Abstract
This article describes a system of diagnostic categories that Medicaid programs can use for adjusting capitation payments to health plans that enroll people with disability. Medicaid claims from Colorado, Michigan, Missouri, New York, and Ohio are analyzed to demonstrate that the greater predictability of costs among people with disabilities makes risk adjustment more feasible than for a general population and more critical to creating health systems for people with disability. The application of our diagnostic categories to State claims data is described, including estimated effects on subsequent-year costs of various diagnoses. The challenges of implementing adjustment by diagnosis are explored.
Introduction
Medicaid programs are increasingly turning to capitated managed care, not only for adults and children receiving Aid to Families with Dependent Children (AFDC) who have, to date, dominated Medicaid managed care enrollment, but also for Medicaid recipients with disability, whom health plans have little experience serving. This article has two purposes. First, we argue that risk adjustment is even more important when contracting with health plans for people with disabilities than when contracting for other populations. Second, we describe the Disability Payment System (DPS), which State Medicaid programs can use to provide financial incentives so that health plans will seek to excel in providing appropriate services for people with disabilities.
Need for Risk-Adjusted Payment
Advocates of managed competition have long argued that risk-adjusted payments are required to make a competitive health care system function properly (Enthoven, 1988). As we shall see, the argument is much more powerful for people with disabilities.
In any year, a small number of people account for a large portion of health care expenditures. If a health plan can avoid these costly people, it can reap large, undeserved profits. Management of competition by public or private purchasers may limit the more egregious tactics used to avoid high-risk enrollees, but without adequate risk adjustment, plans will at best try to stay “in the middle of the pack.” That is, no plan will seek to excel in serving high-risk people, lest it attract a larger share of costly members who would force the plan to lose money or raise premiums. Yet people with serious illness, even more than others, can benefit from the creative efforts of health plans to improve their care (Master et al., 1996). If we want plans to excel in caring for those most in need, sufficient dollars must be allocated to the plans that take on this challenge.
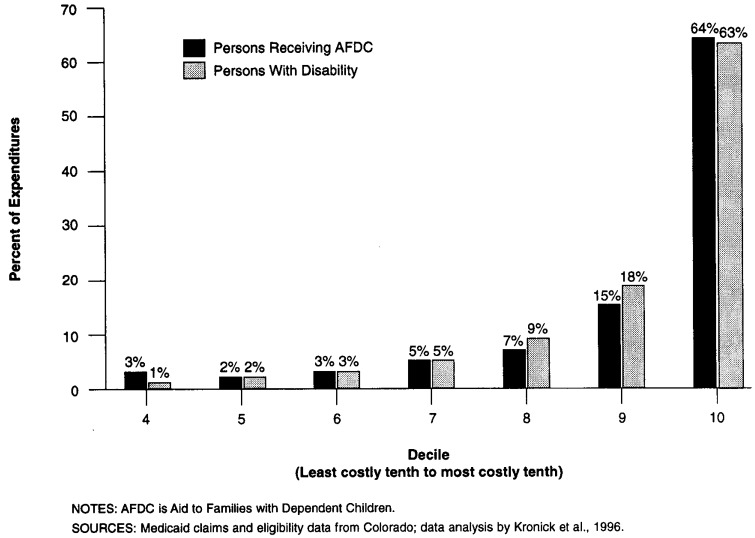
People with disabilities strongly resemble non-disabled populations in that there is a concentration of expenditures among a small fraction of the group. As shown in Figure 1, among Colorado Medicaid recipients with disabilities in 1994, the most expensive 10 percent of recipients accounted for 63 percent of expenditures, and the least expensive 50 percent accounted for only 3 percent of expenditures. This distribution is virtually the same for recipients of AFDC.1 This skew of expenditures reflects the diversity of health status among people with disabilities. Many recipients have little illness requiring extensive medical care and have very low levels of health care expenditures, while smaller numbers have intermediate, high, and very high levels of expenditures. A health plan that can enroll a disproportionately small share of the high-cost cases can make very large profits without making any efforts to improve quality or efficiency.
Figure 1. Distribution of Expenditures by Decile for Colorado Medicaid Recipients With Disability and Receiving AFDC.
Risk adjustment is much more necessary for people with disabilities than for other populations, because expenditures are not only skewed but also much more predictable than expenditures for a non-disabled population. The difference is striking. For people with disabilities, individuals' expenditures in 1 year can be used to predict their expenditures in the following year far more accurately than for the general population. It has been estimated (Newhouse et al., 1989; van Vliet, 1992) that for a general population, the maximum R2 is approximately 15 percent. In most previous research, maximum achieved levels of prediction are in the range of 6-12 percent of observed variation (Newhouse et al., 1989; van Vliet and van de Ven, 1993; Dunn, 1995; Epstein and Cumella, 1988; Ellis and Ash, 1995; Hornbrook and Goodman, 1995). Table 1 shows the adjusted R2 statistics from six regressions in which we used 1 year of Medicaid expenditure data to predict the following year of expenditures, predicting separately for adults receiving AFDC, children receiving AFDC, and recipients with disability in both Colorado and Michigan. In Colorado, the R2 for AFDC adults is 0.04, and for AFDC children, 0.05; for recipients with disability, the R2 is 0.42. In Michigan, the figures are 0.09, 0.04, and 0.29, respectively.2 By comparison, in States where data on AFDC recipients were not available to us, the adjusted R2 statistics for recipients with disability were: Minnesota, 0.51; Missouri, 0.45; New York, 0.39; Ohio 0.29; and Wisconsin, 0.35.3
Table 1. Adjusted R2 (Proportion of Variation Explained) for Annual Individual Expenditures Predicted by Expenditures in the Previous Year, by Type of Medicaid Recipient and State: Colorado and Michigan.
| State and Statistic | Recipients of AFDC | Recipients With Disability | |
|---|---|---|---|
|
| |||
| Adults | Children | ||
| Colorado | |||
| R2 | 0.04 | 0.05 | 0.42 |
| N | 17,706 | 44,911 | 16,660 |
| Michigan | |||
| R2 | 0.09 | 0.04 | 0.29 |
| N | 94,365 | 158,034 | 64,914 |
NOTES: AFDC is Aid to Families with Dependent Children. Regressions include people who were eligible for 24 months, not institutionalized, not in waiver programs, not in health maintenance organizations, and not enrolled in Medicare. In Colorado, the dependent variable is fiscal year 1994 expenditures, and the independent variable is fiscal year 1993 expenditures. In Michigan, the dependent variable is calendar year 1993 expenditures, and the independent variable is 1992 expenditures.
SOURCE: Medicaid claims and eligibility data from Colorado and Michigan; data analysis by Kronick et al., 1996.
This much greater predictability of expenditures results from the much larger proportion of costs among the disabled that are for chronic needs, which are by definition more consistent over time. Acute care still contributes significantly to cost, but even acute costs are more predictable. For example, people with spinal cord injury commonly experience repeated acute episodes of urinary and respiratory tract infections. Similarly, individuals with renal failure, muscular dystrophy, or acquired immunodeficiency syndrome (AIDS) can all be expected to have costs far above average, but people whose conditions have been limited to, for example, uncomplicated epilepsy, anemias, or moderate mental retardation, can be expected to have lower costs.
The greater predictability of expenditures increases the potential rewards to health plans from attempts to win favorable selection. Standard Medicaid practice is to pay plans a percentage (e.g., 95 percent) of the fee-for-service (FFS) average, adjusted in some States for age, gender, and region. For example, in Colorado in 1994, the FFS average for AFDC adults was $1,646 per year, and for recipients with disability, $4,763 per year. If a health plan were somehow able to attract members only from the one-fifth of AFDC adults who were least costly in 1993, the next year the plan could expect to make a profit on each enrollee of $963, or 59 percent of the capitation (Table 2). Conversely, if the plan attracted members only from the most expensive one-fifth of AFDC adults, it would expect to lose $831 per enrollee, or one-half of the capitation. The potential profits and losses for recipients with disabilities are much larger, because of the higher costs involved and because of the much greater predictability. A plan attracting members only from the least expensive one-fifth of recipients with disability in 1993 would earn profits of $4,021 per member, or 84 percent of the capitation; a plan enrolling from the most expensive one-fifth would expect to lose $9,736 per member, or more than twice the capitation.
