Abstract
Aims
Missed diagnosis of maturity-onset diabetes of the young (MODY) has led to an interest in biomarkers that enable efficient prioritization of patients for definitive molecular testing. Apolipoprotein M (apoM) was suggested as a biomarker for hepatocyte nuclear factor 1 alpha (HNF1A)-MODY because of its reduced expression in Hnf1a−/− mice. However, subsequent human studies examining apoM as a biomarker have yielded conflicting results. We aimed to evaluate apoM as a biomarker for HNF1A-MODY using a highly specific and sensitive ELISA.
Methods
ApoM concentration was measured in subjects with HNF1A-MODY (n = 69), Type 1 diabetes (n = 50), Type 2 diabetes (n = 120) and healthy control subjects (n = 100). The discriminative accuracy of apoM and of the apoM/HDL ratio for diabetes aetiology was evaluated.
Results
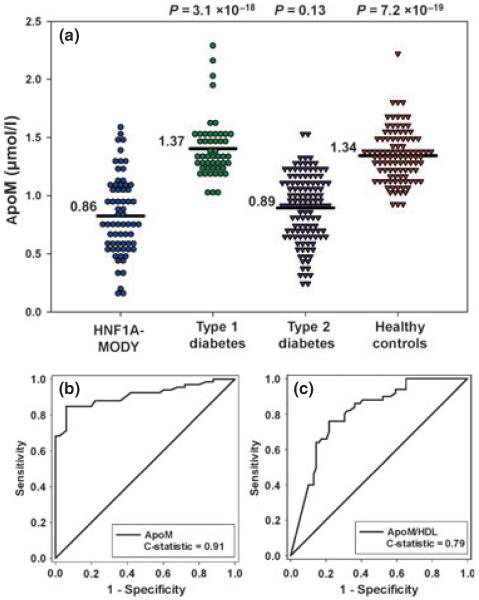
Mean (standard deviation) serum apoM concentration (μmol/l) was significantly lower for subjects with HNF1A-MODY [0.86 (0.29)], than for those with Type 1 diabetes [1.37 (0.26), P = 3.1 × 10−18) and control subjects [1.34 (0.22), P = 7.2 × 10−19). There was no significant difference in apoM concentration between subjects with HNF1A-MODY and Type 2 diabetes [0.89 (0.28), P = 0.13]. The C-statistic measure of discriminative accuracy for apoM was 0.91 for HNF1A-MODY vs. Type 1 diabetes, indicating high discriminative accuracy. The apoM/HDL ratio was significantly lower in HNF1A-MODY than other study groups. However, this ratio did not perform well in discriminating HNF1A-MODY from either Type 1 diabetes (C-statistic = 0.79) or Type 2 diabetes (C-statistic = 0.68).
Conclusions
We confirm an earlier report that serum apoM levels are lower in HNF1A-MODY than in controls. Serum apoM provides good discrimination between HNF1A-MODY and Type 1 diabetes and warrants further investigation for clinical utility in diabetes diagnostics.
Introduction
Maturity-onset diabetes of the young (MODY) is a group of monogenic disorders of pancreatic β-cell function characterized by autosomal dominant inheritance and young-onset of non-insulin-dependent diabetes [1]. Mutations in HNF1A, encoding the transcription factor hepatocyte nuclear factor 1 alpha are the most common cause of MODY and, in a recent study, were shown to account for 1% of those clinically labelled as having Type 1 diabetes and 4% of those diagnosed as having young adult-onset Type 2 diabetes [2]. A correct molecular diagnosis of HNF1A-MODY is important for optimal treatment, predicting clinical course and identifying at-risk family members [1]. However, many patients with HNF1A-MODY are misdiagnosed as having Type 1 or Type 2 diabetes [3].
Identification of non-genetic biomarkers that can facilitate prioritization of patients for molecular diagnostic testing has been a focus of a number of studies in the last decade [4]. One such candidate biomarker is apolipoprotein M (apoM).
ApoM, a 25-kDa apolipoprotein, is found in all major lipoprotein classes, but is mainly associated with HDL [5]. ApoM is regulated by HNF1A [6] and was initially investigated as a biomarker for HNF1A-MODY following the observation of reduced apoM gene expression in Hnf1a−/− knockout mice [6]. Three different studies examining serum apoM in HNF1A-MODY have generated inconsistent results [6-8]. Initially, a 36% lower apoM concentration in subjects with HNF1A-MODY compared with control subjects [6] was reported. In a second study, no significant difference in apoM levels between subjects with HNF1A-MODY, Type 2 diabetes and control subjects was seen [7]. Finally, a third study found 10% lower apoM serum concentration only in women with HNF1A mutations compared with control subjects, while no difference was observed between HNF1A-MODY and Type 2 diabetes [8].
