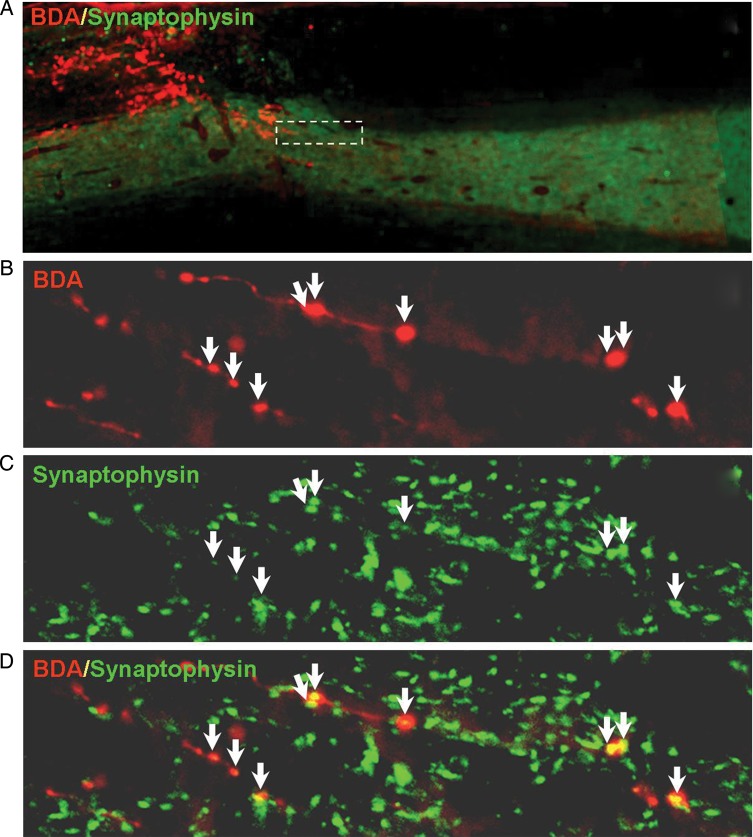
Since the CST axons had successfully regenerated through and beyond the C4 DH in groups treated with cortical injections of Gö6976 alone, or in combination with lesion site injection of lenti-ChABC, our next goal was to determine whether regenerated CST axons in the distal host spinal cord formed new synapses on target neurons. We examined evidence for synaptic formation by immunofluorescence double labeling of BDA to localize regenerated CST axons and synaptophysin to label presynaptic components of new synapses in rats receiving a combined treatment of cortical delivery of GÖ6976 and lesion site delivery of lenti-ChABC (Fig. 4). We found that many regenerated BDA+ axons were colocalized with synaptophysin immunoreactive products within the gray matter where target neurons and dendrites were located (Fig. 4B–D), suggesting the formation of new synapses by regenerated CST axons within the distal spinal cord. Multiple sites of synaptophysin immunoreactivity occurred along the length of many regenerated axons (Fig. 4D).
Figure 4.
Cortical delivery of GÖ6976 promotes the synaptic formation of regenerated CST axons. (A) A low-power photomicrograph shows that, in an animal that received cortical delivery of GÖ6976 and lesion site injection of lenti-ChABC after a C4 DH, many BDA-labeled CST axons (red) regenerated beyond the lesion gap (dashed line). (B) High magnification of a boxed area in A shows several regenerated axons labeled with BDA. (C) Distribution of synaptophysin (a presynaptic marker)-IR in the same field presented in B. (D) Merged image of B and C shows the presence of multiple synaptic contacts (arrows) along the lengths of regenerated axons.