Table 2. Per Capita Expenditures and Potential Profits in 1994 from Enrollment of Medicaid Recipients Recieving AFDC (Adults and Children) and Medicaid Recipients Receiving Disability, by Expenditure Quintiles in 1993: Colorado.
| Type of Recipient and Expenditure Quintile in 1993 | Expenditures in 1994 | Potential Profit (Loss) in 1994 | Percentage of Capitation |
|---|---|---|---|
| AFDC Adults | |||
| All Quintiles | $1,646 | $0 | 0 |
| First | 683 | 963 | 59 |
| Second | 1,103 | 543 | 33 |
| Third | 1,574 | 72 | 4 |
| Fourth | 2,393 | (747) | -45 |
| Fifth | 2,477 | (831) | -50 |
| AFDC Children | |||
| All Quintiles | 649 | 0 | 0 |
| First | 312 | 337 | 52 |
| Second | 375 | 275 | 42 |
| Third | 462 | 187 | 29 |
| Fourth | 622 | 28 | 4 |
| Fifth | 1,476 | (827) | -127 |
| Recipients With Disability | |||
| All Quintiles | 4,763 | 0 | 0 |
| First | 743 | 4,021 | 84 |
| Second | 1,330 | 3,433 | 72 |
| Third | 2,660 | 2,103 | 44 |
| Fourth | 4,584 | 179 | 4 |
| Fifth | 14,499 | (9,736) | -204 |
NOTES: AFDC is Aid to Families with Dependent Children. Analysis includes recipients who were eligible for 24 months in fiscal year 1993 and 1994, not in waiver programs, not in health maintenance organizations, and not enrolled in Medicare.
SOURCE: Medicaid claims and eligibility data from Colorado; data analysis by Kronick et al., 1996.
Not only does greater predictability increase the potential for profits, it also increases the ability to successfully select good risks. In many cases, a brief conversation or look at medical or claims records would suffice to make a decent guess about an individual's approximate future costs. Because the ongoing needs for care vary with the type and severity of disability or chronic illness, it is much easier to predict for people with disabilities than for a general population whether or not an individual is likely to incur high costs in the next year.
Some of the factors that can contribute to a biased selection might be limited by Medicaid purchasing practices, such as third-party management of enrollment, oversight of marketing, monitoring of disenrollment, and requirements for network composition. But other important causes of biased selection, such as service location, network design, and plan quality, will inevitably vary—and should, if recipients are to have meaningful choice among a variety of plans. Plans that are particularly responsive to people with more serious disabilities, that include specialized clinicians and teaching hospitals with expertise in treating people with disabilities and chronic illness, and that maintain a high level of quality and coordination will better meet people's needs but will suffer large financial losses if payments are not risk adjusted. Meanwhile, plans that offer very basic benefits, that exclude providers experienced with people with disabilities, and that make no efforts to maintain quality and coordination will enjoy the large profits that accrue in an unadjusted system to those who avoid high-cost members.
For people with disability, the higher predictability of costs makes adequate risk adjustment a necessary element in developing a system of health plans that will strive to meet the needs of this group rather than avoid them. Fortunately, this predictability allows Medicaid programs to adjust payments to reflect expected needs for care.
Disability Payment System
In developing DPS, we have focused on diagnostic information, which seems to strike the best balance of practicality, accuracy, and appropriate incentives. In contrast with researchers who have worked on the Medicare or privately insured populations, we have developed a risk-adjustment system particular to the conditions of Medicaid recipients with disability.
Creating a System of Diagnostic Categories
The general goals of capitated payment are to give plans flexibility in resource allocation and incentives for efficiency. The more specific goals in developing DPS were to create a classification system that could be readily implemented by State Medicaid programs, that would make reasonably accurate payments, and that would not encourage manipulation by health plans nor undermine incentives for efficiency.
Like other risk-adjustment systems, DPS consists of groups of diagnoses that have been associated with elevated future costs. With claims data from several years for approximately 120,000 Medicaid recipients with disability in Ohio and Missouri, we used regression analysis to estimate the amount of additional expenditures in a given year associated with a person having had a specific diagnosis in the previous year. The diagnoses found to have statistically significant associations with elevated future costs were divided into 18 major categories that correspond either to body systems or to specific types of illness or disability. This effort built on our previous work in developing diagnostic categories for people with disabilities (Kronick, Zhou, and Dreyfus, 1995). Of the nearly 14,900 diagnosis codes in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), approximately 2,400 are used in our system.
Most of the major categories of diagnoses are divided into subcategories according to the degree of elevated future costs. For example, the central nervous system (CNS) diagnoses are divided into three subcategories: CNS, high-cost; CNS, medium-cost; and CNS, low-cost Although the subcategories of DPS were primarily developed using the Ohio and Missouri sample, they were then tested with data from Colorado, Michigan, and New York for approximately 275,000 individuals. In testing DPS, we found that predicted expenditures for some of the subcategories were not significantly different from each other, suggesting that the model had been overfitted on the Ohio and Missouri sample. In these cases, we recombined the subcategories, reducing the total number to the 43 subcategories shown in Table 3, along with sample diagnoses.
Table 3. Disability Payment System Categories With Estimated Additional Annual Costs1.
| Diagnostic Categories | Sample Diagnoses | Percent of Recipients | Model | |
|---|---|---|---|---|
|
| ||||
| Prospective | Concurrent | |||
|
| ||||
| Estimated Additional Cost | ||||
| Fully Counted Categories | ||||
| Central Nervous System | 12.3 | — | — | |
| High-Cost | Quadriplegia | 0.3 | $11,165 | $13,927 |
| Medium-Cost | Paraplegia, muscular dystrophy, amyotrophic lateral sclerosis, other motor neuron disease | 1.7 | 4,320 | 5,446 |
| Low-Cost | Cerebral palsy, multiple sclerosis, epilepsy, spinocerebellar disease | 11.3 | 2,051 | 2,624 |
| Skeletal and Connective | 9.0 | — | — | |
| High-Cost | Juvenile arthritis, osteomyelitis | 0.6 | 3,472 | 6,552 |
| Medium-Cost | Osteoporosis, fractured neck of femur, lupus erythematosus | 2.2 | 1,533 | 2,149 |
| Low-Cost | Rheumatoid arthritis, disc disorders, congenital leg deformities | 6.9 | 683 | 874 |
| Gastrointestinal | 8.5 | — | — | |
| High-Cost | Liver disease, peritonitis, regional enteritis, gastrojejunal ulcers | 3.1 | 3,133 | 4,004 |
| Low-Cost | Intestinal obstruction, diverticula, inguinal and ventral hernias | 6.4 | 1,448 | 2,353 |
| Metabolic | 7.2 | — | — | |
| High-Cost | Pituitary dwarfism, vitamin D deficiencies | 0.1 | 8,818 | 6,475 |
| Medium-Cost | Hyperparathyroidism, malnutrition | 3.2 | 4,504 | 7,266 |
| Low-Cost | Adrenal disorders, electrolyte disorders | 4.4 | 993 | 1,456 |
| Cancer | 5.0 | — | — | |
| High-Cost | Cancers of the nervous system, myeloid and erythroleukemia | 0.2 | 5,620 | 7,585 |
| Medium-Cost | Stomach cancer, multiple myeloma, lymphoid leukemia, lymphomas | 2.1 | 2,548 | 4,027 |
| Low-Cost | Melanoma, cervical cancer, Hodgkin's disease, reticulosarcoma | 3.4 | 1,616 | 2,718 |
| Eye and Ear | Retinal disorders, cataracts | 4.1 | 1,180 | 882 |
| Skin | 1.5 | — | — | |
| High-Cost | Decubitus ulcers | 0.5 | 8,378 | 10,877 |
| Low-Cost | Other chronic skin ulcers | 1.2 | 3,173 | 3,688 |
| Gynecological | Ovarian cysts, cervical dysplasia | 0.8 | 856 | 739 |
| Hierarchic Categories | ||||
| Psychiatric | 22.2 | — | — | |
| High-Cost | Schizophrenia | 10.3 | 4,930 | 6,243 |
| Medium-Cost | Manic, bipolar and major depressive disorders | 5.6 | 2,520 | 3,287 |
| Low-Cost | Neurotic and phobic disorders, hysteria | 6.3 | 776 | 1,291 |
| Pulmonary | 19.2 | — | — | |
| Very High-Cost | Cystic fibrosis, respiratory failure, congenital pneumonia | 0.8 | 11,834 | 17,238 |
| High-Cost | Congenital lung anomalies, tracheostomy status, certain bacterial pneumonias | 0.7 | 6,540 | 12,624 |
| Medium-Cost | Chronic obstructive pulmonary disease | 12.3 | 2,193 | 3,310 |
| Low-Cost | Viral pneumonias, emphysema, simple asthma | 5.4 | 706 | 513 |
| Hierarchic Categories | ||||
| Cardiovascular | 13.2 | — | — | |
| High-Cost | Polyarteritis nodosa, vena cava thrombosis, heart transplant status | 0.4 | 12,103 | 15,207 |
| Medium-Cost | Endocardial disease, arterial embolism, congestive heart failure | 4.5 | 4,512 | 5,783 |
| Low-Cost | Rheumatic fever, dysrhythmias, angina, phlebitis, acute myocardial infarction | 8.4 | 1,619 | 2,303 |
| Diabetes | 11.3 | — | — | |
| High-Cost | Adult-onset diabetes with complications, juvenile-onset diabetes | 3.9 | 3,994 | 3,566 |
| Low-Cost | Adult-onset diabetes without complication | 7.3 | 1,048 | 42 |
| Hematological | 7.1 | — | — | |
| Very High-Cost | Hemophilia (clotting factors VIII and IX) | 0.1 | 35,548 | 29,999 |
| High-Cost | Hemoglobin S sickle cell disease with crisis, hemophilia (other clotting factors) | 0.4 | 14,861 | 12,438 |
| Medium-Cost | Hemoglobin C sickle cell disease, acquired hemolytic anemias | 0.3 | 7,307 | 10,854 |
| Low-Cost | Aplastic anemias, thrombocytopenia, white blood cell disorders | 6.3 | 737 | 1,516 |
| Substance Abuse | 5.4 | — | — | |
| High-Cost | Drug dependence or abuse | 2.6 | 3,646 | 4,889 |
| Low-Cost | Alcohol dependence or abuse | 2.8 | 956 | 1,863 |
| Mental Retardation | 4.4 | — | — | |
| High-Cost | Profound mental retardation | 0.4 | 11,953 | 10,682 |
| Medium-Cost | Severe mental retardation | 0.7 | 6,935 | 7,356 |
| Low-Cost | Mild and moderate mental retardation | 3.3 | 3,551 | 4,124 |
| Renal | 4.4 | — | — | |
| High-Cost | Renal failure, hypertensive renal disease | 1.1 | 11,504 | 11,052 |
| Low-Cost | Nephritis, calculus of kidney and ureter | 3.3 | 1,297 | 1,534 |
| Cerebrovascular | Cerebral thrombosis, subarachnoid hemorrhage | 2.3 | 1,410 | 2,887 |
| AIDS | Kaposi's sarcoma, cytomegalovirus | 1.4 | 13,287 | 10,658 |
| Baseline1 | 34.5 | 1,998 | 630 | |
See the Technical Note at the end of this article for a description of the included population, analytic methods, and baseline.
