Abstract
Medicare patients in five diagnosis-related groups (DRGs) associated with heavy use of post-hospital care discharged from 52 hospitals in 3 cities were followed up at 6 weeks, 6 months, and 1 year to determine the factors associated with their being discharged home with or without home health care and the correlates of improvement in their functional status. Models correctly predicted those discharged home from those going to institutions in a range from 54 to 82 percent of cases. The amount of the variance in the change in function for those who went home (with or without home health care) explained by the models tested ranged from 19 percent to 73 percent. Total Medicare costs for the patients who went home were considerably less in the year subsequent to the hospitalization compared with those discharged to institutional care.
Introduction
Although home health care has been a part of Medicare-covered services since the inception of the program, the changes in hospital payment under the prospective payment system (PPS) and a growing interest in finding ways to keep patients in the community have led to a dramatic growth in this sector of care. This article uses data from a larger study of post-hospital care to examine the factors associated with Medicare patients being discharged from the hospital to their homes with and without home health care and the factors that are associated with better functional outcomes in such patients 6 weeks after their discharge from the hospital.
Home health agencies (HHAs) were originally conceived as a stage in the continuum of care following hospitalization, where the patient's recovery and rehabilitation could be effectively continued at the patient's home at a lower cost than in a hospital or skilled nursing facility (SNF) (Helbing et al., 1992). Through the later 1970s, home care was an undeveloped, fragmented, and poorly financed enterprise referred to as “the empty alternative” to institutional care (Vladeck, 1980). Since 1980, home health care has been a growth industry. Home health care expenditures increased from about $200 million in 1970 to an estimated $9.8 billion in 1991, of which Medicare expenditure on home care increased from about $100 million in 1970 to $5.7 billion in fiscal year 1991 (Helbing et al., 1992; Letsch et al., 1992).
This growth was the result of both serving more people and providing more care. The number of Medicare beneficiaries who received Medicare home health services more than tripled from 1974 to 1990, from 16 per 1,000 to 57 per 1,000. At the same time, the average number of Medicare home health visits per beneficiary served rose from 21 to 36 (Helbing et al., 1992).
The number of Medicare-certified HHAs grew rapidly during the 1980s, almost doubling from 2,924 in 1980 to 5,708 in 1990 (Helbing et al., 1992). Most of this increase, which occurred in the early 1980s, can be attributed to several factors: (1) the introduction of PPS, which encouraged hospitals to use post-acute home health care and nursing home care to reduce length of hospital stay (Kenney, 1991); (2) the expansion of the Medicare HHA benefit; (3) legislative changes that permitted greater involvement of proprietary HHAs to operate in States without licensure laws; and (4) growing numbers of older persons.
Not only has the number of Medicare-certified HHAs substantially increased, but there has been a shift in who provides the home care away from the voluntary and public sector to the private sector; additionally, there has been a rapid growth in proprietary and hospital-based home HHAs. In 1972, government-owned HHAs comprised 57 percent of all Medicare-certified HHAs; however, by 1983, the percent of government HHAs had dropped to 29 percent of all Medicare-certified HHAs (Gornick and Hall, 1988); by 1990, it had further declined to 17 percent (Helbing et al., 1992). Today, proprietary agencies represent the largest share of the national home care market.
Some information about factors associated with home health care use is available, but the results are not especially illuminating or even consistent across studies. Soldo (1985) found five need variables (extreme activity of daily living [ADL] and instrumental activity of daily living [IADL] needs, medical needs, incontinence, and supervision) associated with a greater likelihood of using formal (paid) home care services as well as never being married and living in a central city. Living with a spouse or with other relatives and receiving informal services were related to less likelihood of using formal care. Several investigators have used the Andersen behavioral model to identify a set of predictive variables for using services (Andersen and Newman, 1973). Change in physical health, increased task burden, activity restrictions, incontinence, paralysis, and race were associated with use; but different factors, unrelated to recipient need, predicted the intensity of formal care: household income, number of family-assisted tasks, and mental impairment (Bass and Noelker, 1987). Starrett, Rogers, and Walters (1988) found five predictors, several of which seem confounded with use: need for care, ADL impairment, knowledge of home health resources, age, and use of health appliances. Cognitive impairment in the presence of living arrangements, other helpers, task burden, and depression was associated with hours of personal service, as were pain problems (Bass, Looman, and Ehrlich, 1992).
Within 2 weeks of post-hospital discharge, a large proportion of personal care and housekeeping for elderly patients who were discharged home was provided by relatives (Jones, Densen, and Brown, 1989). However, the proportion of this care provided by relatives decreased, and the proportion of paid home care had increased within 8 months after discharge. The utilization of informal care and home care at both time points was strongly related to the ADLs at the time of hospital discharge. Another study also suggested that discharge ADLs indicate the need for home care (Benjamin, Fox, and Swan, 1993). Among older patients discharged from medical and surgical units, home health care use was related to lower educational levels, prior use of home health care, IADL impairment, and less accessible social support (Solomon et al. 1993).
The use of Medicare HHA services differs with the gender of beneficiary (Helbing, Sangl, and Silverman, 1992). In 1990, the annual utilization rate per 1,000 enrollees was 31 percent higher for females (64 per 1,000) than for males (49 per 1,000). The average payment per enrollee was also higher for females ($1,938) than for males ($1,810).
On a larger scale, supply-and-demand factors play a central role. Swan and Benjamin (1990) note that the use of home health care is related to the size of the population 85 years of age or over, to the number of HHAs per 100,000 population, and inversely to the supply of nursing home beds. This conclusion is supported by the observation that higher proportions of Medicare enrollees use home health services in areas: (1) with fewer nursing home beds, (2) with higher hospital discharge rates and shorter mean lengths of stay, (3) with higher Medicare ceilings for skilled health visits, (4) with more HHAs per enrollee, (5) located in the New England and Mid-Atlantic Regions, and (6) that are urban (Kenney and Dubay, 1992). Kenney (1993a) found an increase in home health care use in both rural and urban areas, with a persistently higher use in urban areas; however, the average number of visits per user in rural areas became higher than that for urban areas. She has also suggested that hospitals are more likely to discharge patients to home health care when they own such care and are less likely to do so when those hospitals operate swing beds or long-term care beds (Kenney, 1993b).
Although home care has received increasing attention, the effect of home care on patient outcomes and costs of care has been controversial (Hedrick and Inui, 1986) and remains controversial today. More recently, Cummings and Weaver (1991) pointed out that early studies on cost effectiveness “not only failed to demonstrate any dramatic reduction in institutional care but also clearly delineated the additional costs that care would produce.” In addition, the randomized study on channeling reinforced the belief that expanding community care increased costs beyond any savings that resulted from decreased nursing home use (Thornton, Miller-Dunston, and Kemper, 1988).
Home care needs to be distinguished from home health care. Although the two forms may often be interspersed, the latter implies medical (actually nursing) supervision and emphasis. Indeed, Greene (1993) has suggested that had more nursing care been used in the channeling demonstration, greater results might have been seen.
The parent study from which the data reported here are drawn was designed to provide a prospective look at the effects of hospital discharge decisions on Medicare patients. Specifically, it examines the factors associated with hospital discharges to home health care and assesses the factors associated with the functional outcomes of such care. In the parent study, Medicare patients with one of five diagnosis-related groups (DRGs), which account for almost 40 percent of all Medicare-supported post-hospital care, were interviewed just prior to their discharge from the hospital and again at 6 weeks, 6 months, and 1 year after discharge from the hospital.
Methods
Sample
The five DRGs were selected on the basis of the volume of post-hospital care used by Medicare beneficiaries (Neu, Harrison, and Heilbrunn, 1989) and represent conditions used by earlier investigators, for largely the same reason (Morrisey, Sloan, and Valvona, 1988; Meiner and Coffey, 1985). The conditions were also chosen to represent patients requiring medical and rehabilitative care. The original DRGs chosen were stroke (DRG 14), chronic obstructive pulmonary disease (DRG 88), congestive heart failure (DRG 127), major joint procedures (DRG 209), and hip and femur fractures with procedures (DRG 210). However, some flexibility in accepting cases with related DRGs was necessary when the investigators encountered some shifts in DRG classification during and after hospitalization. Thus, the basic conditions remained the same but the DRG criteria were broadened. Originally, we chose hip fractures and hip replacements to represent emergency and elective conditions, respectively. However, we found that many fractures were being treated by replacement Therefore, a third category was created: hip fracture treated by arthroplasty (hip fx/A). Patients with hip procedures (DRGs 209, 210, and 211) who also had evidence of a hip fracture were placed in this third category. A patient was considered to have a hip fracture if: (1) there was a DRG 209, 210, or 211; (2) there was a principal diagnosis of hip fracture (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 820); or (3) there was evidence of a hip fracture on X-ray. These hip fracture patients treated with arthroplasty are thought to combine the clinical course of the hip replacement group with the initial frailty and emergent status of the hip fracture group.
