Abstract
Over the past 15 years, antiretroviral treatment guidelines for HIV infection have evolved significantly, reflective of the major advances in this therapeutic area. Evidenced-based recommendations have largely replaced expert opinion, while diagnostic monitoring and therapeutic interventions have become more sophisticated and effective. Just ten years ago, there was a marked difference in access to antiretroviral therapy for patients in wealthy and impoverished countries. The increasing availability of therapy across the globe, however, has made it possible for international guidelines to more closely resemble those in industrialized countries. This article compares the evolution of antiretroviral therapy treatment guidelines from the United States Department of Health and Human Services and the World Health Organization, focusing on when to initiate ART in asymptomatic patients and in those with an opportunistic infection; initial regimens in general population and in special populations; when to change and what to change; and laboratory monitoring.
I. Introduction
Treatment guidelines for HIV have evolved significantly over the past 15 years. Robust clinical trial data have allowed expert committees to provide clinicians with ever improving evidence-based treatment recommendations. National treatment guidelines have varied greatly by region, and are contingent on economic resources, laboratory capabilities, health priorities, patent law, and pharmaceutical manufacturing capacity.
Innovations in and development of antiretrovirals (ARVs) have taken place largely in high-income regions, and the availability of novel agents mirrors this trend. Medications from newer ARV classes (i.e., integrase inhibitors and entry inhibitors) and medications from older classes with extended spectrum of activity (e.g., darunavir, etravirine, etc.) are often inaccessible in low and middle-income countries due to high prices. Only recently, have alternative measures like compulsory licensing and generic manufacturing brought the cost of some drugs within reach.
II. HIV therapy in rich and poor countries: a brief history
A. Evolution of guidelines
The U.S. Department of Health and Human Services (DHHS) guidelines are based on the latest high-quality evidence and generally have not taken cost into consideration. (There are other treatment guidelines for high income settings available including the International AIDS Society-USA guidelines and British and European HIV guidelines, but, in our opinion, the DHHS guidelines are the most comprehensive and widely used, so for clarity and brevity we focus on the DHHS guidelines for this review.) The World Health Organization (WHO) guidelines, on the other hand, take a public health approach, promoting feasible interventions that are expected to lead to the maximal societal benefit, recognizing resource constraints. The differing approach to guidelines had the effect of creating a two-tiered approach for HIV, one for individuals in higher income countries and one for those in resource-limited settings. With continued decreases in the cost of many first-line medications, the WHO guidelines now promote a more idealized or “aspirational” goal for antiretroviral therapy (ART) coverage, with a caveat that not all countries will be able to implement the guidelines fully.
Table 1 displays the evolution of guidelines from the release of the first DHHS and WHO guidelines in 1998 and 2002, respectively, to present. In 2002, ART was routinely available in the West, but it was estimated that of the 6 million individuals requiring therapy for HIV in resource-limited settings, only 230,000 were on ART (WHO, 2002). The availability of funds from the President’s Emergency Plan for AIDS Relief (PEPFAR) and elsewhere and decreasing prices of generics allowed the WHO to embark on the ambitious and symbolically successful “3 by 5” initiative (i.e., a goal to have 3 million individuals on antiretroviral therapy by the end of 2005). While the 2002 WHO guidelines recommended a broad range of antiretroviral treatments, similar to what was recommended in the West at the time, the 2003 WHO guidelines recommended a more narrow range of less expensive but more toxic nucleoside reverse transcriptase inhibitors (NRTIs; e.g., stavudine (d4T), zidovudine (AZT), to be used in combination with lamivudine (3TC)) and non-nucleoside reverse transcriptase inhibitors (NNRTIs; e.g., nevirapine (NVP) and efavirenz (EFV)), with a view that this approach would most successfully allow for massive scale-up of therapy. At the same time, the West was moving away from these medications in favor of better-tolerated alternatives.
Table 1.
