Abstract
Background
Cystic fibrosis (CF) is a multisystem disorder characterised by mutations of the CFTR gene, which encodes for an important component in the coordination of electrolyte movement across of epithelial cell membranes. Symptoms are pulmonary disease, pancreatic exocrine insufficiency, male infertility and elevated sweat concentrations. The CFTR gene has numerous mutations (>1000) and functionally important polymorphisms (>200). Early identification is important to provide appropriate therapeutic interventions, prognostic and genetic counselling and to ensure access to specialised medical services. However, molecular diagnosis by direct mutation screening has proved difficult in certain ethnic groups due to allelic heterogeneity and variable frequency of causative mutations.
Methods
We applied a gene scanning approach using DHPLC system for analysing specifically all CFTR exons and characterise sequence variations in a subgroup of CF Italian patients from the Lazio region (Central Italy) characterised by an extensive allelic heterogeneity.
Results
We have identified a total of 36 different mutations representing 88% of the CF chromosomes. Among these are two novel CFTR mutations, including one missense (H199R) and one microdeletion (4167delCTAAGCC).
Conclusion
Using this approach, we were able to increase our standard power rate of mutation detection of about 11% (77% vs. 88%).
Keywords: Cystic fibrosis, CFTR mutation screening, DHPLC
Background
Cystic fibrosis (OMIM #219700) is one of the most common genetic diseases in Caucasians with a frequency of 1/3500 [1,2]. Affected children have two mutations in the CFTR gene (CFTR/ABCC7, OMIM #602421), a gene that contains 27 exons encompassing approximately 180 kb of DNA on chromosome 7q31.2. CFTR protein is a Cl- channel, which regulates ion flow across the apical membrane of airway, gastrointestinal and reproductive epithelia, and of sweat glands and pancreatic ducts [3-6]. Over 1000 mutations have been described in the Cystic Fibrosis Mutation Consortium [7], mutations that are clustered in six different classes including defective CFTR biosynthesis, defective protein processing, alteration in CFTR regulation, disruption of the pore activity, alteration of CFTR localisation, and genesis of unstable CFTR [8]. The most common mutation is a deletion of three nucleotides (1652delCTT) that leads to a loss of a phenylalanine residue at position 508 (delF508) of the gene product. This is responsible for approximately two thirds of all CF chromosomes, with a clear Northwest to Southeast gradient in its frequency in the human population across Europe [9]. There is a core of 25 "less common" mutations designated by the CF Steering Committee in 2001 that occur with a European frequency of 0.1% or greater. The remainder of the mutations are termed "rare", being found in only one or few individuals [9]. The spectrum of CFTR-associated phenotypes is quite variable, going from classic CF to mild monosymptomatic presentations, like idiopathic pancreatitis, chronic rhinosinusitis, nasal polyposis, asthma, disseminated bronchiectasis and congenital bilateral absence of the vas deferens (CBAVD) [10]. Some mutations are clearly associated with a mild phenotype (with pancreatic insufficiency and a life expectancy over 50 years) [8]. Other attempts to link mutations in CFTR to disease severity have not been successful, suggesting an influence of non-CFTR gene modifiers and environmental factors [11].
CF diagnosis is based on defined phenotypic criteria, on CF history in the family and/or a positive test for hypertrypsinaemia (IRT) in the neonatal period. In the majority of cases, the diagnosis of CF is confirmed by elevated (>60 nmol/l) sweat chloride concentrations, and by a raised electrical potential difference (NPD) across the nasal epithelium. Molecular diagnosis is based on CFTR mutation screening. However, extensive allelic heterogeneity frequently impairs the rate of detection and therefore the value of the genotypic diagnosis. Several mutation scanning methods have been applied to the detection of sequence variations in the entire coding region of CFTR such as heteroduplex analysis and restriction enzyme analysis [12], single-strand conformation polymorphism analysis (SSCP) [13], and the DGGE method [14,15]. Recently, two different groups have used the DHPLC technology to detect CF alleles [16-18]. We analysed a cohort of CF patients clinically defined from Central Italy (an area characterized by an high allelic heterogeneity) [9] for the presence of CFTR mutations by using this technique to optimize mutation detection. The results presented here not only provide a comprehensive spectrum of the molecular basis of CF in the Central Italy, but also underscore the need for multi-approach screening of CFTR gene in heterogeneous populations to reach appreciable levels of detection rates.