Hospital discharges were sampled in three cities between February 1988 and March 1989. A study of three cities cannot claim to be nationally representative. The cities were chosen to represent a cross-section of the United States but were not intended to be a random sample. The criteria for selecting the cities were geographic distribution, availability of acceptable levels of each of three major post-hospital care modalities, adequate numbers of Medicare discharges, and variation in the nature of the dominant medical care system. The three cities selected were the Twin Cities (Minneapolis/St. Paul), Pittsburgh, and Houston. The Twin Cities provided an example of heavy penetration of prepaid care; whereas, Houston had a high prevalence of proprietary hospitals.
Hospital participation was voluntary. All acute general hospitals treating adults in each city were approached. In the Twin Cities and Pittsburgh, almost every eligible hospital agreed to participate (19 of 19 and 18 of 20, respectively). The enrollment in Houston was more difficult because there were a larger number of smaller proprietary hospitals. An active effort was made to obtain a sample of the proprietary hospitals as well as the generally larger voluntary institutions. In the end, the Houston sample was less complete than that for the other sites but did contain hospitals of both types. Of the 31 hospitals eligible in Houston, 15 agreed to participate, 6 of which were proprietary. The participating hospitals in Houston were more likely to be larger and academically affiliated.
Data Sources
The post-hospital care study relied on merging data from several sources. Information was collected directly from patients (or their proxies when the patients were unable to participate) through interviews conducted in person at the time of discharge from the hospital and at 6 weeks, 6 months, and 1 year post-discharge. Telephone interviews were also conducted with the patients' primary informal caregivers to obtain information on care burden and service use. The caregiver interviews were conducted at 6 weeks, 6 months, and 1 year after patients were discharged. Patient data were augmented with information from the patient's medical record using a modification of the Medisgroups approach (Iezzoni and Moskowitz, 1988). Medicare and Medicaid billing data for the year prior to and subsequent to the triggering hospitalization were obtained from HCFA and from the State Medicaid agencies. The Medicare Automated Data Retrieval System (MADRS) data included information about the study subjects and information about all Medicare beneficiaries in the three metropolitan areas included in the analyses.
Because the goal of the study was to predict discharge decisions, great emphasis was placed on collecting information that was available prior to those discharge decisions. Similarly, there was concern about the consistent availability of pertinent and reliable information in the medical records on the variables of interest, especially with regard to items about the patient's current and prior functional status. It was, therefore, imperative to collect information directly from patients during a 72-hour window prior to discharge. The opportunity to interview patients provided a means to ascertain level of function by way of demonstrated performance and self-report of simulated activities necessary for independent living (ADLs such as eating, dressing, and taking medications). Tests were also conducted on cognitive and emotional status, and information was obtained on patient's functional and perceived health status before the acute event that led to the hospitalization. The in-person interviews also provided a chance to query patients about their role in the decisionmaking process concerning discharge placement, their expectations for recovery, and their satisfaction with the care they received.
The patient interviews were conducted by nurses trained to collect information directly from patients after obtaining informed consent. For patients who were cognitively unable to participate, relevant information was collected from proxies. In these cases, family members provided the historical information, and hospital nurses caring for the patients provided information on their functional abilities.
Interviewers were carefully trained to assure reliability across the sites. Regular supervision from central project staff as well as onsite supervisors helped to maintain the quality and the comparability of data. All interview data were entered directly into preprogrammed computers to maximize error identification while there was still an opportunity for correction and to expedite the availability of information for analysis.
Outcome Measures
The outcome measures used here include self-reported functional status as measured by ADLs and community living situation (institutionalized or living in the community). Information on ADL function (which includes basic self-care activities such as feeding oneself, dressing, and bathing) was self-reported, as was information on the somewhat more complex activities of IADLs (which include such things as shopping, house-cleaning, taking medications, and using the telephone). The self-reported measures of ADLs were derived from those used in the Older American Research Study (OARS) battery (Duke, 1978). The self-reported measures were validated against ADL and IADL performance where possible. The areas simulated included eating, dressing, and using medications. However, we recognized that these two approaches addressed slightly different aspects of disability. The self-report describes what an individual does under prevailing circumstances, whereas demonstrations examine performance under controlled conditions (Burns et al., 1992). The performance measures of ADLs were adapted from the CARES scales developed by Kuriansky and Gurland (1976) and used previously in studies of nursing home patients (Kane et al., 1983; Garrard et al., 1990). For similar items, the count of demonstrated dependencies correlated with that of reported dependencies at about 0.70.
The functional-outcomes approach compares the functional score at the time of discharge from hospital with the score 6 weeks, 6 months, and 1 year after discharge. The score used for this analysis is a weighted sum of seven functions (incontinence, bathing, dressing, toileting, transferring, feeding, and walking). No IADL items were used in the dependent-variable version because it was felt that those in nursing homes (and perhaps some other places) would not have an opportunity to employ them. The weighting system used was developed specifically for this study and relies on a technique known as “magnitude estimation.” In brief, a panel of experts was queried using a Delphi approach to produce weights for each of the functional domains and each of the levels within each domain. A detailed description of the technique is presented elsewhere (Finch, Kane, and Philp, 1994). Each patient has a unique score for each relevant point in time: prior to hospitalization; at discharge; and at 6 weeks, 6 months, and 1 year post-discharge. Because the scale measures the level of dependency, a higher score indicates greater dependency.
Death was incorporated into the overall functional score. We tested the effects of assigning different values to death, including the following methods:
A score value just above the maximum aggregate dependency score.
A score value one and two standard deviations above the value for the most dependent summed State.
Eliminating the dead cases from the score altogether.
The different methods for scoring death were compared with the results using surviving patients only (i.e., excluding all patients who had died before the time of each followup). The weighting of death does produce some change in the ADL predictors. We opted to use a level just above the maximum aggregate dependency score as this weighting does not change the pattern of predictors compared with the results when using surviving patients only.
The distributions achieved using the magnitude-estimation approach and the more conventional ADL approach of simply counting dependencies are strongly related. The weighted score correlates with a simple count of dependencies defined as needing any assistance at 0.91 and with a count of dependencies defined as needing complete assistance at 0.94, whereas the two conventional approaches to defining dependency correlate with each other at only 0.77. When the distributions created with this ratio scale were compared with those obtained by simply counting the numbers of disabilities, the ratio scale produced a more normal distribution.
Beyond the general concern with the conventional approach that there is no a priori basis for weighting all domains equally, there is the further problem of determining what constitutes a dependency when one is forced to dichotomize. For example, a cut made at needing any assistance would yield a different result from one made using a need for a great deal of assistance.
The weighted score we produced for this study can be used as a ratio scale—i.e., the distance between the individual components has relative meaning. One can add scores to produce totals that relate directly to each other. The weighted score produces a scale that is highly correlated with a simple count of the number of areas in which the patient is dependent, and provides a distribution that uses the full range of possible values.
To assess outcomes we used as our dependent-variable change scores calculated as the differences between the summed weighted ADL scores obtained at the time of discharge those measured at followup 6 weeks after discharge. The ADL dependency scores reported prior to hospitalization and at discharge are included in the regression equations as independent variables to control for baseline status.
The same general approach was also used to create independent ADL variables. Patients interviewed at the time of discharge were asked about their present level of functional activity, their function prior to the acute event that led to hospitalization (which included both ADL and IADL items), and how well they expected to function 6 weeks after discharge. The latter was used as a proxy for physician prognosis, which was not consistently recorded in the medical chart.
Costs
The major sources of cost data for this study were Medicare records. We received good cooperation from HCFA's Bureau of Data Management and Strategy in developing records on study participants. The plan was to obtain information on all Medicare expenditures for the year prior to the inciting hospitalization and the year after. One of the problems encountered with the Medicare data was the difficulty in identifying all participants. In some cases, Medicare numbers were incorrectly recorded at the hospital or by the interviewers. After confirming the Medicare numbers with respondents in followup telephone calls and using the Medicare locator file to try to identify the Medicare numbers for those where our recorded numbers did not agree with HCFA's, we were able to locate the Medicare records for 2,101 of the 2,248 subjects who had recorded hospitalizations at the time we know they were in the hospital.
Medicare costs from both Part A and Part B were used to compare the costs in the baseline year prior to hospitalization. The costs were analyzed as both means and medians, the latter being less sensitive to outliers. Both Part A (hospital insurance) and Part B (supplementary medical insurance) costs are included in the MADRS data system. The former includes most of the Medicare costs for paid home health care, nursing home care, inpatient rehabilitative care, and, of course, hospital care. (We had deliberately omitted the costs of the specific hospitalization that triggered entry into the study.) Given the relatively low rate of use for rehabilitation, hospital care is the dominant cost captured in Part A data. Part B data includes physician services, ambulatory physical therapy, outpatient laboratory services, and X-ray costs.
Independent Variables
Independent variables were organized into three categories: (1) patient characteristics obtained from interview data; (2) patient-specific severity measures abstracted from the medical record; and (3) hospital-specific characteristics. Missing values were excluded from the analysis, and ordinal/continuous variables were assumed to be linear.