NOTE: AIDS is acquired immunodeficiency syndrome.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
Balancing Accuracy and Resistance to Gaming
In the development of DPS, careful attention was paid to select diagnoses and specify the diagnostic variables so as to increase the accuracy of payments but limit the opportunities for manipulating the system. A number of choices in constructing a risk-assessment system involve tradeoffs between accuracy and resistance to gaming.
To increase the accuracy of expenditure estimates, we included in our categories both very serious, potentially disabling conditions and many less serious conditions that were shown to have significant effects on future expenditures. Some of the included diagnoses are very likely disabling conditions (e.g., muscular dystrophy or cystic fibrosis) but others (e.g., gastric ulcers or pneumonias) are probably not disabling but do appear to reflect health status and increased risk of future expenditures.
To improve accuracy, DPS considers not only a person's single most serious diagnosis, but a variety of conditions with which a person is diagnosed. The inclusion of less serious diagnoses and the counting of multiple diagnoses improve accuracy by distinguishing people with different degrees of illness or disability. For example, DPS would distinguish someone with both sickle cell disease and congestive heart failure from someone who had only sickle cell disease. The use of multiple diagnoses to predict expenditures is supported strongly by our finding that average expenditures are much higher for people with greater numbers of diagnoses in the previous year (Medicaid Working Group, 1995). Clinicians also tend to agree that multiple diagnoses add significantly to complexity and cost.
By excluding some diagnoses, the system sacrifices some accuracy but is made less susceptible to gaming. We found that many less serious diagnoses show statistically significant association with future costs but with very modest actual dollar effects. For example, controlling for other, more serious diagnoses, we found that diagnoses such as hypertension, obesity, dental caries, and joint pain are all predictive of very small increments to future cost, but we did not include them in DPS. A small amount of predictive accuracy is lost, but the exclusion of these common diagnoses will substantially decrease the effort involved in auditing plans' diagnosis reports.
One way to increase apparent accuracy is to distinguish more finely among diagnoses, so that the occurrences of two related diagnoses are counted separately in hopes of indicating a higher overall severity of illness and expected cost On the other hand, if two diagnoses that are clinically difficult to distinguish are separated into different variables, then plans would be paid more for inadvertently or intentionally adding to members' records slightly different diagnoses that were not truly indicative of higher costs. In general, we tended to regard diagnoses coded with the same first three digits in the ICD-9-CM as being a single diagnosis. We hesitated to break up these groups into multiple diagnoses that could be counted separately or placed in different subcategories unless they were readily distinguished by clinicians and were associated with significantly different predicted expenditures, controlling for other diagnoses. Where the data indicated very different predicted expenditures for closely related diagnoses, but clinicians believed that the diagnoses could not easily be distinguished, we did not separate them into distinct diagnostic groups.
For example, the ICD-9-CM system encourages recording diagnoses of malignant neoplasms of the stomach (general code 151) with four-digit codes (from 151.1 to 151.8) to indicate more precisely the location of the cancer or to report it as unspecified (151.9). Although one might imagine that cancers in different parts of the stomach could be more or less difficult to treat, clinicians tend not to specify the location consistently: The unspecified location was by far the most commonly coded and the most statistically valid predictor of future cost. As a result, distinctions by location of stomach cancer could not be made, and we kept all these codes for stomach cancer together as a single variable within the subcategory of medium-cost cancer diagnoses. This indicator for stomach cancer is set to zero if no diagnosis is recorded and is set to one if a single or several different stomach cancer diagnoses are recorded, in which case a count of one is added to the overall cancer-medium variable.4
In some cases, the groupings of diagnoses in the ICD-9-CM system with the same first three digits include much more varied conditions. For example, quadriplegia, paraplegia, diplegia, monoplegia, and cauda equina syndrome are all grouped in the ICD-9-CM under “other paralytic syndromes” (code 344). These conditions are readily distinguishable and appear to have very different future costs associated with them, so we allowed these diagnoses to be categorized in different CNS subcategories according to the level of associated future costs. In other cases, diagnoses with different first three digits are closely related, e.g., chronic nephritis (code 582), and nephritis and nephropathy, not specified as acute or chronic (code 583); we combined the diagnoses under these two codes into a single indicator that can only be counted once. Clinicians specializing in various areas advised us on the appropriateness of separating or combining diagnoses.
Counting Diagnoses Within Categories
Although DPS always includes in its count multiple diagnoses from different major categories, it only sometimes counts multiple diagnoses from within the same major category. To avoid encouraging a proliferation of diagnoses reported for a single disease, in 10 of our major categories we use “hierarchical counting,” by which only the single most severe diagnosis within the major category is counted. For these categories, it was judged that additional diagnoses within the category more likely reflect additional coding of the same underlying condition rather than additional severity of illness. If the diagnoses are from different subcategories within the major category, then the single count is made in the highest cost subcategory in which there is a diagnosis. For example, among people with diabetes, most people with a high-cost diagnosis such as “diabetes with ophthalmic complications” might well also be recorded at a different visit as having a low-cost diagnosis of diabetes without mention of complications; the additional low-cost diagnosis is not indicative of greater illness. Another example is the category of mental retardation: If someone is coded as having profound mental retardation, then any additional diagnosis of severe, moderate, or mild retardation would not reflect a more serious condition or greater need for care. Hierarchical counting is also used within the major categories of pulmonary, renal, hematological, AIDS, substance abuse, psychiatric, and cardiovascular diagnoses.
Within the other eight major categories, each distinct diagnosis is counted separately. In these cases, the diagnoses in the less severe subcategories are not primarily subsets of the diagnoses in the more severe categories. For example, many people with a medium-cost CNS diagnosis such as muscular dystrophy will not also have other high-, medium-, or low-cost CNS diagnoses. But some persons with muscular dystrophy will have other CNS diagnoses, and counting these other diagnoses adds accuracy without encouraging coding proliferation. For example, a person with muscular dystrophy and quadriplegia would be counted as having one medium-cost and one high-cost CNS diagnosis, but a person with muscular dystrophy and paraplegia would be counted as having two medium-cost CNS diagnoses. DPS fully counts each distinct diagnosis in the categories of metabolic, skin, gastrointestinal, cancer, eye and ear, gynecological, and skeletal diagnoses.