Methods
Patients
All CF patients came from the population of children born in Central Italy (diagnosis performed using standard clinical criteria at Ospedale Bambino Gesù, Roma). Samples were included for DHPLC screening on the basis of presentation of family history and/or abnormal sweat tests. All 290 samples were analysed for the 29 common mutations by INNO-LiPA CFTR12 and CFTR17+Tn assays (Innogenetics NV, Zwijndrecht, Belgium) as part of the routine testing service provided at the centre. During the course of the study, 117 samples were shown to have only one or none of the 29 common mutations tested and were selected for further investigation. DNA from healthy subjects was randomly chosen as controls. Their DNA was sequenced and the entire CFTR gene sequence was confirmed to be wild-type with respect to CFTR. Genomic DNA was isolated from peripheral blood leukocytes using a salting-out protocol.
DNA amplification
Coding regions and intron/exon boundaries of the CFTR gene were amplified in 29 reactions using primers described by Le Marechal et al. [17]. A novel primer design was only required for exons 6b and 9 to improve the PCR sensitivity. Fifty to 100 ng of genomic DNA from different patients were used for the PCR reaction. Amplification was performed in a 25 μl reaction volume containing 0.2 mM each of primers, 200 μM dNTPs, 1X reaction buffer (10 mM Tris HCl pH 8.3, 50 mM KCl, 2.5 mM MgCl2) and 1.25 U AmpliTaq Gold™ DNA polymerase (PE Applied Biosystems, Foster City, CA) by a Gene Amp PCR System 9700 (PE Applied Biosystems, Foster City, CA). PCR amplification was carried out at 94°C for 10 min and then cycled 35 times at 94°C for 40 sec, 57°C or 61°C for 40 sec and 72°C for 1 min, followed by 7 min at 72°C in the final cycle. Amplicons were checked by agarose gel electrophoresis before DHPLC analysis, to make sure that only the specific product was amplified.
DHPLC analysis
DHPLC analysis was carried out on a WAVE DNA fragment analysis system (Transgenomic™, Crewe, UK) equipped with a DNASep® Column (Transgenomic™, Crewe, UK). PCR products was denatured for 5 min at 95°C before being gradually reannealed by decreasing sample temperature from 95 to 65°C over a period of 30 min to enable the formation of heteroduplexes. DHPLC analysis was carried out at a flow-rate of 0.9 ml/min and buffer B (0.1 M TEAA, 25% acetonitrile), with a gradient increase of 2% per min for 4 min. The start and end concentration of buffer B and oven temperature for optimal heteroduplex separation have previously been described [17].
Data analysis
Four wild-type samples were always used as negative controls, to ensure that a normal homoduplex profile was reproducibly obtained with regard to retention time and peak profile. Chromatograms were overlaid with one from a wild-type. Samples with extra peaks (one, two or three more), or with a difference in peak appearance, were scored as positive.
Sequence analysis
Samples showing abnormal elution profile were reamplified from genomic DNA. PCR products were purified with GeneDia Seq-Prep Kit and were sequenced in both directions using CEQ™ 2000 (Beckman Coulter Inc. USA).