The aim of the current study was to re-examine the use of apoM as a biomarker for HNF1A-MODY, employing a recently described, highly sensitive and specific ELISA [9]. As none of the previous studies examined subjects with Type 1 diabetes (an important differential diagnosis for HNF1A-MODY), a group with Type 1 diabetes was included, along with those with Type 2 diabetes and control subjects.
Patients and methods
Study participants
Subjects were recruited from the UK and Poland. UK subjects comprised subjects with HNF1A-MODY (n = 22), Type 1 diabetes (n = 50), Type 2 diabetes (n = 50) and controls (n = 100). Subjects with Type 1 or Type 2 diabetes were selected from the Young Diabetes in Oxford (YDX) study comprising individuals diagnosed with diabetes at ≤ 45 years of age. Type 2 diabetes was defined as: C-peptide positive, no requirement for permanent insulin within 3 months of diagnosis and negative glutamic acid decarboxylase (GAD) antibodies. Type 1 diabetes was defined as permanent insulin treatment since diagnosis, with evidence of severe β-cell dysfunction (C-peptide ≤ 0.2 nmol/l) and/or positive GAD antibodies (> 14 WHO units/ml). Controls were normoglycaemic individuals aged 30–50 years from the Oxford Biobank (http://www.oxfordbiobank.org.uk) (Table 1).
Table 1.
Clinical and biochemical characteristics of subjects
| Characteristics | HNF1A-MODY | Type 1 diabetes | Type 2 diabetes | Healthy controls |
|---|---|---|---|---|
| Gender (male/female) | 26/43 | 22/28 | 82/38 | 50/50 |
| Diabetes duration (years) | 9 (19) | 15.4 (8.7) | 12 (13.2) | — |
| P value‡ vs. HNF1A-MODY | 0.01 | 0.29 | — | |
| Age of diagnosis (years) | 20 (16) | 25.5 (11.5) | 40 (8.7) | — |
| P value‡ vs. HNF1A-MODY | 0.83 | 5.2 × 10−12 | — | |
| BMI (kg/m2) | 22.8 (4.8) | 25.7 (5.6) | 31.5 (7.5) | 25.2 (4.5) |
| P value‡ vs. HNF1A-MODY | 0.003 | 1.0 × 10−17 | 0.003 | |
| Fasting glucose (mmol/l) | 6.2 (2.6) | 11.7 (8.5) | 7.2 (2.8) | 5.3 (0.6) |
| P value‡ vs. HNF1A-MODY | 6.7 × 10−5 | 0.01 | 3.5 × 10−6 | |
| ApoM (μmol/l)* | 0.86 (0.29) | 1.37 (0.26) | 0.89 (0.28) | 1.34 (0.22) |
| P-value† vs. HNF1A-MODY | 3.1 × 10−18 | 0.13 | 7.2 × 10−19 | |
| HDL (mmol/l) | 1.4 (0.63) | 1.4 (0.90) | 1.1 (0.40) | 1.4 (0.49) |
| P value‡ vs. HNF1A-MODY | 0.05 | 2.3 × 10−4 | 0.53 | |
| Ratio (apoM/HDL)* | 0.60 (0.26) | 0.80 (0.28) | 0.79 (0.28) | 0.98 (0.24) |
| P value† vs. HNF1A-MODY | 3.3 × 10−6 | 2.3 × 10−5 | 1.8 × 10−16 |
Data are shown as median (interquartile range) unless otherwise stated.
Mean (SD). P value compares type 2 diabetes, type 1 diabetes and healthy controls with HNF1A-MODY subjects and was calculated by
Student’s t-test or
Mann-Whitney U test
Polish subjects included subjects with HNF1A-MODY (n = 47) and Type 2 diabetes (n = 70). The group with Type 2 diabetes were diagnosed with diabetes at ≤ 45 years of age, did not require permanent insulin within 1 year of diagnosis and did not meet clinical criteria for MODY or Type 1 diabetes (Table 1).