Special difficulties arose with human immunodeficiency virus (HIV)-associated diagnoses, which in the past have been recorded either with codes to indicate any one of many specific HIV-related conditions (042-044) or with codes that represent conditions without specifying that they result from HIV. As a result, we were unable to distinguish among individuals with greater and lesser progression of illness based on diagnoses in claims. In addition, our analyses have focused on recipients with more than 1 year of eligibility, which likely excludes many people with AIDS who become eligible and die within a year. More analytic work and the improved coding system now recommended in the ICD-9-CM should allow better risk assessment in this area.
Data and Methods
We report on data from five State Medicaid programs: Colorado, Michigan, Missouri, New York, and Ohio. In each State, we have claims and eligibility data on all recipients who were eligible for Medicaid because of disability, and in Colorado and Michigan, we also have data on all Medicaid recipients.5 The Medicaid programs are not a nationally representative sample but rather are those that expressed interest in developing risk-adjusted capitation systems as part of projects in which we were engaged.6 The Midwest is strongly represented, and there are no southern or southwestern States in the group. Together, these five States account for more than one-quarter of national Medicaid spending on persons with disabilities. Expenditure levels per capita in New York are approximately twice the national average; expenditures in the other four States cluster around the national average.
The claims data contain information on dates of service, charges, Medicaid payments, type of provider, category of service, diagnosis, and procedure. In most States one diagnosis code is available on ambulatory claims and at least two on inpatient claims. We have used the primary and secondary diagnoses from inpatient claims, and the primary diagnosis on ambulatory claims. Data are available, on a date-of-service basis, for calendar years 1991-93 in Michigan, fiscal years 1993 and 1994 in Colorado, 1991-94 in Missouri, 1992-93 in New York, and 1991-93 in Ohio.
We report on results of both prospective and concurrent regressions. In the prospective regressions, we use diagnoses in 1 year to predict expenditures in the subsequent year. In the concurrent regressions, we use diagnoses in 1 year to predict expenditures in the same year. The dependent variable includes all expenditures for which a health plan would typically be responsible: all Medicaid-covered services less dental care, long-term psychiatric care, and institutional long-term care.7
To focus on those who are most likely among the disabled to be enrolled in managed care, we exclude from the analysis recipients in institutions, those receiving home and community-based waiver services, those on spend-down, and those also receiving Medicare coverage. Most State Medicaid programs are not yet ready to enroll people in institutions into capitated managed care, and waiver recipients are already under quasi-capitated financing, at least for their waivered services. In some States, Medicaid recipients with Medicare coverage can enroll in managed care, but they are excluded from the analysis because of incomplete diagnostic information from Medicare-covered services in many State Medicaid data systems. We also excluded recipients enrolled in health maintenance organizations (HMOs), because Medicaid claims data do not include diagnostic information for them. These restrictions exclude approximately 37 percent of recipients with disability.8
To create more reliable diagnostic profiles, the sample is restricted to those with at least 12 months of eligibility in the year diagnoses are counted for both the prospective and concurrent regressions. The prospective regression includes people who are eligible for only part of the subsequent year, for whom the dependent variable is annualized expenditures. These people receive less weight in the prospective regression than persons eligible for the full year.9
In the regressions for individual States, we combine multiple years of data to increase the stability of the estimates. For example, in the prospective regressions for Missouri, we include observations for recipients who were eligible for all of 1991 and part of 1992, with 1992 expenditures as the dependent variable and 1991 diagnoses as the independent variables. We also include observations in which the dependent variable is 1993 expenditures and the independent variables are 1992 diagnoses, and observations in which the dependent variable is 1994 expenditures and the independent variables are 1993 diagnoses. Because two separate observations may include data on a single individual, the error terms in these regressions are not independent, and the standard errors reported from ordinary least-squares regressions are biased downward from their true values. The parameter estimates, however, are unbiased, and maximizing the precision of the point estimates is our major concern. For the prospective regressions, the number of observations and the number of actual individuals are shown in Table 4. Similarly for the concurrent regressions, we combine data from multiple years to increase the stability of the estimates.10
Table 4. Number of Medicaid Recipients With Disability Included in the Prospective Regression, by Year and State.
| State | Unduplicated Count | Total | Individuals With Eligibility in | ||
|---|---|---|---|---|---|
|
| |||||
| 1991 and 1992 | 1992 and 1993 | 1993 and 1994 | |||
| 5-State Sample | 394,777 | 536,245 | 153,660 | 335,685 | 46,900 |
| Colorado | 18,712 | 18,712 | — | — | 18,712 |
| Michigan | 86,517 | 136,871 | 67,717 | 69,154 | — |
| Missouri | 33,643 | 72,698 | 20,807 | 23,703 | 28,188 |
| New York | 169,916 | 169,916 | — | 169,916 | — |
| Ohio | 85,989 | 138,048 | 65,136 | 72,912 | — |
NOTE: See the Technical Note at the end of this article for a description of the included population.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
For the States in which we combine observations across multiple years (Missouri, Michigan, and Ohio), we standardize the expenditure data to 1993 levels. For example, in the Missouri prospective regression, an observation in which diagnoses are measured in 1991 and expenditures in 1992 would have the 1992 expenditures multiplied by the ratio of average 1993 expenditures to average 1992 expenditures. The concurrent regressions are similarly adjusted to the 1993 expenditure level.
In addition to estimating regressions separately for each State, we also combine data from all of the States to increase the size of the sample and the stability of the estimates. In these combined regressions, we standardize each State's expenditure levels to the average expenditure level across the five States, calculated as a simple average of the per capita 1993 expenditures from each State.
Diagnoses in the claims data were summarized for each individual using the subcategories of DPS. For the diagnoses in the fully counted major categories, each distinct diagnosis that a person had in a subcategory caused the subcategory variable to increase one count For example, a person with diagnoses in claims for epilepsy, multiple sclerosis, and heart failure was assigned a count of two low-cost CNS diagnoses and one medium-cost cardiovascular diagnosis. For diagnoses in the hierarchically counted major categories, a single count was made for the highest cost subcategory in which a diagnosis was recorded. For example, a person with diagnoses in claims for hemoglobin S sickle cell anemia with crisis and unspecified sickle cell anemia would be counted as having only a single high-cost hematological diagnosis.
The regression coefficients for the fully counted subcategories, therefore, are the estimated effects on expenditures of having an additional diagnosis in the subcategory; for the hierarchically counted subcategories, the coefficients are the estimated effects of having at least one diagnosis from that subcategory. The regression also produces estimates of additional cost for other included variables such as age and sex, though their values are quite small compared with the additional costs associated with the diagnostic subcategories.11
Results
Parameter Estimates
As shown in Table 3, the parameter estimates from the model appear reasonable in size and have good face validity.12 In both the prospective and concurrent models, the problems that most clinicians would judge to be more serious have larger expenditures associated with them than the problems that most would judge to be less serious.
In the prospective regressions, there are a number of low-frequency diagnoses for which the estimated additional costs are between $10,000 and $14,000 per year (e.g., quadriplegia, very high-cost pulmonary diagnoses, high-cost cardiovascular diagnoses, high-cost hematological diagnoses, and AIDS). More frequently occurring diagnoses tend to have lower additional costs associated with them. We have identified a group of moderate cost diagnoses with estimated expenditures in the subsequent year of approximately $3,000 to $5,000 (e.g., medium-cost CNS diagnoses, medium-cost metabolic diagnoses, schizophrenia, medium-cost cardiovascular conditions). The most frequently occurring diagnoses tend to have the lowest additional costs associated with them, in many cases between $1,000 and $2,000 per year.
Approximately one-third of all recipients had none of the DPS diagnoses recorded on a health care claim over a 12-month period. For this group of recipients, average predicted expenditures in the subsequent year are $1,998 per year.13 Among recipients with at least one diagnosis, approximately 50 percent have only one diagnosis and have predicted expenditures equal to the sum of the estimated additional cost for their diagnosis, plus the baseline amount of approximately $2,000 per year. The remaining one-third of recipients have more than one DPS diagnosis, and these recipients have expected expenditures equal to the sum of the baseline amount and the additional payments associated with these diagnoses.
For people with some diagnoses, such as quadriplegia and high-cost pulmonary diagnoses, the effects of additional diagnoses are substantial. For example, persons with quadriplegia have predicted expenditures, on average, of $23,467 in the prospective regressions, substantially above the $13,155 that would be predicted from the baseline and the additional cost associated with quadriplegia itself. Similarly, persons with high-cost pulmonary diagnoses have average predicted expenditures of $17,841—again, much higher than the $8,356 that would be predicted for those whose only condition was a high-cost pulmonary diagnosis. In contrast, the burden of additional diagnoses is much smaller for people with schizophrenia, whose average predicted expenditure of $9,506 is only somewhat higher than the $6,931 prediction for those with schizophrenia and no other identified conditions.