Results
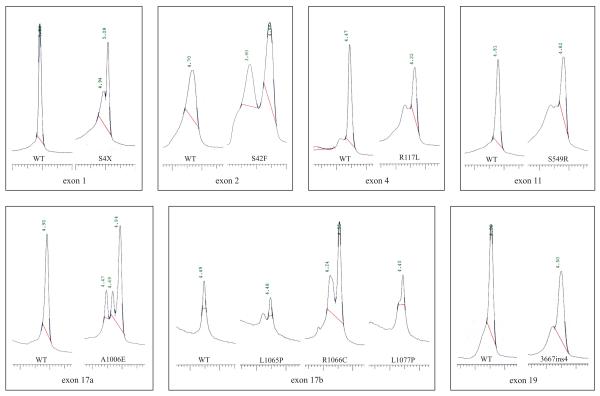
CF patients were firstly screened using a standard panel of 29 different CF mutations (INNO-LiPA CFTR12 and CFTR17+Tn). Of these, 173 were homozygous or compound heterozygous (+/+), 105 were positives for only one mutations (+/-) and 12 were negative for both mutations (-/-). The unknown alleles were screened by DHPLC technology. In order to set-up elution conditions and profiles of known mutations not included in the INNO-LiPA kits, we ran amplicons obtained from DNAs carrying 10 rare CFTR mutations previously found and identified by sequencing in compound heterozygote CF patients from an area of Central Italy [19]. These mutations included S4X (143 C to A), exon 1; S42F (257 C to T), exon 2; R117L (482 G to T), exon 4; S549R (1779 T to G), exon 11; 3667ins4, exon 19; A1006E (3149 C to A), exon17a; L1065P (3326 T to C), R1066C (3328 C to T), L1077P (3362 T to C), exon 17b. DHPLC profiles are shown in Figure 1. In contrast to the normal control that shows a single peak of homoduplex DNA, all samples with previously identified mutations produced chromatograms of a different shape. New primers were synthesised only for exons 6b and 9, and they annealed to the coding sequence (Table 1). DHPLC conditions were set up according to WAVEmaker software (Transgenomic) and the DHPLC Melt program (Table 1) [20]. The complete spectrum of mutations detected in the sample is reported in Table 2. A total of 36 different mutations, including 22 missense, 4 nonsense, 6 micro insertion/deletion and 4 splicing mutations were detected. Only six mutations have a frequency >1%, which account for 77.5 % of the total CF chromosomes. Nineteen mutations have frequencies between 0.9% and 0.4%, and cover 8.36% of the CF chromosomes. Finally, 11 mutations were found only once in the population, increasing the proportion of the characterised CF alleles by 1.87% (data not shown). Among these mutations, we have identified and characterised in the Italian population two novel mutations in two affected children: H199R and 4167delCTAAGCC. An abnormal DHPLC pattern in exon 6a due to the nucleotide change A to G at position 728 of CFTR determines the missense mutation H199R that falls into the transmembrane domain, TM1. This CF mutation was identified in a patient who carries the N1303K (4041 C to G) mutation on the maternal CF chromosome. H199R mutation, to our knowledge, has been detected in a single CF chromosome in a population in Brittany [7]. The other two mutations that affect the same codon are described: H199Y (727 C to T), found in a German CF patient heterozygous for delF508 and who exhibits only mild pancreatic symptoms and borderline sweat chloride values, and H199Q (729 T to G). The microdeletion 4167delCTAAGCC in exon 22 was identified by DHPLC analysis in a CF patient who presents the L1065P (3326 T to C) mutation on the maternal chromosome. This mutation leads to a premature stop codon, resulting in the deletion of 90 carboxyterminal amino acid residues. Consequently, the NBF2 intracellular domain, which constitutes a CFTR ATP binding domain, is lost. These two novel mutations were not found in a group of 100 healthy controls. Furthermore, in both cases, no other alterations were observed after the complete screening of all the coding and flanking regions of CFTR gene. DHPLC analysis also revealed 8 DNA sequence variations (Table 3). In some cases these variations co-segregated with pathogenic CF mutations within the same fragment, resulting in an altered DHPLC profile. Finally, in a single CF patient, we detected two different mutations [G542X (1756 G to T) and S549R (1779 T to G)] in the same exon 11-derived DNA amplicon, which showed a peculiar DHPLC pattern, different from that observed when only one of these mutations was present (data not show).
Figure 1.
DHPLC chromatograms corresponding to nine different CFTR mutations from heterozygous patients compared to a normal sample (WT). The profiles of the mutants show extra peaks or a shoulder and are easily distinguished from profiles of the wild type, which shows a single peak.
Table 1.