Cortical cPKC Inhibition Promoted Forelimb Functional Recovery
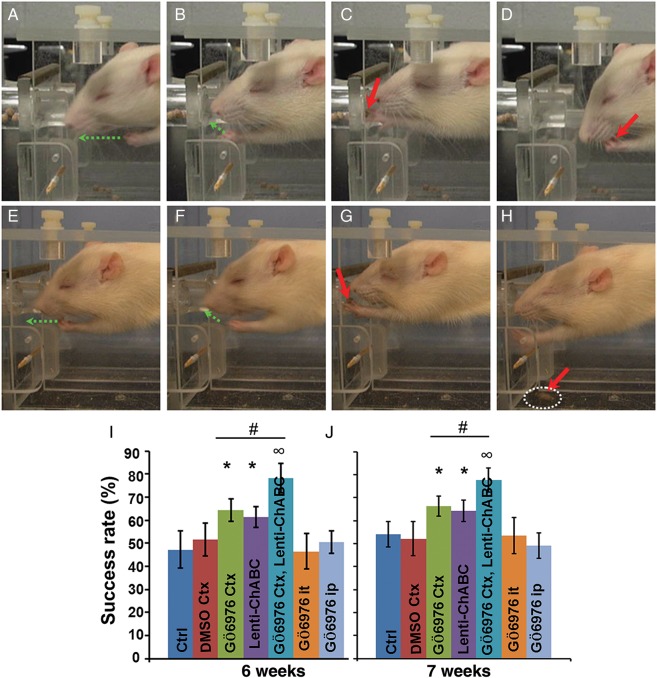
Finally, we examined whether successful regeneration of and new synapse formation by the CST axons could lead to forelimb functional recovery after C4 DH and GÖ6976 or GÖ6976 + lenti-ChABC treatment. Pellet retrieval behavior was assessed at sixth and seventh weeks after these treatments (Fig. 5). We counted the number of eaten pellets of a total of 20 pellets to determine the success rate of pellets retrieved. We found that the success rate was significantly higher in the group treated with the combination of cortical delivery of GÖ6976 and lesion site delivery of lenti-ChABC, when compared with the group that treated with cortical delivery of GÖ6976 alone at both sixth and seventh weeks postinjury (P < 0.05) (Fig. 5I,J). Moreover, both groups showed significantly higher success rate than the other 4 groups in which the CST failed to regenerate through and beyond the lesion (P < 0.05) (Fig. 5I,J).
Figure 5.
Cortical delivery of GÖ6976 promotes the recovery of forelimb function after C4 DH. Representative video captured images of a success (A–D) or failed (E–H) pellet retrieval performance at 6 weeks post-C4 DH. For one successful pellet retrieval, forelimb lift (A, green arrow), reach into the feeding tubes (B, green arrow), forepaw digits open, pronation grasp, supination movements (C, red arrow), and take pellet to mouth (D, red arrow) were observed. For a failed pellet retrieval, forelimb lift (E) and reach into the feeding tubes (F) were observed (green arrows); but the rat could not open the digits and grasp the pellets (G, red arrow). The pellets were dragged out of the feeding tube and fell to the floor out of reach (H, red arrow). Quantification shows that the success rate is significantly higher in groups treated with either cortical delivery of GÖ6976 alone or a combined treatment with cortical delivery of GÖ6976 plus the lesion site injection of lenti-ChABC, when compared with other groups. Moreover, the combined treatment shows even a greater effect in pellet retrieval than the single cortical delivery of GÖ6976 at sixth (I) and seventh (J) weeks after surgery (mean ± SD; n = 6, *, ∞, P < 0.05).
Discussion
Previously, we demonstrated that intrathecal infusion of GÖ6976 into the site of the DH promoted regeneration of dorsal column axons across and beyond the lesion site in adult rats (Sivasankaran et al. 2004). However, in the very same animals that manifested robust regeneration of dorsal column axons, we did not detect any CST axonal regeneration. We reasoned that intrathecal infusion of GÖ6976 to the lesion site may not sufficiently block the endogenous PKC activity of the CST neurons, as it is possible that different neuronal populations may respond differently to the injury and PKC inhibition. We further reasoned that delivering GÖ6976 directly to the cell bodies of axotomized CST neurons may be more effective to block the activation of PKC than delivering the same compound to the damaged nerve terminals. To address this issue, we first examined the activation of 2 cPKC isoforms, that is, p-PKCα and p-PKCβ1, in CST neurons after unilateral pyramidotomy. We used a retrograde tracer FG to prelabel the layer V CST neurons. Compared with uninjured FG-labeled motor neurons, expression of p-PKCα and p-PKCβ1 in the injured CST neurons in the layer V motor cortex were significantly increased and peaked at 4 h following a unilateral pyramidotomy. Cortical delivery of GÖ6976 immediately after injury significantly inhibited the expression of p-PKCα and p-PKCβ1 in axotomized CST neurons. Importantly, when compared with the control groups, the same agent were delivered either to the lesion site or intraperitoneally, inhibition of PKC activation by cortical delivery of GÖ6976 promoted robust axonal regeneration of the CST through and beyond a DH, resulting in new synapse formation and recovery of forelimb function. Thus, we demonstrated that a single treatment agent delivered at different sites can elicit totally different results, emphasizing the importance of site-specific drug delivery. It remains unclear, however, why corticospinal neurons and sensory neurons respond differently to PKC inhibition. We speculate that such difference may be related to that the 2 neuronal populations contain different level, isoform, and distribution of PKCs and, thus, respond to the injury differently.