The core set of independent variables included basic demographic data (age, gender, and race), patients' Medicaid enrollment status (determined by Medicare Denominator files), patients' living arrangements, presence of urinary catheter, and city of residence. Other information included magnitude-estimated forms of prior ADL and IADL dependencies, expected ADL performance, speech and hearing deficiencies, number of errors on a basic 10-item mental status test, and the previous provision of informal support We opted not to ask about available informal assistance but rather whether any had been actually given in the past for fear of eliciting false expectations of assistance. The simple cognitive screening battery was based on the Short Portable Mental Status Questionnaire (MSQ) (Pfeiffer, 1975). The MSQ has been administered to nursing home patients and has proved to correlate highly with more comprehensive measures of cognitive status (Kane et al., 1983). In an effort to account for patients' ability to exercise prudent judgment, we used a test of their awareness of their own body (Fink, Green, and Bender, 1952). A modified version of a scale developed by Coulton and her associates (1988) was used to assess the patient's role in post-hospital care decisionmaking.
Another independent variable was health maintenance organization (HMO) membership. This information was obtained from the Medicare Denominator file, which represents HMO status at the time of discharge from the hospital. If this information was missing, HMO status at 1 year after discharge was used instead. Most HMO members resided in the Twin Cities area. Of the total sample, 465 (20.7 percent) of the patients were enrolled in a Medicare HMO, of whom 427 (92 percent) lived in the Twin Cities area. Pittsburgh had 37 patients (8 percent of their total sample) enrolled in HMOs, and Houston had only 1 HMO patient The high rate of reported membership in the Twin Cities (48 percent) is not surprising, given the area's prevalence of HMOs and its history of early Medicare participation of the HMOs. At the time of the study, there were four Tax Equity and Fiscal Responsibility Act of 1982 HMOs operating in the Twin Cities, of which two were more active.
Severity Measures
One aspect of the analysis of the post-hospital care data is the addition of patient-specific severity scores based on the patient's condition during hospitalization and at the time of discharge. The original Medisgroup severity index (Iezzoni and Moskowitz, 1988) was modified to create a severity index tailored specifically for this study. Information abstracted from the patient's medical records was used to develop several different approaches to measuring severity. The index captures information of the patient's acute status on admission and changes in the patient's clinical condition during hospitalization. Six separate variables were used to measure severity.
Admission Acute Physiology Score (APS)
This variable measured the generic physiologic status of the patient on admission based on the physiologic score used in the Acute Physiology and Chronic Health Evaluation (APACHE II) system (Knaus, Draper, and Wagner, 1985). The APACHE severity index has been used for patients in intensive care and relies heavily on physiologic items, including many laboratory values as well as the Glasgow Coma Scale. Two versions of this variable were tested: one using admission and the other using discharge values. To a large degree, the amount of missing data at the time of discharge made it difficult to base any of the variables solely on discharge information. Thus, this score was limited to the admission values. The values range from 0 to 15.
Comorbidities
This variable, based on previous work, measures the nature and extent of comorbidities present prior to hospitalization as well as those occurring during the hospitalization. Comorbidity was defined as a “patient's other disease burden or conditions which are not related to the principal disease process or the main reason why the patient was admitted to the hospital.” Based on expert clinical opinion, individual comorbidities were assigned a score of 1 to 10 reflecting increasing severity. The final score was established by adding the points assigned to each additional comorbidity. The range of possible scores was from 0 to 20 points.
DRG-Specific Severity Scores
A separate severity score was designed for four of the five DRGs (stroke, chronic obstructive pulmonary disease [COPD], congestive heart failure [CHF], and hip fractures) based on admission information only. (Again, the quality of the data did not permit incorporating discharge information). The lack of adequate data elements for patients with hip procedures prevented the development of a separate severity score for that DRG. Each severity score represented a composite score of clinical items unique to that specific DRGs.
Instability, Sickness, and Laboratory
These variables, adapted from the RAND study of the impact of PPS (Kosecoff, Kahn, and Rogers, 1990), measure patients' clinical status at discharge. The instability score represents a composite score of temperature at admission and discharge, use of catheter, shortness of breath, heart rate, blood pressure, and cardiac arrhythmias, among others. This is a dichotomous variable with potential values of 0 or 1, where a score of 1 indicates that a patient had at least 1 measure of instability. The sickness measure is a composite score of temperature, use of catheter, shortness of breath, heart rate, respiratory rate, blood pressure, and cardiac arrhythmias. This is a dichotomous variable, with potential values of 0 or 1, where a score of 1 indicates that a patient had at least 1 measure of sickness. The laboratory score represents the number of abnormal lab results, including serum potassium, serum sodium, renal lab, hematocrit scores, blood count, CHF, and pulmonary edema present, among others. This is a dichotomous variable with potential values of 0 or 1, where a score of 1 indicates that a patient had at least one abnormal laboratory measure.
Data Analysis
A general model was initially created that identified variables that had been hypothesized as associated with discharge location and later with functional outcomes. Rather than using some of the step-wise-regression approach, this model was tested in its entirety. The analysis of discharge location was done in two stages. First, a logit regression was used to identify the factors associated with a discharge home as opposed to an institution. Because almost all cases of COPD and CHF went home, these patients were eliminated from this step of the analysis. A second logit model was then used among all those who went home (including the conditions earlier eliminated) to distinguish the variables related to the receipt of home health care.
The same basic approach was used to examine the factors related to outcomes, which were expressed as change in the weighted functional status score from hospital discharge to followup. Because the outcome was expressed using a ratio scale, ordinary-least-squares regression was used to distinguish the factors associated with improved function among those who went home (with or without home care) and then specifically among those who received home health care. Except for COPD and CHF cases, where only those who were discharged home were analyzed, a two-step Heckman procedure was used to correct for possible selection bias (Heckman, 1979).
Although the sample size is large, the analysis within DRGs, which we believe essential to separate the different clinical courses of the conditions being examined, results in relatively small effective sample sizes. Although the samples are large enough to use the large numbers of variables included in the models, the adjusted R2 (amount of variance explained) and statistical significance are considerably reduced.
Results
Of the 3,732 patients identified as potential candidates for inclusion and initially contacted to participate in the parent study, 2,865 were interviewed; 31 refused; 318 were interviewed too soon before discharge to be included; and 518 had their DRG changed to something else. Of the 2,865 interviews, 2,611 yielded complete data; 117 could not be completed, and 137 patients subsequently died before discharge. Another 363 cases had to be eliminated; 96 were missing critical elements of their functional scores at discharge; 185 were admitted from a nursing home and felt to be too likely to return there; 80 were discharged to non-post-acute care settings such as transfers to other acute hospitals. Of the final discharge sample of 2,248 patients, 1,837 were successfully contacted at 6 weeks; 118 refused an interview; 125 had died (but were still included in the analyses); 16 were contacted outside the accepted time window for followup; and 152 could not be located. Efforts to compare the study sample with live Medicare discharges in the same DRGs, based on Medicare data files, suggest that the sample had slightly more females (65 percent versus 60 percent), was slightly older (77 years versus 74 years), had a slightly shorter mean length of stay (9.5 days versus 9.9 days), and was more likely to be discharged to some form of institutional care (22 percent to nursing homes and 8 percent to rehabilitation versus 17 percent and 7 percent, respectively). The individual values for each of the variables used in the study are shown in the final report (Kane, 1994).
A major comparison is made between the use of institutional care and going home. Most physicians and discharge planners define the fundamental decision in these terms, although many analyses have addressed the relationship between formal and informal care. Indeed, in our sample among those discharged home, 48 percent received only informal care, 48 percent received some combination formal and informal care, 1 percent got only formal care, and 3 percent got neither during the first 2 weeks after discharge from the hospital. Table 1 shows the factors associated with discharge to community care, as contrasted with institutional care, for each of the DRGs that had sufficient numbers of patients going to institutional care to make the comparison valid. No variable was consistently important across all DRGs. None of the clinical measures of severity and comorbidity was statistically related to going home. Patients who went home had less disability on discharge from the hospital and expected to have less disability at 6 weeks. Among stroke patients, however, disability scores were higher for those who were hospitalized (prior hospitalization) than for those who went to institutional care. Stroke patients discharged home were more often white males with a greater awareness of their bodies. Among hip procedure patients, those going home were less likely to live alone and were younger than were those going to institutions. Hip procedure patients in Pittsburgh were less likely to be discharged home than were those in the Twin Cities. Hip fracture patients who went home were less likely to be female and to have discussed their discharge plans. They were also less likely to belong to an HMO. Beyond the role of disability at discharge, the hip fracture patients treated by arthroplasty who went home were distinguished only by being less likely to live alone.