Primers and DHPLC (oven temperature, gradient) analysis conditions for 6b and 9 exons of the CFTR gene
| exon | Primer 5' → 3' | Amplicon length | Oven temp (°C) | % B buffer start/end |
| 6b | F – CAGAGATCAGAGAGCTGGG | 323 | 56 | 55/63 |
| R – GAGGTGGAAGTCTACCATGA | ||||
| 9 | F – GGGATTTGGGGAATTATTTG | 279 | 55 | 54/62 |
| R – TCTCCAAAAATACCTTCCAG |
Table 2.
CF mutations identified in cohort of 290 patients from the Central Italy
| Mutation | Nucleotide change | Exon/intron | N | % | Method |
| delF508 | 1652delCTT | 10 | 328 | 56.36 | INNO-LiPA, DHPLC |
| N1303K | 4041 C to G | 21 | 51 | 8.76 | INNO-LiPA, DHPLC |
| G542X | 1756 G to T | 11 | 42 | 7.21 | INNO-LiPA, DHPLC |
| W1282X | 3978 G to A | 20 | 15 | 2.60 | INNO-LiPA, DHPLC |
| S549R | 1779 T to G | 11 | 8 | 1.37 | DHPLC |
| 621+1G-T | 621+1 G to T | Intron 4 | 7 | 1.20 | INNO-LiPA, DHPLC |
| 1717-1G-A | 1717-1 G to A | Intron 10 | 5 | 0.86 | INNO-LiPA, DHPLC |
| G85E | 386 G to A | 3 | 4 | 0.69 | INNO-LiPA, DHPLC |
| R553X | 1789 C to T | 11 | 4 | 0.69 | INNO-LiPA, DHPLC |
| H139R | 548 A to G | 6a | 3 | 0.51 | DHPLC |
| R347P | 1172 G to C | 7 | 3 | 0.51 | INNO-LiPA, DHPLC |
| L1065P | 3326 T to C | 17b | 3 | 0.51 | DHPLC |
| L1077P | 3362 T to C | 17b | 3 | 0.51 | DHPLC |
| S4X | 143 C to A | 1 | 2 | 0.34 | DHPLC |
| D110H | 460 G to C | 4 | 2 | 0.34 | DHPLC |
| R334W | 1132 C to T | 7 | 2 | 0.34 | INNO-LiPA, DHPLC |
| M348K | 1175 T to A | 7 | 2 | 0.34 | DHPLC |
| 1259insA | 1259 ins A | 8 | 2 | 0.34 | DHPLC |
| S549N | 1778 G to A | 11 | 2 | 0.34 | DHPLC |
| L558S | 1805 T to C | 11 | 2 | 0.34 | DHPLC |
| 2183+AA-G | 2183 A to G and 2184 del A | 13 | 2 | 0.34 | INNO-LiPA, DHPLC |
| 2789+5G-A | 2789+5 G to A | Intron 14b | 2 | 0.34 | INNO-LiPA, DHPLC |
| R1066C | 3328 C to T | 17b | 2 | 0.34 | DHPLC |
| 3667ins4 | 3667insTCAA | 19 | 2 | 0.34 | DHPLC |
| S42F | 257 C to T | 2 | 2 | 0.34 | DHPLC |
| R117L | 482 G to T | 4 | 1 | 0.17 | DHPLC |
| H199R | 728 A to G | 6a | 1 | 0.17 | DHPLC |
| R334L | 1133 G to T | 7 | 1 | 0.17 | DHPLC |
| T338I | 1145 C to T | 7 | 1 | 0.17 | DHPLC |
| G551D | 1784 G to A | 11 | 1 | 0.17 | INNO-LiPA, DHPLC |
| Q552X | 1786 C to T | 11 | 1 | 0.17 | INNO-LiPA, DHPLC |
| D614G | 1973 A to G | 13 | 1 | 0.17 | DHPLC |
| A1006E | 3149 C to A | 17a | 1 | 0.17 | DHPLC |
| 4016insT | 4016 ins T | 21 | 1 | 0.17 | DHPLC |
| 4040delA | 4040 del A | 21 | 1 | 0.17 | DHPLC |
| 4167del7 | 4167 delCTAAGCC | 22 | 1 | 0.17 | DHPLC |
| Detected | 511 | 88.10 | |||
| Unknown | 69 | 11.90 | |||
| Total | 580 | 100.00 | |||
N = number of CF chromosomes; % = frequency.