In the concurrent regression, the average predicted expenditure for recipients with no identified diagnosis is $630, substantially lower than the approximately $2,000 baseline in the prospective regression. Parameter estimates for the diagnostic variables are generally similar in magnitude in both regressions, although somewhat higher in the concurrent regression than in the prospective regression.
State-Specific Estimates
The expenditure estimates shown in Table 3 describe how expenditures vary with diagnosis in the five-State sample. We have also estimated these relationships on data for individual States (see Tables 7 and 8 in the Technical Note).
Table 7. Percent of Medicaid Recipients With Disability, by State and Diagnostic Category.
| Diagnostic Categories | 5-State Sample | Colorado | Michigan | Missouri | New York | Ohio |
|---|---|---|---|---|---|---|
| Fully Counted Categories | ||||||
| Central Nervous System | 12.3 | 15.9 | 13.8 | 14.5 | 10.2 | 11.9 |
| High-Cost | 0.3 | 0.6 | 0.4 | 0.4 | 0.3 | 0.2 |
| Medium-Cost | 1.9 | 2.5 | 2.3 | 2.5 | 1.5 | 1.7 |
| Low-Cost | 13.3 | 18.5 | 15.0 | 15.9 | 10.9 | 12.4 |
| Skeletal and Connective | 9.0 | 9.2 | 10.4 | 8.9 | 7.9 | 8.8 |
| High-Cost | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | 0.4 |
| Medium-Cost | 2.3 | 2.7 | 2.9 | 2.3 | 1.9 | 2.2 |
| Low-Cost | 7.6 | 7.7 | 9.0 | 7.5 | 6.6 | 7.4 |
| Gastrointestinal | 8.5 | 8.0 | 10.3 | 9.8 | 5.9 | 9.3 |
| High-Cost | 3.6 | 3.4 | 4.8 | 3.5 | 2.6 | 3.7 |
| Low-Cost | 7.7 | 7.6 | 9.6 | 9.6 | 5.0 | 8.2 |
| Metabolic | 7.2 | 6.9 | 8.9 | 9.8 | 4.9 | 6.9 |
| High-Cost | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| Medium-Cost | 3.3 | 3.4 | 3.9 | 3.7 | 2.4 | 3.7 |
| Low-Cost | 4.7 | 4.4 | 6.2 | 7.4 | 2.9 | 3.9 |
| Cancer | 5.0 | 2.9 | 7.8 | 3.6 | 3.1 | 5.6 |
| High-Cost | 0.2 | 0.2 | 0.4 | 0.2 | 0.2 | 0.2 |
| Medium-Cost | 2.4 | 1.8 | 4.9 | 1.7 | 1.5 | 1.7 |
| Low-Cost | 4.1 | 2.1 | 5.6 | 3.7 | 2.7 | 5.1 |
| Eye and Ear | 4.8 | 4.5 | 4.9 | 5.3 | 5.0 | 4.1 |
| Skin | 1.5 | 1.0 | 1.8 | 1.6 | 1.6 | 1.3 |
| High-Cost | 0.5 | 0.4 | 0.6 | 0.6 | 0.5 | 0.4 |
| Low-Cost | 1.2 | 0.7 | 1.5 | 1.2 | 1.2 | 1.0 |
| Gynecologic | 0.8 | 1.0 | 0.8 | 1.2 | 0.5 | 0.9 |
| Hierarchic Categories | ||||||
| Psychiatric | 22.2 | 23.1 | 22.3 | 24.9 | 20.5 | 22.6 |
| High-Cost | 10.3 | 7.6 | 11.1 | 11.9 | 9.4 | 9.9 |
| Medium-Cost | 5.6 | 8.0 | 5.7 | 7.1 | 4.7 | 5.6 |
| Low-Cost | 6.3 | 7.5 | 5.5 | 5.9 | 6.4 | 7.0 |
| Pulmonary | 19.2 | 13.5 | 21.9 | 19.1 | 18.5 | 18.2 |
| Very High-Cost | 0.8 | 0.8 | 0.9 | 0.9 | 0.8 | 0.7 |
| High-Cost | 0.7 | 0.6 | 0.7 | 0.8 | 0.7 | 0.5 |
| Medium-Cost | 12.3 | 7.9 | 16.6 | 14.1 | 9.2 | 11.4 |
| Low-Cost | 5.4 | 4.2 | 3.7 | 3.3 | 7.8 | 5.5 |
| Cardiovascular | 13.2 | 7.2 | 14.6 | 14.3 | 10.1 | 15.9 |
| High-Cost | 0.4 | 0.9 | 0.5 | 0.4 | 0.3 | 0.4 |
| Medium-Cost | 4.5 | 2.7 | 5.7 | 5.6 | 3.5 | 4.0 |
| Low-Cost | 8.4 | 3.6 | 8.5 | 8.3 | 6.2 | 11.5 |
| Diabetes | 11.3 | 5.8 | 13.9 | 12.7 | 9.9 | 10.4 |
| High-Cost | 3.9 | 2.6 | 3.7 | 5.6 | 3.5 | 3.9 |
| Low-Cost | 7.3 | 3.2 | 10.2 | 7.1 | 6.3 | 6.5 |
| Hematologic | 7.1 | 3.7 | 13.8 | 6.7 | 4.6 | 4.1 |
| Very High-Cost | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| High-Cost | 0.4 | 0.1 | 0.5 | 0.4 | 0.5 | 0.4 |
| Medium-Cost | 0.3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.3 |
| Low-Cost | 6.3 | 3.0 | 12.9 | 5.8 | 3.8 | 3.3 |
| Substance Abuse | 5.4 | 4.3 | 5.3 | 5.8 | 6.7 | 3.9 |
| High-Cost | 2.6 | 1.2 | 2.2 | 2.9 | 3.8 | 1.5 |
| Low-Cost | 2.8 | 3.1 | 3.1 | 2.9 | 2.9 | 2.4 |
| Mental Retardation | 4.4 | 2.3 | 6.8 | 7.8 | 2.7 | 2.5 |
| High-Cost | 0.4 | 0.1 | 0.6 | 0.9 | 0.3 | 0.1 |
| Medium-Cost | 0.7 | 0.2 | 1.3 | 1.3 | 0.3 | 0.2 |
| Low-Cost | 3.3 | 2.0 | 4.9 | 5.7 | 2.1 | 2.2 |
| Renal | 4.4 | 2.8 | 4.7 | 4.0 | 2.8 | 6.5 |
| High-Cost | 1.1 | 0.7 | 1.1 | 1.2 | 1.1 | 1.0 |
| Low-Cost | 3.3 | 2.1 | 3.6 | 2.9 | 1.6 | 5.5 |
| Cerebrovascular | 2.3 | 1.6 | 3.5 | 2.0 | 1.5 | 2.2 |
| AIDS | 1.4 | 0.5 | 0.7 | 0.6 | 3.3 | 0.4 |
NOTE: AIDS is acquired immunodeficiency syndrome. See the Technical Note at the end of this article for a description of the included population and analytic methods.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
Table 8. Estimated Additional Cost for Persons With Specific Diagnoses, by State.