Table 1. Factors Significantly Predicting Place of Discharge Community (Home and Home Care) Versus Institution (Nursing Home and Rehabilitation).
| Factor | Stroke | Hip Procedure | Hip Fracture | Hip Fracture Treated by Arthroplasty | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | |
| Discharge ADLs1 | **0.33 | (0.24,0.45) | **0.41 | (0.24,0.71) | 0.94 | (0.88,1.00) | *0.89 | (0.80,0.99) |
| Expected ADLs | *0.65 | (0.45,0.93) | 1.10 | (0.61,1.98) | *0.92 | (0.86,0.99) | **0.86 | (0.78,0.95) |
| Prior ADLs/IADLs | **1.88 | (1.38,2.55) | 0.67 | (0.34,1.32) | 1.05 | (0.99,1.12) | 1.07 | (0.98,1.16) |
| Patient is Female | *0.48 | (0.25,0.90) | 0.92 | (0.39,2.18) | **0.88 | (0.78,1.00) | 0.90 | (0.75,1.09) |
| Patient Lives Alone | 1.03 | (0.55,1.91) | **0.28 | (0.12,0.64) | 0.95 | (0.86,1.06) | *0.84 | (0.73,0.98) |
| Patient is Other Than White | **4.89 | (1.77,13.53) | 1.40 | (0.24,8.09) | 0.92 | (0.70,1.20) | 0.75 | (0.54,1.06) |
| Age | 1.00 | (0.96,1.04) | *0.92 | (0.86,0.98) | **0.99 | (0.98,1.00) | 1.00 | (0.99,1.01) |
| Touch Test Errors | *0.79 | (0.64,0.97) | 1.06 | (0.74,1.53) | 0.98 | (0.94,1.01) | 0.97 | (0.91,1.03) |
| Did Not Discuss Discharge Plan | 0.79 | (0.41,1.53) | 0.83 | (0.22,3.18) | *1.16 | (1.03,1.30) | 0.92 | (0.75,1.13) |
| Patient Lives in Pittsburgh | 0.60 | (0.29,1.27) | **0.21 | (0.08,0.54) | 0.97 | (0.85,1.10) | 1.04 | (0.85,1.26) |
| Patient is HMO Member | 0.65 | (0.30,1.42) | 0.99 | (0.37,2.66) | *0.88 | (0.77,1.00) | 0.95 | (0.77,1.16) |
| Likelihood | -167.82 | -97.06 | -169.08 | -100.11 | ||||
| Prior Probability (Percent) | 55 | 82 | 68 | 54 | ||||
| Percent Correctly Classified | 80 | 82 | 75 | 72 | ||||
Significance level <.05.
Significance level <.01.
The variables Discharge ADLs, Expected ADLs, and Prior ADLs/IADLs use increments of 1,000 points in calculating the odds ratios and confidence intervals.
NOTES: ADLs is activities of daily living. IADLs is instrumental activities of daily living. CI is confidence intervals. HMO is health maintenance organization.
SOURCE: Kane, R.L., Finch, M., and Chen, Q., University of Minnesota School of Public Health; Blewett, L., Minnesota Department of Health; Bums, R., and Moskowitz, M., Boston University School of Medicine.
The strength of the predictive models can be seen in the rate of correct classifications. Because this method is very sensitive to the a priori likelihood of discharge location, it is important to compare the predictive accuracy with the results if one used the modal value. In all but one case in Table 1, the rate of correct classification is substantially better than what would have been predicted by assuming the modal case pertained for all. For hip procedures, however, there was no improvement over the modal case.
Table 2 looks more specifically at factors that distinguish those who went home with home health care from those who did not receive formal services at home. For stroke patients, getting home health care was associated with having less disability at discharge from the hospital but more disability prior to admission to the hospital. Those receiving home care were less likely to live alone, to be younger, and not to have speech problems but to have hearing problems. Those suffering from COPD who received home health care were less likely to live alone and less likely to be of the white race. They made more errors on the hand-face touching test. They were more likely to come from Houston. Their admission APS were somewhat lower. Among CHF patients, those going home with no formal care had poorer function before the hospitalization and were more likely to live alone. They were also less likely to live in Houston. Hip procedure patients who receive home health care had better discharge functional scores than those who go home without formal care. They tended to be younger, to have received less assistance prior to the hospitalization, and less likely to be from Pittsburgh. The only significant characteristic among hip fracture patients to distinguish those who got home health care and those who did not was coming from Pittsburgh. Likewise for those who had a hip fracture treated by arthroplasty, only admission APS was significant. Here again the predictive models did better than relying on the modal case.
Table 2. Factors Significantly Predicting Place of Discharge: Home Versus Home Care.
| Factor | Stroke | COPD | CHF | Hip Procedure | Hip Fracture | Hip Fracture Treated by Arthroplasty | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | Odds Ratio | (CI) | |
| Discharge ADLs1 | **1.96 | (1.38,2.78) | 1.00 | (0.93,1.08) | 1.20 | (0.91,1.58) | 1.84 | (1.10,3.08) | 0.84 | (0.47,1.53) | 2.26 | (0.91,5.59) |
| Expected ADLs1 | 1.35 | (0.81,2.28) | 1.07 | (0.93,1.23) | 1.06 | (0.64,1.76) | 1.69 | (0.90,3.19) | 1.55 | (0.81,2.95) | 0.83 | (0.33,2.08) |
| Prior ADLs/IADLs1 | **0.59 | (0.40,0.88) | 1.05 | (0.97,1.14) | **1.71 | (1.19,2.47) | 0.84 | (0.41,1.72) | 1.00 | (0.56,1.80) | 0.71 | (0.33,1.51) |
| Patient is Female | 0.72 | (0.36,1.46) | 0.95 | (0.84,1.08) | 1.59 | (0.97,2.60) | 0.90 | (0.40,2.01) | 0.88 | (0.28,2.79) | 2.30 | (0.53,10.01) |
| Patient Lives Alone | *2.58 | (1.24,5.37) | *1.16 | (1.02,1.33) | **1.92 | (1.17,3.16) | 2.03 | (0.86,4.78) | 1.26 | (0.45,3.59) | 2.01 | (0.52,7.82) |
| Patient is Other Than White | 1.79 | (0.74,4.28) | **1.32 | (1.10,1.59) | 1.37 | (0.73,2.56) | 3.55 | (0.66,19.01) | 0.33 | (0.01,9.01) | 0.65 | (0.03,16.62) |
| Age | *1.06 | (1.01,1.12) | 0.99 | (0.98,1.00) | 1.01 | (0.97,1.04) | 1.15 | (1.06,1.24) | 0.99 | (0.92,1.07) | 0.96 | (0.86,1.07) |
| Prior Caregiver Help | — | — | — | — | — | — | 2.52 | (1.06,5.98) | 1.18 | (0.43,3.24) | 2.17 | (0.54,8.68) |
| Touch Test Errors | 0.86 | (0.67,1.11) | *0.93 | (0.88,0.99) | 0.95 | (0.80,1.14) | 1.13 | (0.79,1.61) | 0.81 | (0.55,1.19) | 0.88 | (0.51,1.52) |
| Speech Deficiencies | *2.77 | (1.07,7.17) | 0.74 | (0.55,1.00) | 0.64 | (0.23,1.84) | 2.90 | (0.12,71.55) | 0.28 | (0.03,2.24) | 2.06 | (0.08,55.96) |
| Hearing Deficiencies | *0.35 | (0.15,0.82) | 1.01 | (0.85,1.21) | 1.06 | (0.59,1.92) | 0.55 | (0.13,2.35) | 1.64 | (0.44,6.16) | 0.42 | (0.06,2.92) |
| Did Not Discuss Discharge Plan | 0.60 | (0.30,1.21) | 1.02 | (0.89,1.17) | 0.78 | (0.44,1.38) | 2.90 | (0.78,10.83) | 1.12 | (0.33,3.78) | 0.27 | (0.04,2.00) |
| Mental Status Deficiencies | — | — | — | — | — | — | 1.01 | (0.65,1.55) | 1.27 | (0.94,1.72) | 1.12 | (0.71,1.76) |
| Uses Catheter | — | — | — | — | — | — | 0.70 | (0.25,2.01) | 1.41 | (0.53,3.80) | 1.65 | (0.36,7.59) |
| Prior Health Status | — | — | — | — | — | — | 1.37 | (0.85,2.21) | 0.88 | (0.47,1.65) | 0.69 | (0.30,1.58) |
| Patient Lives in Houston | 0.96 | (0.37,2.45) | **0.72 | (0.58,0.89) | *0.46 | (0.22,0.98) | 1.74 | (0.43,7.05) | 3.28 | (0.87,12.42) | 6.51 | (0.64,66.42) |
| Patient Lives in Pittsburgh | 1.32 | (0.58,3.00) | 0.96 | (0.82,1.13) | 0.77 | (0.42,1.43) | 14.18 | (4.85,41.47) | 5.08 | (1.29,20.01) | 2.52 | (0.52,12.24) |
| Patient is HMO Member | 0.53 | (0.22,1.32) | 0.83 | (0.68,1.02) | 0.63 | (0.30,1.34) | 1.59 | (0.59,4.32) | 1.33 | (0.35,5.05) | 1.29 | (0.20,8.48) |
| Admission APS | 1.98 | (1.76,2.22) | 1.02 | (1.00,1.04) | 0.98 | (0.91,1.05) | 0.92 | (0.70,1.21) | 0.92 | (0.74,1.14) | 0.71 | (0.54,0.93) |
| Comorbidity | — | — | — | — | — | — | 1.11 | (1.01,1.22) | 1.03 | (0.92,1.14) | 1.10 | (0.92,1.30) |
| Likelihood | -125.52 | -148.45 | -223.44 | -95.75 | -62.85 | -40.79 | ||||||
| Prior Probability (Percent) | 50 | 54 | 57 | 66 | 50 | 70 | ||||||
| Percent Correctly Classified | 72 | 68 | 65 | 80 | 70 | 73 | ||||||
Significance level <.05.