Evolution of Department of Health and Human Services and World Health Organization Guidelines from 1998-present.*
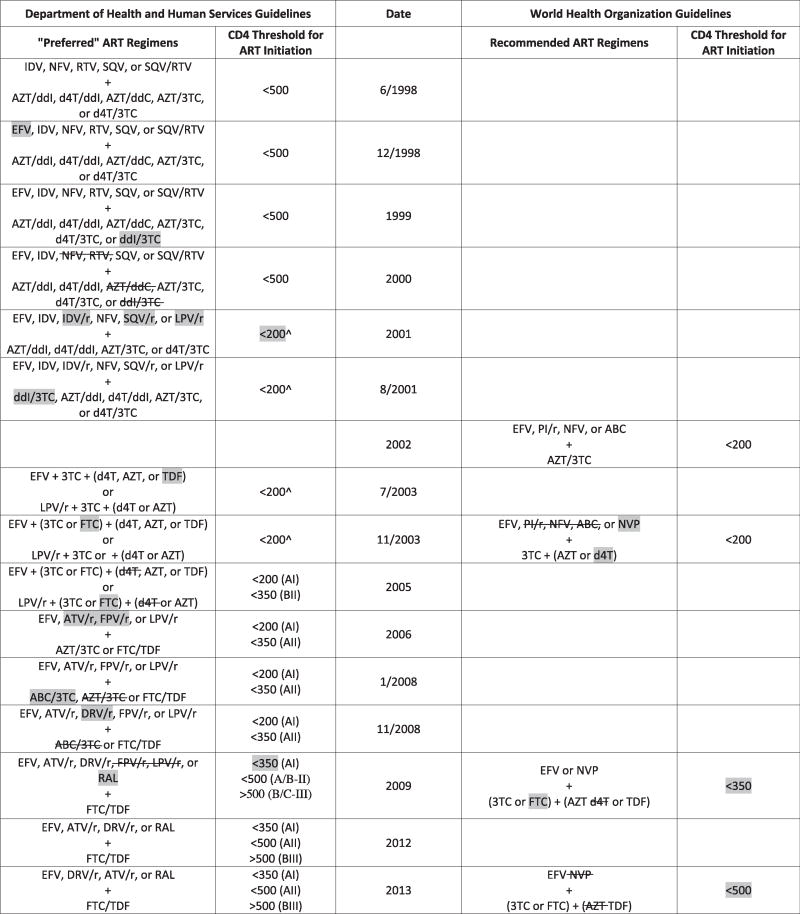
|
ART = combination antiretroviral therapy; IDV = indinavir; NFV = nelfinavir; RTV = treatment-dose ritonavir; SQV = saquinavir; EFV = efavirenz, AZT = zidovudine; ddI = didanosine; d4T = stavudine; ddC = zalcitabine; 3TC = lamivudine; r = pharmacologic-boosting dose of ritonavir; ABC = abacavir; TDF = tenofovir disoproxil fumarate; FTC = emtricitabine; PI = protease inhibitor; NVP = nevirapine; ATV = atazanavir; FPV = fosamprenavir; DRV = darunavir; RAL = raltegravir.
Treatment should generally be offered for 350-500, though controversy existed.
Shading represents addition to guidelines.
In 2003 in resource-limited settings, decisions to treat were for the most part dependent on clinical staging. If CD4 testing was available, a low threshold was used (i.e., CD4<200 cells/μL). In addition, in these countries, there was limited access to HIV viral load and resistance testing and second-line agents, so virologic failure to first-line therapy often left few additional options. However, both the CD4 threshold for treatment initiation and the availability of second-line agents have been increasing with time. Indeed, the number of patients on second-line therapy quadrupled between 2007 and 2012 (World Health Organization HIV/AIDS Programme, 2007, 2013).
In high-income countries, on the other hand, CD4 thresholds for treatment initiation were higher (BHIVA Writing Committee, 2003; EACS Euroguidelines Group, 2003); the availability of resistance testing and multiple ARVs gave greater flexibility in what drugs to initiate; furthermore, a wider array of drugs and close attention to side effects allowed for a more patient-centered approach to treatment. Lastly, the evolution of treatment recommendations has come to espouse ease of administration, with fixed dose combinations (FDC) becoming more widely used globally.