Both UK and Polish cases of HNF1A-MODY had a heterozygous loss-of-function mutation confirmed by sequencing in a certified diagnostic centre. HNF1A mutations were considered pathogenic if they met one or more of these criteria: previously published reports; presence of a truncating mutation; co-segregation of the mutation with a MODY phenotype within the family; and absence of the variant in normal chromosomes. The study was approved by local ethics committees in the UK and Poland and all subjects gave written informed consent.
Apolipoprotein M assay
Serum apoM was measured using an ELISA provided by Roche [9]. Ninety-six-well Nunc Maxisorp plates (Nunc A/S, VWR International AG, Dietikon, Switzerland) were coated with primary apoM antibody in phosphate-buffered saline (PBS) (5 μg/ml) overnight at 4 °C. The plates were blocked in buffer containing 1% bovine serum albumin (BSA) and washed with PBS-Tween (0.05%). Plasma samples were diluted 1:2000 in blocking buffer. Bound apoM was detected with horseradish peroxidise-coupled secondary antibody (1.5 μg/ml) and 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma Aldrich Chemie GmbH, Buchs, Switzerland) as substrate. Absorbance was measured at 450 nm. The standard curve was prepared from recombinant human apoM ranging from 0.1 to 250 ng/ml. The sigmoidal standard curve was fitted by non-linear regression analysis and apoM concentrations calculated in μmol/l. Intra-assay coefficient of variance was 5.5% and interassay coefficient of variance was 11.7%.
Statistical analysis
A t-test or Mann–Whitney U-test was used to compare means/medians. Receiver operating characteristic curve analysis was performed to assess the discriminative accuracy of apoM, apoM/HDL and combined apoM and high-sensitivity C-reactive protein (hsCRP) for diabetes aetiology. Analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered significant.
Results
The results of biochemical investigations are summarized in Table 1.
ApoM results
Mean (sd) serum apoM concentration (μmol/l) was markedly lower in HNF1A-MODY [0.86 (0.29)] than in Type 1 diabetes [1.37 (0.26), P = 3.1 × 10−18]. The receiver operating characteristic curve showed good discrimination (C-statistic = 0.91, with C-statistic of ≥ 0.80 representing a useful test), and identified a cut-off apoM concentration of 1.1 μmol/l for discriminating HNF1A-MODY and Type 1 diabetes, with 94% sensitivity and 85% specificity. No significant differences were observed in serum apoM concentration of HNF1A-MODY compared with Type 2 diabetes [0.89 μmol/l (0.28), P = 0.13]. The C-statistic for HNF1A-MODY vs. Type 2 diabetes was 0.57, indicating that apoM does not distinguish these two groups. Serum apoM concentration in controls [1.34 μmol/l (0.22)] was similar to Type 1 diabetes (P = 0.14) and significantly higher than observed in HNF1A-MODY and Type 2 diabetes (P < 10−18). Serum apoM concentration was markedly lower in Type 2 diabetes than Type 1 diabetes (P = 7.7 × 10−21).
Serum apoM concentration correlated well with total cholesterol (r = 0.46, P = 3.7 × 10−11) and HDL (r = 0.40, P = 1.2 × 10−13) [10]. Adjustment for total cholesterol did not affect the magnitude or significance in apoM differences observed between the study groups. No other significant correlations were found.
HDL results
There was no significant difference in median (interquartile range) HDL (mmol/l) in subjects with HNF1A-MODY [1.4 (0.63)] compared with controls [1.4 (0.49), P = 0.53] and those with Type 1 diabetes [1.4 (0.90), P = 0.05]. As previously reported [11], HDL was significantly higher in HNF1A-MODY compared with Type 2 diabetes [1.1 mmol/l (0.40), P = 2.3 × 10−4).
ApoM/HDL ratio
ApoM constitutes 5% of HDL. Moreover, as apoM and HDL levels are correlated, we hypothesized that, if there was decreased apoM production, apoM might make up a lower proportion of the HDL in HNF1A-MODY and that the apoM/HDL ratio might be a superior biomarker for HNF1A-MODY. Mean (sd) apoM/HDL ratio was lower in HNF1A-MODY [0.60 (0.26)] compared with Type 1 diabetes [0.80 (0.28), P = 3.3 × 10−6], Type 2 diabetes [0.79 (0.28), P = 2.3 × 10−5] and controls [0.98 (0.24), P = 1.8 × 10−16]. The apoM/HDL ratio, however, performed less well than apoM alone in differentiating HNF1A-MODY from Type 1 diabetes (C-statistic = 0.79). The C-statistic for apoM/HDL to discriminate HNF1A-MODY from Type 2 diabetes was 0.68.