Significance level <.01.
The variables Discharge ADLs, Expected ADLs, and Prior ADLs/IADLs use increments of 1,000 points in calculating the odds ratios and confidence intervals.
NOTES: CI is confidence intervals. COPD is chronic obstructive pulmonary disease. CHF is congestive heart failure. ADLs is activities of daily living. IADLs is instrumental activities of daily living. HMO is health maintenance organization. APS is acute physiology score.
SOURCE: Kane, R.L., Finch, M. and Chen, Q., University of Minnesota School of Public Health; Blewett, L., Minnesota Department of Health; Bums, R., and Moskowitz, M., Boston University School of Medicine.
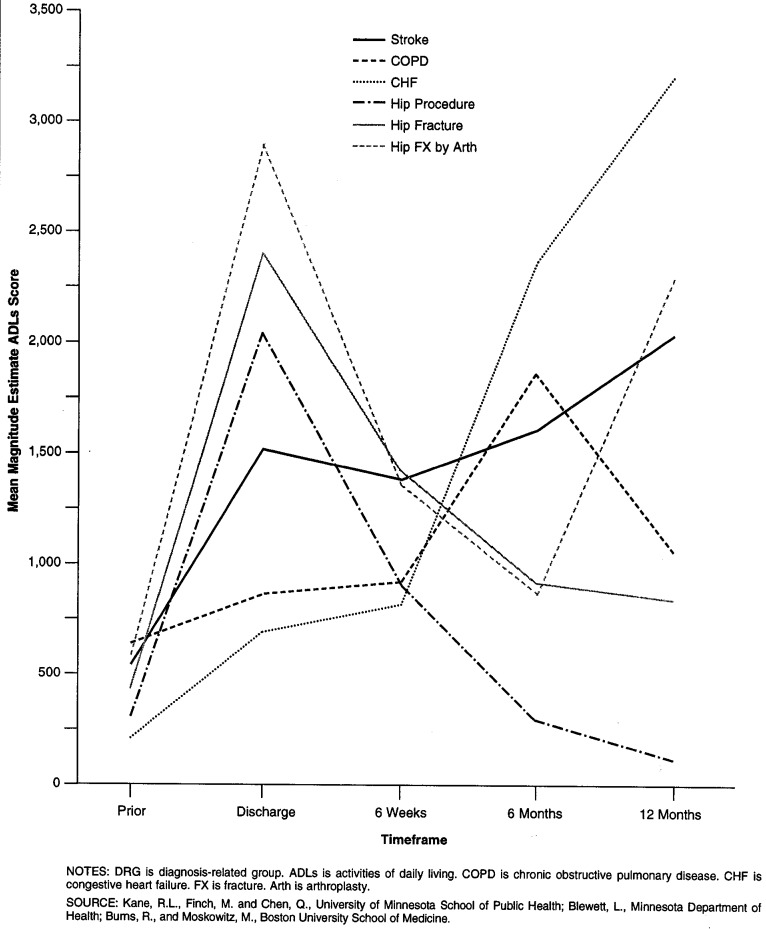
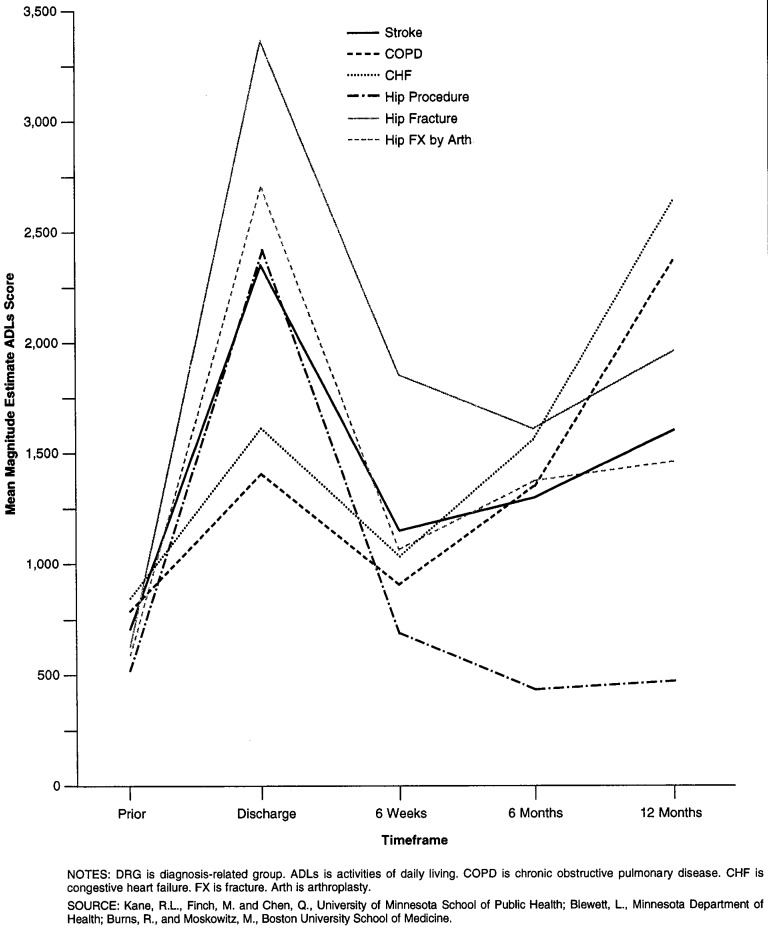
Figures 1 and 2 trace the mean functional status scores for patients discharged home with and without home health care, respectively, in each of the DRGs. The patterns for patients who went home with no formal care varies greatly by the condition examined. Hip procedures and simple hip fractures show a pattern of essentially improvement after discharge. Hip fractures treated with arthroplasty improve until the last 6 months. Stroke, CHF, and COPD patients show an overall pattern of deterioration. In contrast, patients with each of the conditions who received home care show improvement between discharge from the hospital and 6 weeks. That improvement is sustained, however, for those who underwent hip procedures only.
Figure 1. Selection Corrected Mean Functional Dependency Scores for Patients Discharged to Home (Without Home Care), by DRG at Each Time Point.
Figure 2. Selection Corrected Mean Functional Dependency Scores for Patients Discharged to Home Care, by DRG at Each Time Point.
As shown in Table 3, which shows the significant predictors of change in functional status between hospital discharge and 6 weeks followup for patients in each DRG who went home, the models for predicting the outcomes of post-hospital care explain different amounts of the variance, ranging from 19 percent of the adjusted R2 to 73 percent Only one variable is consistently significant across all DRGs. As might be expected, the level of discharge ADL dependency (which makes up part of the definition of the outcome variable) is regularly related to the change score; the greater the disability at the time of discharge, the greater the improvement by 6 weeks. For stroke patients and hip procedure patients, greater disability before the hospitalization is associated with less improvement. Patients who were other than white did less well than white patients. For hip procedure cases, poorer prior health status was associated with more improvement. These patients were also the only ones for whom any of the severity measures showed a significant effect More comorbidity was related to less improvement For hip fracture patients treated with internal fixation, living alone was associated with improved function and poorer mental status with worsening of function. For those treated with arthroplasty, expected disability was correlated with actual increased disability, as was living alone and being older. In two cases, there was evidence that selection bias did occur, i.e., the lambda coefficients for strokes and hip fractures treated with arthroplasty were significant.