Table 3.
Polymorphisms (*) identified in our cohort of CF patients by DHPLC
| Polymorphism | Nucleotide change | Exon/intron |
| 125G/C | 125 G to C | 5' UTR |
| R75Q | 356 G to A | 3 |
| 875+40A/G | 875+40A/G | Intron 6a |
| M470V | 1540 A to G | 10 |
| T854T | 2694 T to G | 14a |
| T966T | 3030 G to A | 15 |
| 4006-199G/A | 4006-199G/A | Intron 20 |
| P1306P | 4050 C to T | 21 |
(*) According to [7].
Overall, these results revealed a detection rate of 88%, increasing the standard level of detection by ~11%.
Discussion
Prior to complete gene analysis ~77% of the mutant alleles could be detected by screening with various commercially available mutation panels. CFTR gene analysis by DHPLC presented here allowed identification of an additional 11% of mutant alleles, including nine rare mutations and 2 novel ones, underlying the high mutational heterogeneity for CF in this Italian population. The two novel mutations identified could be pathogenic because they determine significant alterations in the protein. H199R has a substitution of a conserved amino acid crucial in the TM1 domain, while the other, a 7bp-deletion, introduces a premature STOP codon, resulting in a truncated protein.
Mutation screening of newborns clinically suspected of having cystic fibrosis can contribute to the confirmation of diagnosis, and in some instances may help to predict the severity of the disease. This can lead to early treatment to retard irreversible tissue damage and ensure the highest possible quality of life for these individuals. The level of detection is therefore important, and this will depend on the sensitivity of the method used. Routine methods, i.e. INNO-LiPA CFTR assay, include a bias since they are based on a selection of known mutations, established on the basis of mutations frequently occurring in North Caucasians. Due to the large number of mutations found in the CFTR gene and their variable frequency among different ethnic and racial groups, it is difficult to develop a suitable assay for all populations that covers all known mutations. With the exception of a few genetically homogeneous populations (i.e. Ashkenazi Jewish, Brittany and Quebec populations), the detection rate of CFTR mutations in heterogeneous populations varies between 88 and 90% [17,21-27]. Our experience is that the DHPLC technique is useful in mutation detection in heterogeneous ethnic populations in Central and South Italy. In these populations, we have now reached a detection sensitivity similar to that observed in other Italian regions and other Mediterranean populations, where a relatively low frequency of delF508 mutation is detected.
Conclusions
In this study, we scanned the entire exonic region of the CFTR gene in genomic DNA obtained from a cohort of 290 CF patients. We were able to identify two pathogenic mutations by DHPLC technique in 211 subjects. In the remaining cases, at least a single CF allele was detected. The pattern of the elution profile for heteroduplex analysis has shown that this technique has proved to be able to distinguish the disease-causing mutations of the CFTR gene. However, in our experimental conditions, sometimes we found difficult to correlate the profile of DHPLC chromatograms with the mutation type. Consequently, further confirmation of the sequence variants by direct sequencing is necessary. Overall, DHPLC has been shown to be a reliable, sensitive, and specific screening method to rapidly detect CFTR mutations, that account for about 88% of CF alleles in our population. In addition, this semi-automated method would also be quite suitable for rapid mutational screening of unknown mutations.
These data also illustrate the need for a multi-system scanning approach. In fact, although the sensitivity was elevated, a significant rate of failure in 69 alleles analysed out of 580 (11.90%) was observed. The extension of DHPLC analysis to the non-coding regions, including promoter and regulatory regions, further increases the detection rate. However, our opinion is that even extensive DHPLC analysis of the whole CFTR gene would not increase the detection rate above 95% in our population. This is because larger genomic rearrangements occurring in some CF cases, and/or technical reasons (i.e. DNA quality, primers design, fragment length), can affect DHPLC.