Combined effect of apoM and hsCRP
HsCRP is a promising biomarker for HNF1A-MODY [12,13]. Logistic regression models were used to calculate the combined contribution of apoM and hsCRP in discriminating HNF1A-MODY from both Type 1 and Type 2 diabetes. For HNF1A-MODY vs. Type 2 diabetes, there was no significant benefit in adding apoM measurements to a model using hsCRP as a predictor of HNF1A-MODY (receiver operating characteristic curve C-statistic increased from 0.88 to 0.89, P = 0.16). For HNF1A-MODY vs. Type 1 diabetes, adding hsCRP to the model using apoM as a predictor of HNF1A-MODY resulted in a small improvement in C-statistic from 0.91 to 0.94, P = 0.048.
Discussion
This study confirms the result of an initial report that subjects with HNF1A-MODY have lower serum apoM concentration than control subjects [6]. We show for the first time that serum apoM concentration is lower in subjects with HNF1A-MODY than in those with Type 1 diabetes and that apoM provided good discrimination between these two patient groups. As low apoM levels were also observed in subjects with Type 2 diabetes, apoM does not distinguish HNF1A-MODY from Type 2 diabetes. We also report a lower apoM/HDL ratio in the HNF1A-MODY group compared with all other study groups, but this was not highly discriminative between the groups.
It is of interest that apoM can discriminate HNF1A-MODY from Type 1 diabetes. Recently described biomarkers for HNF1A-MODY, hsCRP, C-peptide and islet autoantibodies either do not provide good discrimination from Type 1 diabetes or their performance is dependent on diabetes duration [2,12-14]. ApoM could have a useful role here, particularly in the first 3–5 years after diagnosis when endogenous insulin secretion persists in those with Type 1 diabetes.
Low apoM in Type 2 diabetes has been previously observed [15]. Indirect evidence suggests this is probably caused by the combination of chronic inflammation and hyperinsulinaemia found in Type 2 diabetes. The APOM promoter contains a dual specificity regulatory region that binds both HNF1A and pro-inflammatory transcription factors (c-Jun, JunB) [16]. HNF1A leads to activation and Jun leads to repression of APOM promoter activity. Inflammation-induced over-expression of Jun in HepG2 cells lead to transcriptional repression of APOM [16]. In murine models, ApoM expression was reduced by hyperinsulinaemia via a FOXA2-mediated mechanism [17].
The features of a clinically useful biomarker would include reproducibility of results and a widely available, economical assay. Although Richter et al. reported low apoM concentration in HNF1A-MODY compared with controls, two later studies did not show differences in apoM between HNF1A-MODY, Type 2 diabetes and controls [6-8]. The Swedish researchers [8] employed a very similar type of assay to the one used in this study and have previously shown that apoM levels are lower in subjects with Type 2 diabetes than controls [15]. There may be other confounding features, such as differences in the baseline characteristics of the subjects studied.
The apoM assay used in this study currently has limited availability. However, with increasing interest in apoM as a marker for cardiovascular risk [9,18], apoM may be more routinely measured.
This study included highly selected groups of subjects therefore these results need to be interpreted with caution before applying to general diabetes population.
In conclusion, this study demonstrates that serum apoM concentration is lower in HNF1A-MODY than in both controls and Type 1 diabetes. Serum apoM provides good discrimination between HNF1A-MODY and Type 1 diabetes and can help prioritize patients with early-onset diabetes for definitive molecular testing.
FIGURE 1.
(a) Dot histogram illustrating apoM concentration for the study groups; mean values are indicated by the black line. (b) Receiver operating characteristic curve illustrating the capacity of apoM to distinguish between HNF1A-MODY and Type 1 diabetes. The C-statistic for this comparison is 0.91. (c) Receiver operating characteristic curve illustrating the capacity of apoM/HDL to distinguish between HNF1A-MODY and Type 1 diabetes. ApoM, apolipoprotein M; HNF1A, hepatocyte nuclear factor 1 alpha; MODY, maturity-onset diabetes of the young.
Acknowledgements
We thank the patients and families taking part in this study. We are grateful to our research nurses for their help in subject recruitment. Recruitment in Oxford was supported by the NIHR Thames Valley Diabetes Local Research Network, part of the UK Clinical Research Network.