B. History of drug development
Since the efficacy of zidovidine (AZT)—however limited—was demonstrated in the late 1980’s (Fischl et al., 1987), there have been continual improvements in antiretroviral therapy, initially marked by the development of protease inhibitors (PIs) in the mid-1990’s (Hammer et al., 1997; Hirsch et al., 1999; Cameron et al., 1998). Antiviral effectiveness has been further enhanced with the introduction of the second-generation PI, darunavir (DRV) (Katlama et al., 2009); the second-generation NNRTI, etravirine (ETR) (Madruga et al., 2007); and newer class agents like the integrase inhibitors – raltegravir (RAL) (Steigbigel et al., 2008; Lennox et al., 2009), elvitegravir (EVG) (Zolopa et al. 2013), and dolutegravir (DTG) (Eron et al., 2013); and the CCR5 antagonist, maraviroc (MVC) (Gulick et al., 2008; Cooper et al., 2010).
III. Comparison of current DHHS and WHO guidelines
A. When to initiate ART
There continues to be uncertainty as to the optimal timing for initiating ARVs in the setting of asymptomatic infection with the most uncertainty in those individuals with a CD4 count greater than 500 cells/μL (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2013). There remains some disagreement between guidelines for initiation in the CD4 range of >350 to 500 cells/μL range as well. For individuals with CD4 counts in this range there is mounting data supporting earlier initiation of ART although primarily from large observational studies (Sterne et al., 2009; Baker et al., 2008; Palella et al., 2003; Cain et al., 2011; CASCADE Collaboration, 2011; Kitahata et al., 2009) and secondary analyses of randomized controlled trials (Emery et al., 2009; Grinsztejn et al., 2012). For individuals with a CD4 count below 350 cells/μL, the most convincing evidence for the benefit of ART initiation is from a large randomized trial that showed mortality reductions with ART (Severe et al., 2010).
While the DHHS guidelines recommend ART for individuals with a CD4 count above 500 cells/μL, the data on the clinical benefits of ART for individuals with a CD4 count in this range are mixed. The NA-ACCORD observational study showed a reduction in all-cause mortality with earlier ART within this CD4 stratum (Kitahata et al., 2008), but other studies have not shown this benefit (Palella et al., 2003; CASCADE Collaboration, 2011). Based on the conflicting data, guidelines from resource-rich countries outside the U.S. do not universally recommend ART for individuals with CD4 counts within this stratum (Sterne et al., 2009; Gazzard et al., 2008). The START Study, an ongoing randomized clinical trial evaluating the optimal timing of ART in individuals with CD4 counts greater than 500 cells/μL, may provide more definitive data on this question (START Collaboration, 2013).
Guidelines also emphasize that earlier ART initiation should be considered in certain patient groups who have co-morbid conditions and those with increased risk of disease progression (e.g., viral load > 100,000 copies/mL, co-infection with hepatitis B or C, HIV-associated nephropathy, and/or a rapid decline in CD4). Given the potential link between untreated HIV infection and cardiovascular events, the DHHS guidelines recommend considering the presence of multiple cardiac risk factors as a factor favoring earlier ART initiation.
In addition to these personal health-related factors, prevention considerations now influence current guidelines in both high- and low-income settings. Based on evidence documenting the benefits of ART as prevention (Cohen et al., 2011; Tubiana et al., 2010), the DHHS and WHO guidelines recommend treatment in pregnant women. The WHO guidelines also single out individuals in serodiscordant sexual relationships to reduce transmission.
In acute HIV infection, recent studies suggest that initiation of treatment within days to months of HIV acquisition may reduce seeding of long-lived latently-infected cellular reservoirs and potentially lead to a functional cure in a small percentage of patients (Ananworanich et al., 2013; Sáez-Cirión et al., 2013). Current DHHS guidelines only provide a weak recommendation that treatment should be offered in early infection (BII evidence), but there is gathering evidence that treatment in the very earliest stages of infection can reduce the viral set point (Hogan et al., 2012), decrease the size of the viral reservoir (SPARTAC Trial Investigators, 2013), and reduce transmission risk.