To maximize mutation detection, different techniques need to be employed, including DHPLC, Southern analysis, and expression studies. Recently, it has been demonstrated that the screening of large deletions by quantitative multiplex polymerase chain reaction of short fluorescent fragment (QMPSF) can improve the detection of uncharacterised CF alleles [28]. Therefore, on this basis, we recommend caution in diagnostic use of DHPLC method.
Competing interests
None declared.
Authors' contributions
MRD participated in design and coordination of the study and drafted the manuscript; SG carried out the DHPLC and sequence analysis; MB as a clinical geneticist providing genetic counselling and consultation to the CF patients and families; AMN carried out the DNA amplification; SR carried out the genomic DNA extraction; VL carried out the CF clinical diagnosis; FS participated in design and coordination of the study; GN revised the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
This work was supported by Italian Ministry of Health, Italian Ministry of Education, University and Research and Regione Lazio. We are extremely grateful to the patients and their families for their invaluable participation and cooperation and to TRANSGENOMIC, INC. (Omaha, NE) for the support and help in DHPLC set up.
Contributor Information
Maria Rosaria D'Apice, Email: d.apice@med.uniroma2.it.
Stefano Gambardella, Email: stefanogamb@libero.it.
Mario Bengala, Email: mario.bengala@ptvonline.it.
Silvia Russo, Email: silviarusso@yahoo.it.
Anna Maria Nardone, Email: annamaria.nardone@ptvonline.it.
Vincenzina Lucidi, Email: lucidi@opbg.net.
Federica Sangiuolo, Email: sangiuolo@med.uniroma2.it.
Giuseppe Novelli, Email: novelli@med.uniroma2.it.
References
- Welsh MJ, Tsui LC, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editor. In The metabolic and molecular bases of inherited disease. 7. McGraw Hill, New York; 1995. pp. 3799–3876. [Google Scholar]
- Doull I. Recent advances in cystic fibrosis. Arch Dis Child. 2001;85:62–66. doi: 10.1136/adc.85.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem BS, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan J, Tsui LC, Collins FS. Identification of cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm M, Iannuzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Zielenski J, Rozmahel R, Bozon D, Kerem B, Grzelczak Z, Riordan JR, Rommens J, Tsui LC. Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics. 1991;10:214–228. doi: 10.1016/0888-7543(91)90503-7. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Mutation Database. http://www.genet.sickkids.on.ca/cftr/
- Haardt M, Benharouga M, Lechardeur D, Kartner N, Lukacs GL. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem. 1999;274:21873–21877. doi: 10.1074/jbc.274.31.21873. [DOI] [PubMed] [Google Scholar]
- Estivill X, Bancells C, Ramos C. Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. The Biomed CF Mutation Analysis Consortium. Hum Mutat. 1997;10:135–54. doi: 10.1002/(SICI)1098-1004(1997)10:2<135::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Cystic fibrosis: a disease of electrolyte transport. FASEB J. 1990;4:2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Salvatore F, Scudiero O, Castaldo G. Genotype-phenotype correlation in cystic fibrosis: the role of modifier genes. Am J Med Genet. 2002;111:88–95. doi: 10.1002/ajmg.10461. [DOI] [PubMed] [Google Scholar]
- Dequeker E, Cassiman JJ. Evaluation of CFTR gene mutation testing methods in 136 diagnostic laboratories: report of a large European external quality assessment. Eur J Hum Genet. 1998;6:165–175. doi: 10.1038/sj.ejhg.5200195. [DOI] [PubMed] [Google Scholar]