Funding sources: The study was funded by the Oxford (NIHR) Biomedical Research Centre and the European Community FP7 programmes CEED3 (HEALTH-F2-2008-223211). KRO is an NIHR-funded Clinician Scientist. ALG is a Wellcome Trust Senior Research Fellow in Basic and Biomedical Science (095101/Z/10/Z).
Footnotes
Competing interests: None declared.
This study was presented in abstract form at the Diabetes UK Annual Professional Conference, Glasgow, Scotland, 7–9 March 2012 and at the Third Meeting of European Association for Study of Diabetes-Study Group on Genetics of Diabetes (EASD-SGGD), Smolenice, Slovakia, 30 September-3 October 2011.
References
- 1.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343:d6044. doi: 10.1136/bmj.d6044. [DOI] [PubMed] [Google Scholar]
- 2.Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset Type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206–1212. doi: 10.2337/dc11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504–2508. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- 4.Owen KR, Skupien J, Malecki MT. The clinical application of non-genetic biomarkers for differential diagnosis of monogenic diabetes. Diabetes Res Clin Pract. 2009;86:S15–21. doi: 10.1016/S0168-8227(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 5.Xu N, Dahlback B. A novel human apolipoprotein (apoM) J Biol Chem. 1999;274:31286–31290. doi: 10.1074/jbc.274.44.31286. [DOI] [PubMed] [Google Scholar]
- 6.Richter S, Shih DQ, Pearson ER, Wolfrum C, Fajans SS, Hattersley AT, et al. Regulation of apolipoprotein M gene expression by MODY3 gene hepatocyte nuclear factor-1alpha: haploinsufficiency is associated with reduced serum apolipoprotein M levels. Diabetes. 2003;52:2989–2995. doi: 10.2337/diabetes.52.12.2989. [DOI] [PubMed] [Google Scholar]
- 7.Skupien J, Kepka G, Gorczynska-Kosiorz S, Gebska A, Klupa T, Wanic K, et al. Evaluation of apolipoprotein M serum concentration as a biomarker of HNF-1α MODY. Rev Diabet Stud. 2007;4:231–235. doi: 10.1900/RDS.2007.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cervin C, Axler O, Holmkvist J, Almgren P, Rantala E, Tuomi T, et al. An investigation of serum concentration of apoM as a potential MODY3 marker using a novel ELISA. J Intern Med. 2010;267:316–321. doi: 10.1111/j.1365-2796.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 9.Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, et al. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219:855–863. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 10.Axler O, Ahnstrom J, Dahlback B. An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J Lipid Res. 2007;48:1772–1780. doi: 10.1194/jlr.M700113-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.McDonald TJ, McEneny J, Pearson ER, Thanabalasingham G, Szopa M, Shields BM, et al. Lipoprotein composition in HNF1A-MODY: differentiating between HNF1A-MODY and type 2 diabetes. Clin Chim Acta. 2012;413:927–932. doi: 10.1016/j.cca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Owen KR, Thanabalasingham G, James TJ, Karpe F, Farmer AJ, McCarthy MI, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010;33:1919–1924. doi: 10.2337/dc10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanabalasingham G, Shah N, Vaxillaire M, Hansen T, Tuomi T, Gasperikova D, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011;54:2801–2810. doi: 10.1007/s00125-011-2261-y. [DOI] [PubMed] [Google Scholar]
- 14.McDonald TJ, Colclough K, Brown R, Shields B, Shepherd M, Bingley P, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med. 2011;28:1028–1033. doi: 10.1111/j.1464-5491.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 15.Plomgaard P, Dullaart RPF, de Vries R, Groen AK, Dahlback B, Nielsen LB. Apolipoprotein M predicts pre-β-HDL formation: studies in type 2 diabetic and nondiabetic subjects. J Intern Med. 2009;266:258–267. doi: 10.1111/j.1365-2796.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 16.Mosialou I, Krasagakis K, Kardassis D. Opposite regulation of the human apolipoprotein M gene by hepatocyte nuclear factor 1 and Jun transcription factors. J Biol Chem. 2011;286:17259–17269. doi: 10.1074/jbc.M110.200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfrum C, Howell JJ, Ndungo E, Stoffel M. Foxa2 activity increases plasma high density lipoprotein levels by regulating apolipoprotein M. J Biol Chem. 2008;283:16940–16949. doi: 10.1074/jbc.M801930200. [DOI] [PubMed] [Google Scholar]
- 18.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]