The current WHO guidelines now make a strong recommendation to start ART in individuals with a CD4 count of ≤ 500 cells/μL (WHO, 2013), influenced by clinical trial data that supports the personal and public health benefits of earlier ART initiation. This rapid evolution over the past decade to guidelines that now more closely resemble the DHHS guidelines contrast markedly with the first set of guidelines in 2002 that endorsed treatment initiation only in those patients with WHO Stage IV disease or with a CD4 count of less than 200 cells/μL (WHO, 2002).
B. Starting ART in the setting of an acute AIDS-related opportunistic infection (OI)
Despite the substantial advances in HIV care, many patients even in high-income countries still present to care with late-stage HIV infection (Giardi et al., 2007; Sabin et al., 2004; Schwarcz et al., 2006; Antiretroviral Therapy Cohort Collaboration, 2007).
Many of these late-presenters only come to medical attention when they develop an acute AIDS-related complication. Since randomized controlled trials evaluating ART generally excluded patients with acute AIDS-related OIs, the optimal timing for initiation of ART in the setting of an OI was a long-standing and unanswered question.
Now, based on recent trial evidence, treatment guidelines recommend that ART be initiated early during treatment of an AIDS-related OI as long as there is no compelling clinical contraindication. The exception to this rule may be in the treatment of CNS opportunistic infections such as cryptococcal meningitis where early ART has been shown to increase mortality, at least in resource-limited settings (Makadzange et al., 2010; Boulware et al. 2013).
ACTG A5164, the first randomized trial evaluating the optimal timing of ART initiation in the setting of an active OI, demonstrated that early ART (given within 2 weeks of treatment initiation for the index OI) produced a 50% reduction in the composite endpoint of AIDS progression or death compared to deferred ART (given a median of approximately 6 weeks after treatment initiation for the index OI) (Zolopa et al., 2009). This reduction in clinical progression and death was associated with a more rapid increase in CD4 counts – which likely reduced the “window of vulnerability” to additional complications and/or death associated with advanced immunodeficiency.
More recently, three different randomized trials evaluated the impact of early ART in the setting of active tuberculosis (TB) in HIV-infected patients, mostly enrolled from lower-income countries (Abdool Karim et al., 2011; Havlir et al., 2011; Blanc et al., 2011). Although there were some differences in the design of these studies and subjects enrolled, all three trials demonstrated that early ART led to important clinical benefits (either a reduction in mortality or in the composite endpoint of mortality/AIDS progression, depending on the study) in those patients with a CD4 count of less than 50 cells/μL at ART initiation. TB meningitis may again be the exception to the rule of early ART in the setting of an active OI; a study from Vietnam showed no reduction in mortality with early ART in patients with TB meningitis (Torok et al., 2011)
C. Initial regimens
More than a quarter century after the introduction of AZT, twenty-seven HIV medications in six different classes have been approved for the treatment of HIV, a rate of approximately one new drug per year. Table 2 shows current DHHS guidelines for treatment-naïve adults and adolescents. Currently, the preferred regimens combine tenofovir disoproxil fumarate (TDF)/ emtricitabine (FTC) with either efavirenz (EFV), darunavir/ritonavir (DRV/r), atazanavir/ritonavir (ATV/r), RAL, EVG/cobicistat or DTG. DTG can also be used in combination with ABC/3TC. A rilpivirine (RPV)-based regimen is now recommended as an alternative NNRTI-based regimen in patients with a pre-treatment HIV RNA level of < 100,000 copies/mL. Lastly, the guidelines continue to recommend against triple NRTI regimens and unboosted PIs.
Table 2. DHHS Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Treatment-Naïve Adults and Adolescents*.
Revised February 12, 2013. Selection of a regimen should be individualized on the basis of virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance testing results, and comorbid conditions.