The expression and activation of PKCα and PKCβ1 in the cell body of cortical neurons after injury indicate that cortical delivery of the PKC inhibitor is more efficient than when it is delivered into the lesion site. Notably, PKCα and PKCβ1 inhibition and CST axonal regeneration were achieved only after a single delivery of GÖ6976 to cortical neurons. This indicates that injury-induced PKC activation within the period of a few hours is the key to launching CST growth inhibition, and that suppression of PKC activation at this early stage may disable the initiation of growth inhibition, which allows a lengthy effect of CST axonal regeneration observed at seventh week post-SCI. Our previous study indicates that, once initiated, the rate of axonal growth can be quick. For example, axonal growth into a Schwann cell bridge transplant was observed as early as 2 days posttransplantation and axons reached the distal end of the 3-mm long graft by 7 days (Hsu and Xu 2005). Once axonal growth is launched, the subsequent axonal regeneration and synaptogenesis may be mediated by other mechanisms unrelated to PKC inhibition. The beneficial effect of one single injection of a treatment agent is obvious since it is convenient and easy to deliver. Such a strategy was also used in many other studies including the injection of ChABC in promoting regeneration of serotonergic fibers after SCI (Tom et al. 2009) and injections of polylaminin in stimulating long axonal growth after SCI (Menezes et al. 2010).
It has been well established that CSPGs, the major class of inhibitor in the glial scar (Sivasankaran et al. 2004), play a key role in restricting axonal regeneration in the adult CNS, as enzymatic removal of these molecules from the lesion sites improves axonal regeneration and functional recovery (Brittis et al. 1992; Bradbury et al. 2002; Chau et al. 2004). Since the conventional isoforms of PKC are key components in the signaling pathway that mediates the activity of CSPGs, we examined whether a combined treatment with cortical delivery of GÖ6976 and lesion site delivery of lenti-ChABC would produce a synergistic effect on axonal regeneration of the CST and, subsequently, enhanced recovery of forelimb function. Indeed, the combined treatment with site-specific delivery of GÖ6976 and lenti-ChABC enhanced both anatomical regeneration and recovery of forelimb function, when compared with the single cortical treatment of GÖ6976. Thus, our study also supports the rationale for using combinatorial repair strategies, particularly when the individual treatment strategies are site- and/or target-specific.
We previously demonstrated that treatment with GÖ6976 profoundly mitigated outgrowth inhibition and promoted significant neurite outgrowth of postnatal day 7–9 rat CGNs on CNS myelin substrates. The effects of these PKC inhibitors were comparable with that of Y-27632, an inhibitor of Rho-associated kinase, which has previously been shown to block the inhibitory activities of CNS myelin and its components (Lehmann et al. 1999; Sivasankaran et al. 2004). In this study, we examined whether GÖ6976 had a similar effect on neurite outgrowth on myelin substrates in cortical neurons (E16), as it did on CGNs. Our results clearly indicate that PKC signaling involves in the inhibitory activities of CNS myelin in cortical neurons, as GÖ6976 treatment significantly promoted neurite outgrowth of cortical neurons on CNS myelin substrates.
Previous studies demonstrated that dorsal CST transiently expressed strong PKCα and PKCβ at early postnatal developmental stages, but thereafter, the immunoreactivity rapidly declined (Jaken 1996). In this study, we examined the expression of p-PKCα and PKCβ1 in layer V motor neurons, retrogradely labeled with FG. Compared with contralateral FG-labeled motor neurons, the expression of p-PKCα and p-PKCβ1 in the ipsilateral layer V motor neurons were increased at 1 h, peaked at 4 h, and returned to the normal level at 24 h following a unilateral medullary pyramidotomy. Immediate cortical delivery of GÖ6976 significantly inhibited the phosphorylation of PKCα and PKCβ1 in corticospinal neurons induced by pyramidotomy. More importantly, the activation of PKCα and PKCβ1 were predominately distributed within the cell body, and we did not observe the expression of p-PKCα and p-PKCβ1 in axons particularly those at the injury site. This may explain why GÖ6976 promoted the CST regeneration when it was delivered into the sensorimotor cortex and did not do so when it was delivered into the lesion site. A previous study also demonstrated that PKCα and PKCβ1 are among the growth-associated proteins that are upregulated and heavily localized on the membrane of retinal ganglion cells after optic nerve crush injury (Wu et al. 2003). However, GÖ6976 has been profiled against 300 kinases (Anastassiadis et al. 2011), and other pathways besides PKCα and PKCβ1 may be involved in the effect that we found in the present study.