Table 3. Predictors for Change in Function for Patients Who Went Home (With or Without Home Care), From Discharge to 6 Weeks Post-Discharge.
| Predictor | Hip Procedure | Hip Fracture | Hip Fracture Treated by Arthroplasty | Stroke | COPD | CHF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Coeff | (CI) | Coeff | (CI) | Coeff | (CI) | Coeff | (CI) | Coeff | (CI) | Coeff | (CI) | |
| Discharge ADLs | **-1.03 | (-1.14,-0.91) | **-0.87 | (-1.20,-0.53) | *-0.52 | (-1.00,-0.04) | **-1.14 | (-1.41,-0.87) | **0.71 | (0.92,0.51) | **0.87 | (1.07,0.68) |
| Expected ADLs | 0.11 | (0.00,0.23) | 0.11 | (-0.35,0.58) | *0.65 | (0.04,1.25) | 0.00 | (-0.28,0.28) | **0.67 | (0.31,1.04) | *0.44 | (0.08,0.79) |
| Prior ADLs/IADLs | *0.19 | (0.04,0.33) | 0.13 | (-0.17,0.44) | 0.07 | (-0.30,0.44) | **0.56 | (0.32,0.80) | *0.25 | (0.04,0.46) | 0.00 | (0.25,0.25) |
| Patient is Female | 134.72 | (-9.07,278.51) | 333.24 | (-268.28,934.76) | 398.19 | (-351.90,1148.28) | -218.49 | (-599.12,162.14) | 152.25 | (488.39,183.89) | **487.63 | (833.96,141.30) |
| Patient Lives Alone | -199.37 | (-404.78,6.04) | *-676.59 | (-1244.01,-109.17) | *905.76 | (117.64,1693.88) | 114.21 | (-244.67,473.09) | 47.25 | (411.81,317.31) | 101.50 | (451.36,248.36) |
| Patient is Other Than White | *377.62 | (50.89,704.35) | -490.18 | (-1961.94,981.58) | 1516.30 | (-206.15,3238.75) | **731.12 | (225.05,1237.19) | 422.65 | (919.90,74.60) | 339.29 | (788.91,110.33) |
| Age | 3.10 | (-12.42,18.62) | 52.23 | (-0.45,104.92) | *56.05 | (12.18,99.91) | -8.14 | (-31.78,15.50) | 21.59 | (49.36,6.19) | 2.18 | (26.74,22.38) |
| Mental Status Deficiencies | -4.97 | (-79.73,69.78) | *116.65 | (8.40,224.90) | 46.33 | (-86.21,178.86) | 65.25 | (-2.23,132.73) | 15.95 | (101.51,133.41) | *109.05 | (12.95,205.15) |
| Prior Health Status | *-91.51 | (-174.28,-8.74) | 24.43 | (-270.75,319.60) | 60.02 | (-302.77,422.82) | 99.04 | (-99.90,297.98) | 111.90 | (304.63,80.83) | 26.45 | (168.45,221.35) |
| Comorbidity | **25.02 | (7.93,42.10) | 34.20 | (-21.76,90.16) | -25.29 | (-106.67,56.09) | -5.08 | (-37.80,27.63) | 0.98 | (31.91,29.95) | 32.24 | (0.69,65.16) |
| Lambda1 | -296.64 | (-905.02,311.74) | 19.35 | (-1483.97,1522.67) | **3,012.30 | (962.14,5062.46) | **-1,348.40 | (-2260.19,-436.61) | ||||
| R-square | 0.75 | 0.44 | 0.56 | 0.43 | 0.26 | 0.29 | ||||||
| Unbiased Adjusted R-square | 0.73 | 0.32 | 0.45 | 0.37 | 0.19 | 0.24 | ||||||
| F-value | **31.62 | **3.02 | **3.65 | **7.12 | **3.39 | **5.79** | ||||||
| P-value | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||
| N | 230 | 104 | 82 | 222 | 258 | 367 | ||||||
Significance level <.05.
Significance level <.01.
The variables Discharge ADLs, Expected ADLs, and Prior ADLs/IADLs use increments of 1,000 points in calculating the odds ratios and confidence intervals.
NOTES: Coeff is coefficient. CI is confidence intervals. COPD is chronic obstructive pulmonary disease. CHF is congestive heart failure. ADLs is activities of daily living. IADLs is instrumental activities of daily living. HMO is health maintenance organization. APS is acute physiology score.
SOURCE: Kane, R.L., Finch, M. and Chen, Q., University of Minnesota School of Public Health; Blewett, L., Minnesota Department of Health; Bums, R., and Moskowitz, M., Boston University School of Medicine.
The results for COPD and CHF are shown separately because they were calculated without the selection-bias adjustment The COPD patients did less well when they were expected to fare poorly and when their status prior to the hospital episode was more disabled. The CHF patients' courses were also related to poorer prognoses. Females did better, whereas those with poor cognitive status did worse.
Table 4 displays the predictors for decreased function between hospital discharge and the 6-week followup separately for those getting home care and those going home without formal care in each DRG. For stroke patients, the significant predictors are quite different for the two groups. For those going home without formal care, the only general variable that is significant is the discharge functional score, but two severity measures are significant: the admission APS and the sickness score. By contrast, among those getting home health care at discharge, expected and prior functional scores are all related to the change in function, as is living in Houston, but none of the severity measures is related to the change in function.
Table 4. Significant Predictors for Decrease in Function, From Discharge to 6 Weeks Post-Discharge.
| Predictor | Home | Home Care | ||
|---|---|---|---|---|
|
|
|
|||
| Coeff | (CI) | Coeff | (CI) | |
| Stroke | ||||
| Discharge ADLs | **-0.78 | (-1.24,-0.32) | **-0.79 | (-1.10,-0.49) |
| Expected ADLs | 0.14 | (-0.47,0.76) | *0.31 | (0.05,0.58) |
| Prior ADLs/IADLs | 0.24 | (-0.25,0.73) | *0.32 | (0.06,0.58) |
| Patient Lives in Houston | 5.01 | (-794.67,804.69) | *701.41 | (109.88,1,292.94) |
| Admission APS | *118.60 | (14.58,222.62) | -27.23 | (-97.08,42.63) |
| Sickness | *-1,144.80 | (-2,264.55,-25.05) | -301.53 | (-983.61,380.55) |
| Lambda1 | -425.79 | (-1,863.45,1,011.87) | -185.05 | (-1,286.37,916.27) |
| R-square | 0.40 | 0.55 | ||
| Unbiased Adjusted R-square | 0.29 | 0.47 | ||
| F-value | 2.82 | 5.29 | ||
| P-value | 0.000 | 0.000 | ||
| N | 110 | 112 | ||
| COPD | ||||
| Discharge ADLs | **-0.62 | (-0.97,-0.26) | **-0.77 | (-0.95,-0.60) |
| Expected ADLs | **1.63 | (0.77,2.49) | **0.43 | (0.18,0.69) |
| Prior ADLs/IADLs | 0.23 | (-0.20,0.65) | *0.19 | (0.02,0.36) |
| Uses Catheter | *-1,145.10 | (-2,184.10,-106.10) | 224.50 | (-229.44,678.44) |
| Lambda1 | 681.89 | (-901.59,2,265.37) | 54.18 | (-592.42,700.79) |
| R-square | 0.30 | 0.54 | ||
| Unbiased Adjusted R-square | 0.20 | 0.46 | ||
| F-value | 2.41 | 5.49 | ||
| P-value | 0.002 | 0.000 | ||
| N | 139 | 119 | ||
| CHF | ||||
| Discharge ADLs | **-0.55 | (-0.96,-0.14) | **-0.84 | (-1.04,-0.64) |
| Expected ADLs | 0.57 | (-0.04,1.18) | **0.41 | (0.13,0.69) |
| Prior ADLs/IADLs | *0.90 | (0.03,1.78) | *0.47 | (0.10,0.84) |
| Patient Lives Alone | *1,237.10 | (140.87,2,333.33) | 198.03 | (-356.26,752.32) |
| Mental Status Deficiencies | *175.21 | (15.00,335.42) | 16.79 | (-48.98,82.57) |
| Uses Catheter | *1,257.60 | (306.80,2,208.40) | 131.68 | (-226.41,489.77) |
| Patient is HMO Member | *-1,380.50 | (-2,465.95,-295.05) | -150.46 | (-653.59,352.67) |
| Comorbidity | *62.07 | (2.39,121.76) | -4.94 | (-28.85,18.97) |
| Lambda1 | *5,081.80 | (818.80,9,344.80) | -1,133.30 | (-2,924.94,658.34) |
| R-square | 0.23 | 0.61 | ||
| Unbiased Adjusted R-square | 0.16 | 0.55 | ||
| F-value | 2.72 | 9.96 | ||
| P-value | 0.000 | 0.000 | ||
| N | 209 | 158 | ||
| Hip Procedure | ||||
| Discharge ADLs | **-1.04 | (-1.17,-0.90) | **-1.01 | (-1.18,-0.84) |
| Prior ADLs/IADLs | *0.21 | (0.03,0.40) | *0.27 | (0.06,0.48) |
| Patient is Other Than White | 11.27 | (-586.53,609.07) | *521.43 | (112.18,930.68) |
| Lambda1 | -502.08 | (-1,075.77,71.61) | -13.35 | (-459.45,432.75) |
| R-square | 0.71 | 0.86 | ||
| Unbiased Adjusted R-square | 0.68 | 0.82 | ||
| F-value | 18.04 | 17.44 | ||
| P-value | 0.000 | 0.000 | ||
| N | 151 | 79 | ||
| Hip Fracture | ||||
| Discharge ADLs | **-0.89 | (-1.47,-0.31) | -0.95 | (-1.25,-0.65) |
| Age | **99.11 | (44.92,153.31) | -28.64 | (-78.56,21.29) |
| Lambda1 | -1371.60 | (-3931.36,1188.16) | 289.47 | (-1308.91,1887.85) |
| R-square | 0.60 | 0.71 | ||
| Unbiased Adjusted R-square | 0.44 | 0.59 | ||
| F-value | 2.19 | 3.43 | ||
| P-value | 0.02 | 0.001 | ||
| N | 52 | 52 | ||
| Hip Fracture Treated by Arthroplasty | ||||
| Discharge ADLs | 0.44 | (-3.26,4.13) | **-1.27 | (-1.61,-0.93) |
| Prior ADLs/IADLs | 0.88 | (-0.09,1.86) | *0.62 | (0.34,0.91) |
| Mental Status Deficiencies | *-296.66 | (-476.20,-117.12) | 97.81 | (-24.30,219.91) |
| Prior Health Status | **1,955.00 | (1,375.23,2,534.77) | -138.25 | (-472.63,196.13) |
| Admission APS | *717.84 | (324.66,1,111.02) | 69.98 | (-76.47,216.43) |
| Lambda1 | 459.32 | (-5093.36,6,012.00) | 763.49 | (-675.74,2,202.72) |
| R-square | 0.99 | 0.76 | ||
| Unbiased Adjusted R-square | 0.99 | 0.67 | ||
| F-value | 2.01 | 5.32 | ||
| P-value | 0.008 | 0.000 | ||
| N | 25 | 57 | ||
Significance level <.05.