Available at: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/11/what-to-start
| Preferred Regimens | Alternative Regimens |
|---|---|
|
| |
| NNRTI-Based Regimen | NNRTI-Based Regimens |
| • EFV + TDF/FTC | • EFV + ABC/3TC |
| • RPV + TDF/FTC | |
| PI-Based Regimens | • RPV + ABC/3TC |
| • ATV/r (daily) + TDF/FTC | PI-Based Regimens |
| • DRV/r (daily) + TDF/FTC | • ATV/r + ABC/3TC |
| • DRV/r + ABC/3TC | |
| INSTI-Based Regimen# | • FPV/r + ABC/3TC or TDF/FTC |
| • RAL + TDF/FTC | • LPV/r + ABC/3TC or TDF/FTC |
| • EVG/COBI + TDF/FTC | INSTI-Based Regimen |
| • DTG + ABC/3TC | • RAL + ABC/3TC |
| • DTG + TDF/FTC | |
NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; EFV=efavirenz; TDF=tenofovir disoproxil fumarate; FTC=emtricitabine; ATV=atazanavir; r=low-dose ritonavir; DRV=darunavir; RAL=raltegravir; ABC=abacavir; 3TC=lamivudine; RPV=rilpivirine; LPV=lopinavir; EVG=elvitegravir; COBI=cobicistant; DTG=dolutegravir
In low-income settings, TDF with either FTC or lamivudine (3TC) have become the standard NRTIs. EFV has largely supplanted nevirapine (NVP) as the third agent given the toxicity of the latter and decreased evidence of teratogenicity with the former. These guidelines have evolved from when NVP/ stavudine (d4T)/3TC was the most commonly used regimen in the developing world. Current WHO guidelines recommend EFV/TDF/(3TC or FTC) as first-line; for second-line regimens, the guidelines recommend ATV/r or lopinavir/ritonavir (LPV/r) and two NRTIs (Table 3) (WHO, 2013).
Table 3. WHO Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents (WHO, 2013)*.
Selection of a regimen should be individualized on the basis of virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance testing results, and comorbid conditions.
| First Line | Second Line | Third Line |
|---|---|---|
| TDF/ (3TC or FTC) / EFV If contraindicated, then AZT + 3TC + EFV AZT + 3TC + NVP TDF + (3TC or FTC) + NVP |
2 NRTIs + LPV/r or ATV/r | Decided by national programs (should include integrase inhibitors and second-generation NNRTIs and PIs) |
TDF=tenofovir disoproxil fumarate; 3TC=lamivudine; FTC=emtricitabine; EFV=efavirenz; AZT=zidovudine; NVP=nevirapine ; NRTI=nucleoside/tide reverse transcriptase inhibitor; LPV=lopinavir; r=low-dose ritonavir; ATV=atazanavir; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor.
D. When to change and what to change
In high-income settings, changes in ART are common and are primarily done for virologic failure adherence challenges, or intolerance/toxicity. Currently, given the number of new drugs available in resource-rich countries, it is possible to provide a fully active ART regimen that will suppress HIV plasma viral loads to <50 copies/mL in nearly every patient—even in those harboring multi-drug resistant HIV. The same cannot be said for patients failing ART in resource-limited settings where the limited availability of second-generation ritonavir-boosted PIs and newer class agents makes it difficult to manage those patients failing first- and second-line regimens.
Ideally, when a patient is on a failing regimen, a resistance test is performed to help guide the selection of a new regimen (Tural et al., 2002). Fortunately, resistance testing along with viral load monitoring is expanding in some low-income countries (mostly programs associated with NGO’s or academic research). Careful review of the patient’s adherence patterns is also required to ensure that the new regimen meets the needs of the patient. In addition, the patient’s entire ARV treatment history should be reviewed along with any prior resistance tests. A new regimen generally should contain at least two and preferably three active drugs for patients to obtain optimal results. (The SECOND-LINE study, however, suggests that two active drugs may be enough, if one of them is a boosted PI. (Boyd et al., 2013).) In the absence of newer agents, a combination that includes partially active NRTIs produces superior results to an NRTI-sparing regimen (Paton et al., 2013). However, partially active NRTIs do not appear beneficial in high-income settings when more than two active agents are included in a salvage regimen (Tashima et al., 2013). Available agents to construct a potent new regimen include integrase inhibitors (i.e., RAL, EVG, DTG), the CCR5 antagonist (i.e., MVC) and new generation agents (i.e., ETR, DRV, and tipranavir).