Several studies explored CST regeneration using a spinal cord crush or contusion model in which the lesion cavity is usually a big physical obstacle for regeneration (Bradbury et al. 2002; Teng et al. 2002; Ruitenberg et al. 2005), and therefore, corresponding regenerative evidence supported by quantification of labeled CST axons below the injury site is indirect and difficult to interpret because some intact CST axons may remain below the injury site. In our case, we produced the dorsal spinal hemisection using a VibraKnife lesioning device (Sivasankaran et al. 2004; Zhang et al. 2008; Hill et al. 2009), which can be advanced stereotaxically to produce precise, consistent, and reliable lesions. When the VibraKnife was advanced to a depth of 1.6 mm from the dorsal surface of the spinal cord, it transected the entire bilateral dorsal funiculi including the CST (Sivasankaran et al. 2004). Additionally, the VibraKnife produced a very narrow lesion gap (Fig. 3), even at 2 months postinjury, possibly due to the minimal damage to the surrounding tissue at the initial cut by the VibraKnife, providing a unique model for studying axonal regeneration, particularly that of the CST and dorsal column axons. Using this model, we have provided clean and definitive anatomical evidence of CST axonal regeneration from the cut end all the way to their innervation on target neurons. In this study, we found that cortical injection of GÖ6976, combined with lesion site delivery of lenti-ChABC, resulted in best regeneration of the CST after SCI. In contrast, lesion site delivery of lenti-ChABC alone failed to promote CST axonal growth through and beyond the lesion gap. Likewise, CST axons failed to regenerate through and beyond the lesion gap when GÖ6976 was delivered at the lesion site or intraperitoneally. The molecular weight of GÖ6976 is 378.4, which may allow it to pass freely through the blood–brain barrier following the intrathecal (IT) or intraperitoneal (IP) delivery. Due to the extensive tissue distribution of PKC and possible side effect of PKC inhibition, we applied 2.50 mg/kg body weight of GÖ6976 daily as a previous study suggested (Marzioni et al. 2006). The inhibitor at this dose may not completely block the injury-induced PKC activation, and this could be the reason why we did not observe obvious CST regeneration in the IT and IP delivery groups. In contrast, although only injecting once, cortical delivery of high concentration of GÖ6976 (1 mM) effectively block the phosphorylated activation of PKCα and PKCβ1 in cortical neurons that was specifically induced by the CST injury.
Since the CST damage in rodents primarily causes deficits in the fine control of the forearm muscles, which are required for grasping and holding objects (Anderson et al. 2007; Courtine et al. 2007), we employed skilled paw use relevant to the CST for evaluating the treatments (Garcia-Alias et al. 2009). We established a modified pellet retrieval device to evaluate the forelimb use in various treatment conditions (Titsworth et al. 2007). Using this method, we found that the success rate of pellet retrieval was significantly higher in the group treated with cortical delivery of GÖ6976 than the other 4 control groups. Moreover, the combined treatment group with cortical delivery of GÖ6976 and lesion site delivery of lenti-ChABC showed even greater effect on CST axonal regeneration and recovery of forelimb function, when compared with the cortical GÖ6976 treatment alone. These data collectively suggest that rebuilding the neural network by successful regeneration and reinnervation of the CST axons, as demonstrated in the present study, can lead to the recovery of forelimb function, a behavior that is critically dependent on the function of the CST.
Impairments in voluntary motor function after SCI in humans are often attributed to disruption of CST projections (Clark et al. 1985; Farmer et al. 1993; Nathan 1994). Therefore, experimental therapeutic interventions in animal models often focus on promoting regeneration of this pathway. After experimental SCI, the cut ends of the CST axons branch extensively, but do not advance through the damaged area (Li and Raisman 1994; Hill et al. 2001). Recent literature suggests that SCI does not cause death of the CST neuron cell bodies in the motocortex (Nielson et al. 2010), and injured axons retain the ability to respond to growth signals over the extended distances of the primate CNS (Brock et al. 2010). These findings indicate that a separate intervention to prevent retrograde degeneration of upper motoneurons in the cortex may not be required. On the other hand, a therapeutic strategy aimed at controlling special targets, such as PKC, expressed abundantly in the cell body may be essential for promoting axonal regeneration through and beyond the level of injury. In fact, enhancing intrinsic abilities of CST axons to regenerate, such as through increased activity of the mammalian target of rapamycin pathway, has recently been shown to promote CST axons to regenerate across a DH (Liu et al. 2010).