Significance level <.01.
Lambda represents the selection coefficient.
NOTES: Coeff is coefficient. CI is confidence intervals. ADLs is activities of daily living. IADLs is instrumental activities of daily living. COPD is chronic obstructive pulmonary disease. CHF is congestive heart failure.
SOURCE: Kane, R.L., Finch, M., and Chen, Q., University of Minnesota School of Public Health; Blewett, L., Minnesota Department of Health; Burns, R., and Moskowitz, M., Boston University School of Medicine.
For COPD patients, the significant predictors are similar for both those who did and did not receive home care. Discharge disability was associated with improved function at 6 weeks, and more expected disability proved to be an accurate prophecy. In addition, among those who went home with no formal care, being catheterized on discharge from the hospital was related to an improved outcome; whereas for those who did receive home care, more disability prior to the hospitalization was associated with a decline in function at 6 weeks.
For CHF patients who went home without formal care, better function at discharge, more disability prior to the hospital episode, poorer mental functioning, using a catheter, not being an HMO member, and having more comorbidities were each associated with a decline in function at 6 weeks. For those getting home care, the same pattern of prior and discharge functioning applied as well as a role for expected decline.
Hip procedure patients also showed a similar influence from discharge and prior functioning. In addition, for those receiving home care, white patients were more likely to improve by 6 weeks. Few variables were significant for the hip fracture patients. Among the internally reduced patients, there were no significant predictors for those who got home care. For those who did not get home care, better function at discharge was related to improved function at 6 weeks, whereas older patients were more likely to decline. For those treated with arthroplasty who went home with no formal care, cognitive impairment, worse prior health status, and a poorer admission APS were associated with improved function at 6 weeks, whereas for those who got home care more disability at discharge and less prior to admission were associated with improvement.
The Medicare Parts A and B costs for the year prior to and after (but not including) the hospitalization are shown in Table 5 for patients who went home with and without home care and those who went to institutions. The Medicare payments in the year prior to the hospitalization are quite similar with each condition among the those discharge home without formal care, those who received home health care, and those who went to institutions. The exception to this rule is found with the CHF patients, where those who received home health care had substantially higher costs in the year before hospitalization, and those who went home with no formal care had higher costs for the prior year than those who went to institutions. In every instance, the costs in the year after hospitalization for those receiving home health care are intermediate between those for patients discharged home with no formal care and those sent to institutions. In three instances (CHF and both types of hip fracture), however, the costs for the home health group in the year after hospitalization are not significantly higher than those for the year prior.
Table 5. Median Medicare Expenditures (per Patient per Year) During the Year Prior To and After the Study Hospitalization1.
| DRG | Place of Discharge | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Home | Home Care | Nursing Home/Rehabilitation | ||||
|
|
|
|
||||
| Prior | After | Prior | After | Prior | After | |
| Stroke | $1,256 | **$3,801 | $1,126 | **$6,178 | $1,359 | **$13,600 |
| COPD | $3,786 | $5,415 | $4,992 | **$11,784 | — | — |
| CHF | $3,946 | *$6,119 | $6,240 | $8,747 | — | — |
| Hip Procedure | $2,188 | **$93 | $3,085 | *$1,405 | $3,234 | **$6,722 |
| Hip Fracture | $1,747 | $2,134 | $2,559 | $3,294 | $1,960 | **$6,964 |
| Hip Fracture Treated by Arthroplasty | $2,257 | $3,456 | $2,590 | $4,560 | $2,106 | **$9,431 |
Significance level < .05 compared with prior using Wilcoxon's Rank test.
Significance level < .01 compared with prior using Wilcoxon's Rank test.
Does not include the cost of the hospitalization itself.
NOTES: DRG is diagnosis-related group. COPD is chronic obstructive pulmonary disease. CHF is congestive heart failure.
SOURCE: Kane, R.L., Finch, M., and Chen, Q., University of Minnesota School of Public Health; Blewett, L., Minnesota Department of Health; Bums, R., and Moskowitz, M., Boston University School of Medicine.
Discussion
This study was deliberately designed to follow a cohort of patients prospectively. It used information available to discharge planners to model not only the factors associated with the post-discharge location decisions but also the functional outcomes associated with them.
The findings here suggest that certain patient characteristics are associated with patients being discharged home and even getting home health care. Likewise, it is possible to predict with substantial power which patient characteristics are associated with a change in functional status between hospital discharge and followup 6 weeks later. However, the predictive variables are, for the most part, different with each DRG studied. Moreover, the variables associated with a discharge home are not necessarily the same ones that predict a better outcome for such patients.
This discrepancy between the factors that are related to post-hospital discharge location and those related to functional improvement, together with the modest ability to correctly identify those patients discharged home with and without formal care, can be interpreted as indicating that discharge decisions are not being made solely on the basis of patient characteristics and are not being made consistently. Although it makes sense to assume that other factors influence hospital discharge decisions, more work is needed to understand this process better, especially in the context of the pressures under PPS to discharge patients from the hospital as quickly as possible.
In this regard, the factors that are not significantly associated with discharge decisions may be more informative than those that are. For example, living alone was significant only with hip patients, and receipt of informal care prior to hospitalization was not a significant factor in any case. Although one might assume that impaired cognitive status would be a deterrent to discharge home, it too was not significant. The clinical measures of severity and comorbidity generally proved unhelpful.
The findings from this study may help to explain the inconsistent results of others. Determining the need for home health care is not a simple process. The relevant factors to be considered appear to vary with the patient's underlying medical problem as well as with the circumstances of the case. To the extent that the discharge planning process does not depend on simply a few consistent factors can be interpreted as implying it is more of an art than a science, or at least that up until now, there has not been a wealth of information on which to base prudent decisions. Discharge planners have had to rely on clinical intuition and basic problem-solving skills for guidance.
In most cases, it is more complicated to discharge home a patient who needs post-hospital care. Coordinated arrangements must be made with family and formal service providers. It is often easier to organize a transfer to a nursing home. When time pressures are felt, the easier path may be the one taken.
The outcomes relationships presented here imply that identifying those patients who are most likely to benefit from home health care will not be accomplished by looking at only a few selected variables. For example, the predictive variables differ by DRG. Appropriately identifying those most likely to benefit will therefore involve the use of more complex algorithms. It is possible to imagine constructing algorithms that employ data like those noted here and using computer technology to assist discharge planners in selecting the best modality of post-hospital care to maximize the speed and extent of recovery.
This study did not examine the nature of the home health care provided. Nothing is known about the participation of physicians or the intensity of primary care. Likewise nothing is known about what level of personnel provided the services, although there is no reason to believe the care covered in this study differs from what is generally provided.
Likewise, this study addresses only that portion of home health care that emanates from hospital discharges. There is at least as much growth in Medicare-financed home health care for patients coming directly from the community. Here too, there is a need for better information on what sorts of factors can be used to predict those who will benefit most from such care. Although there should be considerable overlap with the findings from this study, the situations are not entirely comparable and, hence, different factors may play a greater role in a community admission.
This is effectively an epidemiological analysis of extant efforts. It does not directly test an intervention designed to demonstrate how such care could be best delivered or even who would most benefit from the care. Such demonstrations are needed, but more attention needs to be paid to finding ways to make existing home care efforts more effective. If home care is to become a major modality for providing needed post-hospital care, much more can be done to strengthen home care programs, including better integration with medical efforts and better coordination with hospital care.