- Orita M, Suzuki Y, Sekija T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989;5:874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Lerman LS, Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Cremonesi L, Carrera P, Cardillo E, Fumagalli A, Lucchiari S, Ferrari M, Righetti SC, Righetti PG, Gelfi C. Optimized detection of DNA point mutations by double gradient denaturing gradient gel electrophoresis. Clin Chem Lab Med. 1998;36:959–961. doi: 10.1515/CCLM.1998.165. [DOI] [PubMed] [Google Scholar]
- Xiao W, Oefner PJ. Denaturing High-Performance Liquid Chromatography: a review. Hum Mutat. 2001;17:439–474. doi: 10.1002/humu.1130. [DOI] [PubMed] [Google Scholar]
- Le Marechal C, Audrezet MP, Quere I, Raguenes O, Langonne S, Ferec C. Complete and rapid scanning of the cystic fibrosis transmembrane conductance regulator (CFTR) gene by denaturing high-performance liquid chromatography (D-HPLC): major implications for genetic counselling. Hum Genet. 2001;108:290–298. doi: 10.1007/s004390100490. [DOI] [PubMed] [Google Scholar]
- Ravnick-Glavac M, Atkinson A, Glavac D, Dean M. DHPLC screening of Cystic Fibrosis Gene Mutations. Hum Mutat. 2002;19:374–383. doi: 10.1002/humu.10065. [DOI] [PubMed] [Google Scholar]
- Il sito dei laboratori di genetica molecolare CF. http://spazioweb.inwind.it/laboratoriCF/
- DHPLC Melt Program. http://insertion.stanford.edu/melt.html
- Abeliovich D, Pashut Lavon I, Lerer I, Cohen T, Springer C, Avital A, Cutting GR. Screening for five mutations detects 97% of cystic fibrosis (CF) chromosomes and predicts a carrier frequency of 1:29 in the Jewish Ashkenazi population. Am J Hum Genet. 1992;51:951–956. [PMC free article] [PubMed] [Google Scholar]
- de Braekeleer M, Mari C, Verlingue C, Allard C, Leblanc JP, Simard F, Aubin G, Ferec C. Complete identification of cystic fibrosis transmembrane conductance regular mutations in the CF population of Saguenay Lac St Jean (Quebec, Canada) Clin Genet. 1998;53:44–46. doi: 10.1034/j.1399-0004.1998.531530108.x. [DOI] [PubMed] [Google Scholar]
- Casals T, Ramos MD, Gimenez J, Larriba S, Nunes V, Estivill X. High heterogeneity for cystic fibrosis in Spanish families: 75 mutations account for 90% of chromosomes. Hum Genet. 1997;101:365–370. doi: 10.1007/s004390050643. [DOI] [PubMed] [Google Scholar]
- Claustres M, Laussel M, Desgeorges M, Giansily M, Culard JF, Razakatsara G, Demaille J. Analysis of the 27 exons and flanking regions of the cystic fibrosis gene: 40 different mutations account for 91.2% of the mutant alleles in Southern France. Hum Mol Genet. 1993;2:1209–1213. doi: 10.1093/hmg/2.8.1209. [DOI] [PubMed] [Google Scholar]
- Kanavakis E, Tzetis M, Antoniadi T, Traeger-Synodinos J, Doudounakis S, Adam G, Matsaniotis N, Kattamis C. Mutation analysis of 10 exons of the CFTR gene in Greek cystic fibrosis patients: characterization of 74.5% of CF alleles including 1 novel mutation. Hum Genet. 1995;96:364–366. doi: 10.1007/BF00210426. [DOI] [PubMed] [Google Scholar]
- Bonizzato A, Bisceglia L, Marigo C, Nicolis E, Bombieri C, Castellani C, Borgo G, Zelante L, Mastella G, Cabrini G, Gasparini P, Pignatti PF. Analysis of the complete coding region of the CFTR gene in a cohort of CF patients from North-Eastern Italy: identification of 90% of the mutations. Hum Genet. 1995;95:397–402. doi: 10.1007/BF00208963. [DOI] [PubMed] [Google Scholar]
- Bombieri C, Pignatti PF. Cystic fibrosis mutation testing in Italy. Genetic Testing. 2001;5:229–233. doi: 10.1089/10906570152742281. [DOI] [PubMed] [Google Scholar]
- Audrezet MP, Chen JM, Raguenes O, Chuzhanova N, Giteau K, Le Marechal C, Quere I, Cooper DN, Ferec C. Genomic rearrangements in the CFTR gene: extensive allelic heterogeneity and diverse mutational mechanisms. Hum Mutat. 2004;23:343–357. doi: 10.1002/humu.20009. [DOI] [PubMed] [Google Scholar]