In summary, we report that CST axons can successfully regenerate through and beyond a lesion gap of the DH after cortical delivery of a PKC inhibitor, GÖ6976, into the motocortex. Such regeneration led to significant forelimb functional recovery, a task critically dependent on the intactness of the CST. Moreover, a combined strategy with cortical delivery of GÖ6976 and lesion site delivery of ChABC had a synergistic effect on promoting greater anatomical regeneration and functional recovery than when GÖ6976 was used alone. Thus, our study has provided morphological and functional evidence for reestablishing neural circuit and functional recovery of an important descending pathway, the CST. Our study also emphasizes the importance of selecting an appropriate site for drug delivery, as well as the concept of using combinatorial strategies to achieve maximal therapeutic effects.
Funding
This work was supported by the National Institutes of Health (NIH/NINDS NS052290, NS052290-06S1, NS050243, and NS059622); The Indiana Spinal Cord and Brain Injury Research Funds (SCBI 200-98); and The Mari Hulman George Endowment Funds.
Notes
We would like to thank Jay E. Oakes for his excellent work on animal behavior tests. We appreciate the use of the Core facility of the Spinal Cord and Brain Injury Research Group/Stark Neurosciences Research Institute at Indiana University. Conflict of Interest: None declared.
References
- Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Goldberg DE, Madsen JR, Soni D, Irwin N. Inosine stimulates extensive axon collateral growth in the rat corticospinal tract after injury. Proc Natl Acad Sci USA. 1999;96:13486–13490. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Brock JH, Rosenzweig ES, Blesch A, Moseanko R, Havton LA, Edgerton VR, Tuszynski MH. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30:9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Clark JB, Bellegarrigue RB, Salcman M. Gunshot wound to the pons with functional neuroanatomical and electrophysiological correlation. Neurosurgery. 1985;16:607–611. doi: 10.1227/00006123-198505000-00004. [DOI] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol. 2001;171:153–169. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- Hill RL, Zhang YP, Burke DA, Devries WH, Zhang Y, Magnuson DS, Whittemore SR, Shields CB. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J Neurotrauma. 2009;26:1–15. doi: 10.1089/neu.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Xu XM. Early profiles of axonal growth and astroglial response after spinal cord hemisection and implantation of Schwann cell-seeded guidance channels in adult rats. J Neurosci Res. 2005;82:472–483. doi: 10.1002/jnr.20662. [DOI] [PubMed] [Google Scholar]
- Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Raisman G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J Neurosci. 1994;14:4050–4063. doi: 10.1523/JNEUROSCI.14-07-04050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G, et al. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Menezes K, de Menezes JR, Nascimento MA, Santos Rde S, Coelho-Sampaio T. Polylaminin, a polymeric form of laminin, promotes regeneration after spinal cord injury. FASEB J. 2010;24:4513–4522. doi: 10.1096/fj.10-157628. [DOI] [PubMed] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117(Pt 2):337–346. doi: 10.1093/brain/117.2.337. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Nielson JL, Sears-Kraxberger I, Strong MK, Wong JK, Willenberg R, Steward O. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci. 2010;30:11516–11528. doi: 10.1523/JNEUROSCI.1433-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain. 2005;128:839–853. doi: 10.1093/brain/awh424. [DOI] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif GI, Nomura H, Tator CH. Retrograde axonal degeneration “dieback” in the corticospinal tract after transection injury of the rat spinal cord: a confocal microscopy study. J Neurotrauma. 2007;24:1513–1528. doi: 10.1089/neu.2007.0323. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Spencer T, Domeniconi M, Cao Z, Filbin MT. New roles for old proteins in adult CNS axonal regeneration. Curr Opin Neurobiol. 2003;13:133–139. doi: 10.1016/s0959-4388(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Abe Y, McGehee DS, Keath JR, Yajima H, Sharma K, Brorson JR. Long-lived retrograde fluorescent labeling of corticospinal neurons in the living animal. Brain Res Brain Res Protoc. 2004;13:183–188. doi: 10.1016/j.brainresprot.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thallmair M, Metz GA, Z'Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Titsworth WL, Onifer SM, Liu NK, Xu XM. Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Exp Neurol. 2007;207:150–162. doi: 10.1016/j.expneurol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Kadakia R, Santi L, Houle JD. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J Neurotrauma. 2009;26:2323–2333. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wu DY, Zheng JQ, McDonald MA, Chang B, Twiss JL. PKC isozymes in the enhanced regrowth of retinal neurites after optic nerve injury. Invest Ophthalmol Vis Sci. 2003;44:2783–2790. doi: 10.1167/iovs.02-0715. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Burke DA, Shields LB, Chekmenev SY, Dincman T, Zhang Y, Zheng Y, Smith RR, Benton RL, DeVries WH, et al. Spinal cord contusion based on precise vertebral stabilization and tissue displacement measured by combined assessment to discriminate small functional differences. J Neurotrauma. 2008;25:1227–1240. doi: 10.1089/neu.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]