Acknowledgments
This work was conducted with the support of the Health Care Financing Administration (HCFA) and the Office of the Assistant Secretary for Planning and Evaluation of the Department of Health and Human Services under Cooperative Agreement Number 17-C-98891 with the Institute for Health Services Research, University of Minnesota School of Public Health. Robert L Kane, Michael Finch, and Qing Chen are with the Institute for Health Services Research, University of Minnesota School of Public Health; Lynn Blewett is with the Minnesota Department of Health; and Risa Burns and Mark Moskowitz are with the Boston University School of Medicine. The opinions expressed are those of the authors and do not necessarily reflect the views or policy positions of HCFA, the Institute for Health Services Research, the Minnesota Department of Health, the Boston University School of Medicine, or their sponsors.
Footnotes
Reprint Requests: Robert L. Kane, M.D., Institute for Health Services Research, University of Minnesota School of Public Health, 420 Delaware Street, SE., Mayo Box 197, Minneapolis, Minnesota 55455.
References
- Andersen R, Newman J. Societal and Individual Determinants of Medical Care Utilization in the United States. Milbank Quarterly. 1973;51:95–124. [PubMed] [Google Scholar]
- Bass DM, Looman WJ, Ehrlich P. Predicting the Volume of Health and Social Services: Integrating Cognitive Impairment Into the Modified Andersen Framework. The Gerontologist. 1992;32(1):33–43. doi: 10.1093/geront/32.1.33. [DOI] [PubMed] [Google Scholar]
- Bass DM, Noelker LS. The Influence of Family Caregivers on Elder's Use of In-Home Services: An Expanded Conceptual Framework. Journal of Health and Social Behavior. 1987;28(2):184–196. [PubMed] [Google Scholar]
- Benjamin AE, Fox PJ, Swan JH. The Posthospital Experience of Elderly Medicare Home Health Users. Home Health Care Services Quarterly. 1993;14(2/3):19–35. doi: 10.1300/j027v14n02_03. [DOI] [PubMed] [Google Scholar]
- Burns RB, Moskowitz MA, Ash A. Self-Report Versus Medical Record Functional Status. Medical Care. 1992;30:89–95. doi: 10.1097/00005650-199205001-00008. [DOI] [PubMed] [Google Scholar]
- Coulton CJ, Dunkle RE, Chun-Chun J, et al. Dimensions of Post-Hospital Care Decision-Making: A Factor Analytic Study. The Gerontologist. 1988;28(2):218–223. doi: 10.1093/geront/28.2.218. [DOI] [PubMed] [Google Scholar]
- Cummings JE, Weaver FM. Cost-Effectiveness of Home Care. Clinics in Geriatric Medicine. 1991;7(4):865–874. [PubMed] [Google Scholar]
- Duke University Center for the Study of Aging and Human Development. Multidimensional Functional Assessment: The OARS Methodology. Duke University; Durham, NC.: 1978. [Google Scholar]
- Finch M, Kane RL, Philp I. Developing a New Metric for ADLs. Appendix A from A Study of Post-Acute Care: Final Report. 1994 May; Prepared for the Health Care Financing Administration. University of Minnesota School of Public Health. [Google Scholar]
- Fink M, Green M, Bender MB. The Face-Hand Test as a Diagnostic Sign of Organic Mental Syndrome. Neurology. 1952;2:46–59. doi: 10.1212/wnl.2.1-2.46. [DOI] [PubMed] [Google Scholar]
- Garrard J, Kane RL, Radosevich DM, Skay CL. Impact of Geriatric Nurse Practitioners on Nursing-Home Residents' Functional Status, Satisfaction, and Discharge Outcomes. Medical Care. 1990;28(3):271–283. doi: 10.1097/00005650-199003000-00007. [DOI] [PubMed] [Google Scholar]
- Gornick M, Hall MJ. Trends in Medicare Utilization of SNFs, HHAs, and Rehabilitation Hospitals. Health Care Financing Review. 1988;(1988 Annual Supplement):27–38. [PMC free article] [PubMed] [Google Scholar]
- Greene VL, Lovely ME, Ondrich JI. The Cost Effectiveness of Community Services in a Frail Elderly Population. The Gerontologist. 1993;33(2):177–89. doi: 10.1093/geront/33.2.177. [DOI] [PubMed] [Google Scholar]
- Heckman J. Sample Selection Bias as a Specification Error. Econometrica. 1979;47:153–61. [Google Scholar]
- Hedrick SC, Inui TS. The Effectiveness and Cost of Home Care: An Information Synthesis. Health Services Research. 1986;20:851–880. [PMC free article] [PubMed] [Google Scholar]
- Helbing C, Sangl JA, Silverman HA. Home Health Agency Benefits. Health Care Financing Review. 1992;(1992 Annual Supplement):125–148. [PubMed] [Google Scholar]
- Iezzoni LI, Moskowitz MA. A Clinical Assessment of Medisgroups. Journal of the American Medical Association. 1988;260:3159–3163. doi: 10.1001/jama.260.21.3159. [DOI] [PubMed] [Google Scholar]
- Jones EW, Densen PM, Brown SD. Posthospital Needs of Elderly People at Home: Findings From an Eight-Month Follow-Up Study. Health Services Research. 1989;24(5):642–664. [PMC free article] [PubMed] [Google Scholar]
- Kane RL. A Study of Post-Acute Care: Final Report. University of Minnesota; 1994. [Google Scholar]
- Kane RL, Bell R, Riegler S, et al. Assessing the Outcomes of Nursing Home Patients. Journal of Gerontology. 1983;38:385–393. doi: 10.1093/geronj/38.4.385. [DOI] [PubMed] [Google Scholar]
- Kenney GM. Understanding the Effects of PPS on Medicare Home Health Use. Inquiry. 1991;28(2):129–139. [PubMed] [Google Scholar]
- Kenney GM. How Access to Long-Term Care Affects Home Health Transfers. Journal of Health Politics, Policy and Law. 1993b;18(14):937–965. doi: 10.1215/03616878-18-4-937. [DOI] [PubMed] [Google Scholar]
- Kenney GM. Rural and Urban Differentials in Medicare Home Health Use. Health Care Financing Review. 1993a;14(4):39–57. [PMC free article] [PubMed] [Google Scholar]
- Kenney GM, Dubay LC. Explaining Area Variation in the Use of Medicare Home Health Services. Medical Care. 1992;30(1):43–57. doi: 10.1097/00005650-199201000-00004. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP. APACHE II: A Severity of Disease Classification System. Critical Care Medicine. 1985;13:818–829. [PubMed] [Google Scholar]
- Kosecoff J, Kahn KL, Rogers WH, et al. Prospective Payment System and Impairment at Discharge. “The-Quicker-and-Sicker” Story Revisited. Journal of the American Medical Association. 1990;264(15):1980–1983. [PubMed] [Google Scholar]
- Kuriansky JB, Gurland B. Performance Test of Activities of Daily Living. International Journal of Aging and Human Development. 1976;7:343–352. doi: 10.2190/x45l-tww7-wxxy-ka6k. [DOI] [PubMed] [Google Scholar]
- Letsch SW, Lazenby HC, Levit KR, Cowan CA. National Health Expenditures, 1991. Health Care Financing Review. 1992;14(2):1–30. [PMC free article] [PubMed] [Google Scholar]
- Meiner MR, Coffey RM. Hospital DRGs and the Need for Long-Term Care Services: An Empirical Analysis. Health Services Research. 1985;20:359–384. [PMC free article] [PubMed] [Google Scholar]
- Morrisey MA, Sloan FA, Valvona J. Medicare Prospective Payment and Posthospital Transfers to Subacute Care. Medical Care. 1988;26(7):685–698. doi: 10.1097/00005650-198807000-00004. [DOI] [PubMed] [Google Scholar]
- Neu CR, Harrison S, Heilbrunn JZ. Medicare Patients and Postacute Care: Who Goes Where? The Rand Corporation; Santa Monica, CA.: 1989. [Google Scholar]
- Pfeiffer E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. Journal of the American Geriatrics Society. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Soldo BJ. In-Home Services for the Dependent Elderly: Determinants of Current Use and Implications for Future Demand. Research on Aging. 1985;7(2):281–304. doi: 10.1177/0164027585007002007. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Wagner DR, Marenberg ME, Acampora D. Predictors of Formal Home Health Care Use in Elderly Patients After Hospitalization. Journal of the American Geriatrics Society. 1993;41(9):961–966. doi: 10.1111/j.1532-5415.1993.tb06762.x. [DOI] [PubMed] [Google Scholar]
- Starrett RA, Rogers D, Walters G. Home Health Care Utilization: A Causal Model. Home Health Care Services Quarterly. 1988;9(4):125–140. doi: 10.1300/j027v09n04_06. [DOI] [PubMed] [Google Scholar]
- Swan JH, Benjamin AE. Medicare Home Health Utilization as a Function of Nursing Home Market Factors. Health Services Research. 1990;25:479–500. [PMC free article] [PubMed] [Google Scholar]
- Thornton C, Miller-Dunston S, Kemper P. The Effect of Channeling on Health and Long-Term Care Costs. Health Services Research. 1988;23(1):129–142. [PMC free article] [PubMed] [Google Scholar]
- Vladeck BC. Unloving Care: The Nursing Home Tragedy. New York: Basic Books; 1980. [Google Scholar]