| Diagnostic Categories | 5-State Sample | Colorado | Michigan | Missouri | New York | Ohio |
|---|---|---|---|---|---|---|
| Fully Counted Categories | ||||||
| Central Nervous System | ||||||
| High-Cost | $11,165 | $20,315 | $4,930 | $8,788 | $19,482 | $6,565 |
| Medium-Cost | 4,320 | 4,270 | 2,711 | 3,966 | 7,314 | 3,578 |
| Low-Cost | 2,051 | 2,456 | 1,233 | 1,970 | 3,027 | 1,871 |
| Skeletal and Connective | ||||||
| High-Cost | 3,472 | 5,062 | 3,384 | 4,613 | 2,445 | 5,706 |
| Medium-Cost | 1,533 | 1,990 | 1,056 | 1,694 | 1,337 | 2,328 |
| Low-Cost | 683 | 1,708 | 721 | 1,025 | 472 | 894 |
| Gastrointestinal | ||||||
| High-Cost | 3,133 | 2,364 | 2,546 | 2,825 | 2,936 | 4,927 |
| Low-Cost | 1,448 | 1,120 | 1,403 | 1,620 | 699 | 2,229 |
| Metabolic | ||||||
| High-Cost | 8,818 | 19,288 | 6,756 | 13,339 | 7,736 | 9,799 |
| Medium-Cost | 4,504 | 4,549 | 3,489 | 3,803 | 6,185 | 4,908 |
| Low-Cost | 993 | 560 | 1,134 | 858 | 1,135 | 1,272 |
| Cancer | ||||||
| High-Cost | 5,620 | 10,058 | 2,560 | 6,475 | 7,542 | 9,766 |
| Medium-Cost | 2,548 | 3,077 | 2,179 | 3,622 | 2,756 | 4,609 |
| Low-Cost | 1,616 | 4,872 | 1,279 | 1,699 | 2,098 | 1,440 |
| Eye and Ear | 1,180 | 773 | 1,538 | 1,422 | 669 | 1,507 |
| Skin | ||||||
| High-Cost | 8,378 | 14,909 | 6,803 | 4,269 | 12,578 | 6,790 |
| Low-Cost | 3,173 | 2,450 | 2,070 | 3,016 | 4,063 | 3,251 |
| Gynecologic | 856 | 1,136 | 120 | 1,424 | (1,080) | 2,317 |
| Hierarchic Categories | ||||||
| Psychiatric | ||||||
| High-Cost | 4,930 | 8,476 | 5,925 | 5,821 | 4,030 | 3,918 |
| Medium-Cost | 2,520 | 3,030 | 2,549 | 3,823 | 1,522 | 2,931 |
| Low-Cost | 776 | 1,048 | 1,367 | 1,003 | 94 | 1,002 |
| Pulmonary | ||||||
| Very High-Cost | 11,834 | 30,003 | 7,907 | 16,382 | 9,555 | 13,412 |
| High-Cost | 6,540 | 9,305 | 5,628 | 4,569 | 7,377 | 7,157 |
| Medium-Cost | 2,193 | 3,129 | 1,807 | 2,083 | 2,383 | 2,721 |
| Low-Cost | 706 | 2,213 | 689 | 1,470 | 417 | 913 |
| Cardiovascular | ||||||
| High-Cost | 12,103 | 12,286 | 11,385 | 10,578 | 9,184 | 17,102 |
| Medium-Cost | 4,512 | 5,228 | 4,307 | 3,567 | 4,531 | 5,413 |
| Low-Cost | 1,619 | 2,157 | 1,275 | 1,473 | 1,504 | 1,855 |
| Diabetes | ||||||
| High-Cost | 3,994 | 4,529 | 3,292 | 2,941 | 4,228 | 4,933 |
| Low-Cost | 1,049 | 1,243 | 520 | 964 | 1,119 | 1,982 |
| Hematologic | ||||||
| Very High-Cost | 35,548 | 44,692 | 20,941 | 76,197 | 18,251 | 58,286 |
| High-Cost | 14,861 | 12,281 | 15,542 | 21,548 | 13,723 | 11,613 |
| Medium-Cost | 7,307 | 14,129 | 4,442 | 4,362 | 7,734 | 9,841 |
| Low-Cost | 737 | 2,451 | 342 | 939 | 1,245 | 1,944 |
| Substance Abuse | ||||||
| High-Cost | 3,646 | 2,598 | 2,503 | 3,774 | 4,347 | 2,033 |
| Low-Cost | 956 | 661 | 1,096 | 675 | 899 | 706 |
| Mental Retardation | ||||||
| High-Cost | 11,953 | 48,936 | 11,804 | 15,966 | 8,001 | 1,274 |
| Medium-Cost | 6,935 | 17,564 | 8,997 | 5,354 | 5,658 | 967 |
| Low-Cost | 3,551 | 5,785 | 5,012 | 2,787 | 2,995 | 1,006 |
| Renal | ||||||
| High-Cost | 11,504 | 9,065 | 11,176 | 6,518 | 11,655 | 14,799 |
| Low-Cost | 1,297 | 2,299 | 623 | 1,474 | 1,195 | 2,018 |
| Cerebrovascular | 1,410 | 995 | 1,451 | 1,342 | 1,627 | 1,915 |
| AIDS | 13,287 | 5,257 | 8,902 | 5,946 | 13,510 | 13,522 |
NOTE: AIDS is acquired immunodeficiency syndrome. See the Technical Note at the end of this article for a description of the included population and analytic methods.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
The prevalence of diagnoses in the diagnostic subcategories is, for the most part, similar in the five States. The diagnoses that are relatively frequent in one State tend to be so in all the States. A notable exception is AIDS, which was recorded for 3.3 percent of New York recipients, but no more than 0.7 percent of recipients in any other State.14 For many diagnoses, prevalence is somewhat lower among Colorado recipients than in other States, but overall, the diagnostic profiles are quite similar across States.15
Diagnostic subcategories that are high-cost in the combined regression are high-cost in each of the State-specific regressions as well (see Tables 7 and 8). Those that are low tend to be low in all the States.16 For example, the coefficient for high-cost hematological diagnoses is $9,622 in Ohio and more than $10,000 for all the other States. For medium-cost cardiovascular diagnoses, the coefficients are all in the $3,000-$5,000 range. For low-cost substance-abuse diagnoses, the coefficients are all in the $600-$l,100 range. For some diagnoses, however, the estimated effects do vary across States. We are interested in examining the extent to which these differences may be the result of differences in practice patterns, the burden of disease across States (even controlling for diagnosis), Medicaid benefit packages or payment rates, or random variation.
Goodness of Fit
Adjusted R2 statistics for the prospective and concurrent regressions, for individual States and all States combined, are presented in Table 5. Also shown are the adjusted R2 statistics from regressions that include only age and gender variables, and, for comparison purposes, regressions with prior expenditures as the independent variable.
Table 5. Percentage of Variance Explained (Adjusted R2) by Selected Models.
| State | Model | |||
|---|---|---|---|---|
|
| ||||
| Demographic | Prospective DPS | Concurrent DPS | Prior-Expenditures | |
| All Expenditures | ||||
| 5-State Sample | 0.007 | 0.173 | 0.338 | 0.275 |
| Colorado | 0.004 | 0.222 | 0.362 | 0.395 |
| Michigan | 0.013 | 0.208 | 0.415 | 0.276 |
| Missouri | 0.001 | 0.222 | 0.347 | 0.420 |
| New York | 0.015 | 0.192 | 0.352 | 0.361 |
| Ohio | 0.007 | 0.156 | 0.317 | 0.172 |
| Expenditures Truncated at $100,000 | ||||
| 5-State Sample | 0.008 | 0.216 | 0.397 | 0.359 |
| Colorado | 0.004 | 0.258 | 0.446 | 0.413 |
| Michigan | 0.015 | 0.240 | 0.462 | 0.333 |
| Missouri | 0.002 | 0.235 | 0.394 | 0.420 |
| New York | 0.016 | 0.212 | 0.382 | 0.387 |
| Ohio | 0.013 | 0.241 | 0.457 | 0.311 |
NOTES: DPS is Disability Payment System. See the Technical Note at the end of this article for a description of the variables in the prospective and concurrent regressions. State-specific regressions are estimated on data normalized to average payment levels across the States. In the truncated regressions, we have included recipients with expenditures of more than $100,000 but recoded their expenditures to $100,000. The prior-expenditure regressions are estimated on the same sample as the prospective regression. The independent variables in the regression with prior expenditures include a continuous variable for prior expenditures as well as four dummy variables if prior expenditures were less than $300 per year, between $300 and $1,000, between $1,000 and $4,000, or more than $9,000, to account for non-linearities in the relationship between prior and current expenditures.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
In the prospective regression, in which observations from all five States are combined, the adjusted R2 is 0.17. This is much higher than the R2 of 0.01 using demographic information alone and lower than the R2 using concurrent diagnoses or information on prior expenditures.17 With the exception of Ohio, the explanatory power of the prospective diagnostic regressions is similar in each of the States. The relatively low R2 in Ohio for both the prospective DPS and the prior-utilization regression is accounted for by two recipients, each of whom had more than $1 million per year in annualized expenditures. Each of these recipients was eligible for only part of a year, and hence was excluded from the concurrent regressions. When expenditures are truncated at $100,000 per year (as they might be if a health plan purchased reinsurance above this level), the R2 statistics increase substantially in Ohio and modestly in other States.
The explanatory power of the concurrent regressions is similar to the explanatory power of concurrent regressions on expenditure data from non-disabled populations, but the prospective regressions have much higher R2 statistics for the disabled than for other populations. This reinforces the argument already made that diagnoses among persons with disabilities are much more likely to be indicative of chronic problems with persistent effects than are such diagnoses in non-disabled populations.
Predictive Ratios
Table 6 shows the actual expenditures for a variety of subsets of persons with disabilities, and the ratio of predicted to actual expenditures for the demographic model, the prospective DPS model, the concurrent DPS model, and a model using prior-year expenditures.