Single drug switches are not recommended and generally should only be done when the patient has achieved and maintained optimal viral suppression (i.e., plasma HIV viral load of < 50 copies/ml). These types of switches are primarily done for toxicity or tolerability issues.
In some resource-limited areas, the older PIs (LPV/r, ATV/r) are available for second-line regimens at lower cost than the newer generation PIs. Lastly, given the difficulty in acquiring newer generation drugs, the 2013 WHO guidelines leave third-line regimens to the discretion of national governments (WHO, 2013).
E. Special populations: pregnant women
The most significant shift in the recommendations regarding pregnant women is cited in the DHHS Perinatal Guidelines: “Because the risk of neural tube defects is restricted to the first 5 to 6 weeks of pregnancy and pregnancy is rarely recognized before 4 to 6 weeks of pregnancy, EFV can be continued in pregnant women receiving an EFV-based regimen who present for antenatal care in the first trimester, provided the regimen produces virologic suppression (CIII)" (Panel on Antiretroviral Guidelines for Pregnant HIV-1-Infected Women, 2013). Women should receive intravenous AZT intrapartum if the viral load is above 400 copies/mL. In addition, all exposed infants should receive 6 weeks of AZT prophylaxis postpartum.
The WHO guidelines also recommend EFV (in combination with TDF and 3TC (or FTC)) for pregnant women. This recommendation will help streamline care in high prevalence TB areas, as drug-drug interactions preclude the use of nevirapine in combination with standard TB treatment. They guidelines also state that breastfeeding infants of mothers on ART should receive 6 weeks of daily NVP as prophylaxis (or 4–6 weeks of either daily NVP or twice-daily AZT if they are receiving replacement feeding).
F. Laboratory monitoring
In addition to ART management, the DHHS guidelines provide recommendations for laboratory monitoring. According to the DHHS guidelines, HIV RNA levels should be monitored prior to ART initiation/modification and 2–8 weeks subsequently (DHHS, 2013). After suppression of HIV RNA levels, HIV RNA levels are recommended to be monitored every 3–6 months. CD4 counts should be evaluated every 3–6 months, with the caveat that in virologically-suppressed patients CD4 counts can be checked annually. Genotypic resistance testing should be performed at baseline and potentially repeated prior to initiating ART so as to tailor the regimen to the results. Resistance testing should also be performed in patients whose viral load rebounds above 500–1000 copies/mL after being suppressed or in patients who fail to achieve a viral load of <200 copies/ml by 24 weeks. Tropism tests (genotypic or phenotypic) are recommended when considering a CCR5 antagonist as part of a treatment regimen. HLA-B-5701 testing is recommended prior to initiating abacavir as the hypersensitivity reactions to abacavir are strongly linked with this haplotype. Therapeutic drug monitoring should not be used routinely but may be helpful if pharmacokinetic drug-drug interactions or impaired drug absorption leading to decreased ARV exposure is suspected.
While many of the above mentioned tests are not available or recommended in low-income settings, the WHO guidelines do recommend viral load testing as the preferred monitoring approach to diagnose and confirm ARV treatment failure. If this is not available, then CD4 count and clinical monitoring should be used (WHO, 2013).
IV. Conclusions
Current treatment guidelines have continued the trend of recommending earlier initiation of ART, with a growing cognizance that such a practice also has utility in preventing new HIV infections. In addition, more potent and better-tolerated medications and increasing laboratory capacity have allowed regimens to be used effectively earlier in the course of therapy.
It is interesting to note that the WHO guidelines have actually superseded those in high-income countries by prioritizing the recommendation of initiating ART at CD4 counts less than 500 cells/mm3. That there is weaker evidence for this cutoff than for 350 cells/mm3 underscores the WHO’s evolution towards aspirational recommendations, as opposed to those that are solely evidence based or what is possible in individual countries. Recent literature has questioned the application of high-income country guidelines to resource-limited settings (Gallant et al., 2013; Lundgren and Wood, 2013). We agree that the incremental clinical benefit gained by early treatment would likely be outweighed by the potential danger of exacerbating resource disparities.