Table 6. Predictive Ratios of Selected Models Tested on Age-Gender, Diagnostic, and Prior-Expenditure Groups.
| Group | Average Expenditures | Predictive Ratios for Models1 | |||
|---|---|---|---|---|---|
|
| |||||
| Demographic | Prospective DPS | Concurrent DPS | Prior-Expenditure | ||
| Age-Gender Groups | |||||
| Under Age 15 | $4,861 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 15-24, Male | 4,534 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 15-24, Female | 4,857 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 25-44, Male | 5,816 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 25-44, Female | 6,219 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 45-64, Male | 5,989 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age 45-64, Female | 6,413 | 1.0 | 1.0 | 1.0 | 1.0 |
| Prior-Year Diagnoses by DPS Categories | |||||
| Quadriplegia | 23,467 | 0.23 | 1.0 | 0.87 | 0.77 |
| Schizophrenia | 9,506 | 0.63 | 1.0 | 0.98 | 1.00 |
| High-Cost Pulmonary | 17,841 | 0.34 | 1.0 | 0.87 | 0.93 |
| High-Cost Diabetes | 12,678 | 0.49 | 1.0 | 0.95 | 0.87 |
| Low-Cost Substance Abuse | 8,397 | 0.72 | 1.0 | 1.00 | 1.09 |
| AIDS | 22,836 | 0.27 | 1.0 | 0.90 | 0.65 |
| Prior-Year Diagnoses by Other Diagnostic Samples | |||||
| No Diagnoses | 1,954 | 2.89 | 1.02 | 1.13 | 1.17 |
| Any Pulmonary and Cardiology | 14,208 | 0.43 | 0.98 | 0.93 | 0.92 |
| Any Psychiatric and Substance Abuse | 12,353 | 0.49 | 0.97 | 0.88 | 1.02 |
| Any Metabolic and Diabetes | 13,928 | 0.44 | 1.04 | 1.00 | 0.88 |
| Prior-Year Expenditures | |||||
| First Quintile | 1,164 | 4.82 | 1.95 | 1.63 | 1.05 |
| Second Quintile | 2,062 | 2.76 | 1.71 | 1.66 | 0.99 |
| Third Quintile | 3,526 | 1.66 | 1.41 | 1.47 | 1.11 |
| Fourth Quintile | 6,134 | 0.96 | 1.10 | 1.15 | 0.95 |
| Fifth Quintile | 16,335 | 0.37 | 0.71 | 0.71 | 0.99 |
Predictive ratios are the ratio of predicted to actual expenditures.
NOTES: DPS is Disability Payment System. AIDS is acquired immunodeficiency syndrome. See the Technical Note at the end of this article for a description of the included population and analytic methods.
SOURCE: Medicaid claims and eligibility data from Colorado, Michigan, Missouri, New York, and Ohio; data analysis by Kronick et al., 1996.
Each of the models provides accurate payment for subsets defined by age and gender. Indicator variables for the age-gender groups shown in Table 6 are included in each of the models, and if the predictive ratios were not uniformly 1.0, we would know that our statistical package was not working correctly. Note that there is relatively little difference across age and gender subgroups in average expenditure levels.
When diagnostic subgroups are considered, the demographic model underpredicts actual expenditures for persons with diagnoses and greatly overpredicts expenditures for persons without diagnoses. For the higher cost diagnostic categories, the demographic model predicts expenditures that are approximately 25 percent of the actual amounts. In contrast, for persons without any DPS diagnosis included in the prior year, the demographic model predicts expenditures that are almost three times the actual level.
By construction, the prospective DPS model predicts accurately when recipients are divided into subsets based on prior-year diagnoses, and the diagnostic categories are the categories that are included in the model. A slightly more difficult test is posed when the diagnostic subsets do not correspond exactly to indicator variables in the model. For example, the model does not include a variable for persons with no diagnosis and does not include variables to indicate interactions between major categories. Predictive ratios for these subgroups are reassuringly close to 1.0. A more difficult test, which we have not yet had time to perform, could be made by examining subgroups of ICD-9-CM codes that were not included at all in DPS. If predictive ratios significantly far from 1.0 were found, these codes could be added to the system.
The concurrent DPS model performs reasonably well when recipients are divided into groups based on diagnoses in the previous year. But the model over-predicts (by about 13 percent) actual expenditures for recipients who had no diagnosis in the previous year, and tends to underpredict expenditures for recipients in many of the diagnostic subcategories. It appears that recipients who had no diagnosis in the previous year have slightly lower expenditures in the current year than recipients with the same diagnostic profile in the current year but who also had some diagnosis in the previous year. A model using prior expenditures performs similarly to the concurrent DPS model when recipients are divided based on prior diagnostic history: It overpredicts expenditures for those recipients with no prior diagnosis and underpredicts for many diagnostic categories.
When recipients are divided into quintiles based on their prior-year expenditures, the demographic model performs extremely poorly. It overpredicts expenditures for the lowest quintile by a ratio of 5:1, resulting in a potential profit of more than $4,000 per person for every first-quintile recipient that a health plan enrolls. The demographic model predicts less than 40 percent of actual expenditures for recipients in the fifth quintile, leaving plans with a $10,000 loss per person for these enrollees.
The prospective DPS model performs much better but still leaves room for profits or losses based on successful selection. Overpayments for the least expensive quintile are reduced from 5:1 to 2:1, with per capita profits for enrollees in this quintile reduced to approximately $1,100. Underpayments for recipients in the fifth quintile are reduced to $5,000 per enrollee—significantly better than the demographic model, but still a big enough number to be of concern to plans. Despite its much higher R2, the concurrent DPS performs similarly.
The test based on prior expenditures is perhaps overly severe. In real application, Medicaid programs will need to use risk adjustment as part of a broader strategy to limit opportunities for risk selection. By managing enrollment and monitoring disenrollment, the State can make it difficult for a plan to attract recipients whose costs are lower than predicted by their diagnoses. States can use third-party enrollment brokers, depriving plans of the ability to discourage the enrollment of evidently high-cost individuals. States can also analyze the expenditures of disenrollees and sanction plans whose disenrollees are disproportionately high-cost.
Even with State oversight, we expect that plan design and individual choice will still work to distribute risks unequally among plans. For example, a plan might be unattractive to people with cystic fibrosis if the best pulmonologists are excluded from its network. Or a plan might attract people with AIDS or severe physical disabilities by developing a responsive home care system. It is more difficult to imagine how, among people with a certain diagnosis, a plan could selectively attract the low-cost but avoid the high-cost individuals. When State oversight limits the ability of plans to select risks by prior expenditure, diagnostic adjustment should provide appropriate resources to plans that attract diagnostic subgroups with high levels of need.
Implementation
Although diagnostically adjusted payments are conceptually straightforward, their implementation involves a number of important choices.
Moving from Risk Assessment to Risk Adjustment
The subcategories of DPS, defined as lists of ICD-9-CM codes, can be used to count diagnoses in claims records for all Medicaid recipients with disability in a State. For ratesetting purposes, a large State such as Michigan or New York might estimate regression coefficients using its own data, while a small State might make more reliable predictions by averaging estimates from its own data with estimates from a multistate sample, adjusted to its own expenditure levels. A regression predicting expenditures should also include demographic variables, such as age and sex, though their values will be small compared with the additional costs associated with the diagnostic subcategories. Within many States, differences in expenditure levels across regions, especially between rural and urban areas, even controlling for diagnosis, are large enough that variables for region should also be used to adjust payments. Such a regression also will yield an estimate of costs for individuals with no diagnoses in any of the categories in the previous year—a baseline value (the regression intercept).
For those with diagnoses, the estimated effects on expenditure for the subcategories in which the person has diagnoses can be added to the baseline to produce a total expenditure estimate for each individual. For an example, consider the estimates in Table 3 as the possible regression results from a particular State. An individual with diagnoses in claims of severe mental retardation and epilepsy would be counted as having a medium-cost mental retardation diagnosis and a low-cost CNS diagnosis. On top of the baseline amount of approximately $2,000, adjustments of $6,935 and $2,051 would be added, yielding an estimate of annual expenditures at $10,986. Small additional adjustments could be made for age, sex, and region. For a second example, consider an individual with a diagnosis in claims for juvenile-onset diabetes, which is a high-cost diabetes diagnosis. On top of the $2,000 baseline, an adjustment of $3,994 would be added, giving an estimate of annual expenditures at $5,994.