Guidelines in high-income vs. impoverished regions have evolved in different environments. The ecological boundaries, so to speak, are framed by broad forces such as financial resources, international patent laws, and local government oversight. Organizations like the Treatment Action Campaign, Clinton Foundation, and MSF Campaign for Access to Essential Medicines as well as larger programs such as PEPFAR and the Global Fund to Fight AIDS, Tuberculosis and Malaria have altered the landscape dramatically and allowed for the evolution in guidelines highlighted above; however, many of the newer medicines remain out of reach in low-income regions and viral load monitoring and resistance testing are far too limited. Only continued efforts at expanding generic production and compulsory licensing will obviate the need for two-tiered guidelines in the future and ensure that all human beings infected with HIV—no matter where they live—are treated the same.
Highlights.
HIV treatment guidelines in rich and poor countries are becoming more aligned.
We review early antiretroviral therapy for HIV infection in rich and poor countries.
We review early ART for active opportunistic infections and TB, except for CNS infections.
We discuss HIV viral load monitoring in rich and poor countries.
Early HIV therapy has both clinical and public health benefits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of Antiretroviral Therapy with Tuberculosis Treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46:607–15. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananworanich J, Vandergeeten C, Chomchey N, et al. Early ART Intervention Restricts the Seeding of the HIV Reservoir in Long-lived Central Memory CD4 T Cells. Presented at the 20th Conference of Retroviruses and Opportunistic Infections; Atlanta, Georgia. March 3–6, 2013; 2013. Abstract. 47. [Google Scholar]
- Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHIVA Writing Committee. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2003;4:1–41. [PubMed] [Google Scholar]
- Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D, et al. ART initiation within the first 2 weeks of cryptococcal meningitis is associated with higher mortality: a multisite randomized trial. 20th Conference on Retroviruses and Opportunistic Infections; 3–6 March 2013; Atlanta. 2013. Abstract 144. [Google Scholar]
- Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, Mohapi L, Martin A, Kerr S, Sohn AH, Teppler H, Van de Steen O, Molina JM, Emery S, Cooper DA. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND) Lancet. 2013;381:2091–9. doi: 10.1016/S0140-6736(13)61164-2. [DOI] [PubMed] [Google Scholar]
- Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154(8):509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S. Randomized placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171(17):1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DA, Heera J, Goodrich J, et al. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis. 201(6):803–13. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- EACS Euroguidelines Group European guidelines for the clinical management and treatment of HIV-infected adults in Europe. AIDS. 2003;17:3–26. [PubMed] [Google Scholar]
- Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. JID. 2009;197(8):1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–8. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard C, Colin C, Charpentier C, et al. Long-term efficacy and safety of raltegravir, etravirine, and darunavir/ritonavir in treatment-experienced patients: week 96 results from the ANRS 139 TRIO trial. J Acquir Immune Defic Syndr. 2012;59(5):489–93. doi: 10.1097/QAI.0b013e31824bb720. [DOI] [PubMed] [Google Scholar]
- Fischl M, Richman D, Grieco M. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–91. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Gallant JE, Mehta SH, Sugarman J. Universal antiretroviral therapy for HIV infection: should US treatment guidelines be applied to resource-limited settings? Clin Infect Dis. 2013;57:884–7. doi: 10.1093/cid/cit382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzard BG, Anderson J, Babiker A, et al. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9(8):563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- Giardi E, Sabin C, Monforte A. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(Suppl 11):S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- Grinsztejn B, Swindells S, et al. Effect of early versus delayed initiation of antiretroviral therapy (ART) on clinical outcomes in the HPTN 052 randomized clinical trial. Paper presented at the XIX International AIDS Conference; 2012; Washington, DC. 2012. Abs ThLBB05. [Google Scholar]
- Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for Previously Treated Patients with R5 HIV-1 Infection. N Engl J Med. 2008;359(14):1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer S, Squires K, Hughes M, Grimes J, Demeter L. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubit millimeter or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M, Steigbigel R, Staszewski S, Scerpella E, Hirschel B. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis. 1999;180:659–665. doi: 10.1086/314948. [DOI] [PubMed] [Google Scholar]
- Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlama C, Haubrich R, Lalezari J, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 2009;23(17):2289–300. doi: 10.1097/QAD.0b013e3283316a5e. [DOI] [PubMed] [Google Scholar]
- Kitahata M, Gange S, Abraham A. Effect of Early versus Deferred Antiretroviral Therapy for HIV on Surviva N. Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patientswith HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Wood R. Editorial commentary: universal antiretroviral therapy for HIV infection? Clin Infect Dis. 2013;57:888–90. doi: 10.1093/cid/cit381. [DOI] [PubMed] [Google Scholar]
- Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370(9581):49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 50(11):1532–8. doi: 10.1086/652652. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138(8):620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf Section accessed 30 March 2013. [Google Scholar]
- Panel on Antiretroviral Guidelines for Pregnant HIV-1-Infected Women. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines/0/Section accessed 30 March 2013. [Google Scholar]
- Paton N, Kityo C, Hoppe A, et al. A pragmatic randomised controlled strategy trial of three second-line treatment options for use in public health rollout programme settings: the Europe-Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial. Program and abstracts of the 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; June 30 – July 3, 2013; Kuala Lumpur, Malaysia. 2013. Abstract WELBB02. [Google Scholar]
- Sabin C, Smith C, Gumley H, Murphy G, Lampe F. Late presenters in era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18:2145–2151. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog. 2013;3:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz S, Hsu L, Dilley J, Loeb L, Nelson K. Late diagnosis of HIV infection: trends, prevalence, and characteristics of persons whose HIV diagnosis occurred within 12 months of developing AIDS. J Acquir Immune Defic Syndr. 2006;43:491–494. doi: 10.1097/01.qai.0000243114.37035.de. [DOI] [PubMed] [Google Scholar]
- Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SPARTAC Trial Investigators. Short-Course Antiretroviral Therapy in Primary HIV Infection. N Engl J Med. 2013;368(3):207–217. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- START Collaboration. Strategic Timing of Antiretroviral Treatment (START) Study. Available at http://www.clinicaltrials.gov/ct2/show/NCT00867048?term=hiv+start+study&rank=4. Accessed 9/28/2013.
- Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima K, Smeaton L, Andrade A, et al. Omitting NRTI from ARV regimens is not inferior to adding NRTI in treatment-experienced HIV+ subjects failing a protease inhibitor regimen: the ACTG OPTIONS study. Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, Georgia. 2013. Abstract 153LB. [Google Scholar]
- Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tural C, Ruiz L, Holtzer C, et al. Clinical utility of HIV-1 genotyping and expert advice: the Havana trial. AIDS. 2002;16:209–218. doi: 10.1097/00002030-200201250-00010. [DOI] [PubMed] [Google Scholar]
- Tubiana R, Le Chenadec J, Rouzioux C, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load <500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1) Clin Infect Dis. 2010;50(4):585–96. doi: 10.1086/650005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Scaling up antiretroviral therapy in resource- limited settings: guidelines for a public health approach: executive summary. 2002 [PubMed] [Google Scholar]
- World Health Organization HIV/AIDS Programme. Demand forecast for antiretroviral drugs in low- and middle income countries, 2007–2008. Geneva: 2007. [Google Scholar]
- World Health Organization HIV/AIDS Programme. Antiretroviral medicines in low-and middle-income countries: Forecasts of global and regional demand for 2012–2015. Geneva: 2013. [Google Scholar]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 [PubMed] [Google Scholar]
- Zolopa A, Andersen J, Powderly W. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PloS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolopa AR. The evolution of HIV treatment guidelines: Current state-of-the-art of ART. Antivir Res. 2010;85(1):241–244. doi: 10.1016/j.antiviral.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Zolopa A, Sax PE, DeJesus E, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63:96–100. doi: 10.1097/QAI.0b013e318289545c. [DOI] [PubMed] [Google Scholar]