Instead of making a payment at a different level for each individual, States can more conveniently average the expenditure estimates for all individuals enrolled in a particular plan and set a single case-mix adjusted rate for the plan. The goal of the system is not to attach a precise estimate to each individual, which is beyond the capability of any predictive system. Rather, the purpose of risk adjustment is to assess the overall risk of health care needs among all the enrollees of a health plan and to provide a level of resources commensurate with their needs.
Start-Up and Continued Use
The ways in which risk assessment is used for paying plans will vary depending on whether substantial numbers of people with disabilities are already enrolled in plans and on whether enrollment in plans is mandatory or voluntary. In some States, there are few people with disabilities enrolled in plans, and recipients will retain a choice between FFS (including primary-care case management) and capitated managed care. In this situation in the first year, with prospective adjustment, the State can make interim payments for new plan enrollees as some percentage of the FFS average for people with disabilities. Toward the end of the year, when FFS claims for the preceding year are available, the State can use the diagnoses in claims to estimate the case mix of each plan's group of members, summarizing it as a percentage of the average cost for recipients with disability in FFS, e.g., 110 percent for a plan with an adverse selection or 85 percent for a plan with a favorable selection. The interim payments can then be adjusted to final payments based on the case-mix percentage.18
Payment rates must also be determined for plan members whose claims are unavailable or incomplete. This group includes new Medicaid recipients and recipients with Medicare benefits whose diagnoses from Medicare claims may not be available. For these individuals, the case mix of members with claims history can be used to estimate the case mix of members without history. If a plan receives an adverse selection of members with history it is likely receiving a similarly adverse selection of members without history. The State should estimate the FFS average for new Medicaid recipients and for dually eligible persons and multiply these averages by the plan's case-mix percentage estimated for enrollees with claims history. (Policy questions about capitating care for Medicare beneficiaries are addressed in the “Discussion” section.)
An important virtue of the diagnostic approach is its continued effectiveness in subsequent years using diagnostic information reported by the health plans. In this respect, adjustment by diagnosis is superior to adjustment by prior expenditures. Adjustment by prior expenditures allows accurate estimates, but if based on expenditures made by the health plans themselves, it would recreate FFS incentives to overserve, merely lagging the rewards: The more services provided this year, the more payment received next year. By contrast, diagnostic adjustment can be started using diagnoses in FFS claims and continued using diagnoses reported by plans without encouraging plans to provide unnecessary services. For diagnostic adjustment to work on an ongoing basis, plans will need to report annually the diagnoses made in health care encounters that are on the DPS list.
However, care needs to be taken in how the diagnostic information provided by plans is used. When payments are based on the diagnoses reported by plans, diagnostic reporting will almost certainly be more complete than it is in FFS. We have found, for example, that not all of the people who have a diagnosis in claims for quadriplegia in 1 year also have that diagnosis in claims the next year. When plans are paid an extra $930 per month for members with quadriplegia, they will report this diagnosis for almost every member who has the condition. The increased intensity of diagnosis results naturally from changing the focus of payment from procedures to health status. Unnecessary diagnoses are generally less harmful and less costly than unnecessary procedures, and the focus on chronic diagnoses may even help plans think more about conditions that deserve ongoing attention.
When case-mix assessment is used to allocate a fixed amount of money among plans, responding to increased diagnostic reporting should not be difficult. For example, if enrollment in managed care is mandatory and the trended FFS average sets total spending, then diagnostic adjustment can be used to allocate the fixed amount of money. Regardless of how many additional diagnoses plans report, DPS can be used to measure the relative case mix of each plan and to allocate the overall budget for people with disabilities among the plans according to the selections of members they have enrolled. Payments would be based on the ratio of each plan's case-mix percentage to the average case-mix percentage. Training of coders and audits of medical records would be needed to help keep plans competing fairly.
Additional adjustments are required when diagnostic assessment not only allocates a fixed pot of money but also affects the overall level of payments to plans. For example, people with disabilities might still be able to choose FFS or quasi-FFS options, such as primary care case management. As a result of increased diagnostic reporting, plans will appear to have a more adverse selection of enrollees than they actually do. In this case, to maintain levels of spending across managed care and FFS, the increased intensity of diagnosis needs to be measured and adjusted for. The extent of increased diagnosis can be measured for individuals who move from FFS to managed care by comparing the predicted expenditures that result from their FFS claims and from their managed-care diagnosis reports. A small additional adjustment needs to be made for the tendency of average expenditures for individuals with disability to rise slightly each year.
Using Only Inpatient Diagnoses or All Diagnoses
Payments to plans do not necessarily need to be adjusted using both inpatient and ambulatory diagnoses. Payments could be adjusted using only inpatient diagnoses, as the Health Insurance Plan of California is testing for small employee groups. This approach would make sense where ambulatory diagnoses are either unreliable or unavailable and would ease the burden (especially on plans that make capitated payments to provider groups) of gathering diagnoses from ambulatory encounters. Some plans may object to the burden of collecting ambulatory diagnoses, but States should be willing to require this information. Plans can hardly claim that they are adding management value to State purchase of medical services if they cannot report on the most basic medical characteristics of their members.
Initially, the use of inpatient diagnoses alone would appropriately bring higher payments for hospitalized individuals whose costs on average are greater than for individuals who receive the same diagnoses outside the hospital. But reliance on inpatient diagnoses is not advisable for long. A major way in which managed care can reduce costs and improve quality is by shifting care out of the hospital and into the community and home. Paying plans more money only for diagnoses made in an inpatient setting encourages hospitalization and may reduce the savings that are needed to finance alternative care. In many situations where the advantages of hospitalization are uncertain, decisions would be biased away from care management at home. Plans that are able to prevent hospitalization would be penalized. This incentive problem would be aggravated under concurrent adjustment (discussed in the next section), because the added costs associated in the same year with a diagnosis made in the hospital would be even greater than for a prospective system.
Prospective Versus Concurrent Adjustment
Although much risk-adjustment research has focused on predicting future service needs, there are advantages to concurrent adjustment Concurrent adjustment means using diagnoses recorded by a health plan to adjust payments for the same year, rather than for the following year. One advantage of concurrent adjustment is that it can be done for all enrollees, not just for those with adequate claims history. Many Medicaid recipients move in and out of eligibility, especially recipients of AFDC, but also those with disability. In a system in which payments this year are based on the diagnoses that health-plan enrollees were given last year, many members will have no diagnostic information available because they were not recipients in the previous year.
The R2 statistics from concurrent regressions are higher than for prospective regressions. As shown in the predictive ratio tables, however, concurrent adjustment does not do significantly better than prospective adjustment for samples biased by prior expenditures or diagnoses. The choice between prospective and concurrent adjustment would seem to depend mostly on questions of implementation.
Some might be concerned that concurrent adjustment would create incentives to provide more service or would unnecessarily complicate risk assessment by including diagnoses, such as trauma, for which risk adjustment is not needed. But the diagnoses in DPS were chosen for their predictive value, so that adjusting payments concurrently would not be tantamount to FFS payment.19 Plans would be paid more not for purely acute diagnoses but for diagnoses associated with ongoing need.
One disadvantage of concurrent adjustment is the need to more quickly deal with the increased intensity of diagnoses that will follow implementation of any diagnosis-based system. With prospective adjustment, payments in the first year will be based on claims, and the problem of increased intensity of diagnosis needs attention only in the second year of the program. With concurrent adjustment, analysis will be needed in the first year to measure and adjust for the increased intensity of reported diagnoses.
Persons Eligible for Medicare
An important problem in the development of managed-care programs for Medicaid recipients with disabilities is the lack of coordination of funds for those who are also Medicare beneficiaries. These dually eligible persons are a large portion of Medicare recipients with disability—about 30 percent. For this group Medicare benefits limit the effectiveness of capitated programs because the availability of Medicare payment for hospital care strongly discourages plans from shifting care to the home and community. By maximizing hospital use, the plan reduces its expenses, increases costs to Medicare, and subverts the goal of the program to develop capacity for delivering care in the most appropriate setting. If the plan does seek to reduce hospitalization of its dually eligible enrollees, it receives none of the savings and is less able to finance the expansion of primary care and home and community options.
The low level of the Medicaid capitation for dually eligible persons further discourages plans from providing this group with the most appropriate services. The Medicaid capitation for people who are dually eligible, intended to cover non-hospital services, is based on the smaller proportion of non-hospital services in the traditional hospital-focused delivery system. The financial incentives strongly encourage Medicaid programs and providers to avoid enrolling persons who are dually eligible or to maintain old patterns of care. To resolve these problems, health plans that enroll the dually eligible disabled should receive capitated payments from both Medicaid and Medicare, preferably both adjusted by diagnosis.
Risk Sharing
Although diagnostic risk adjustment will do far better than demographic adjustment in matching payments to the needs of recipients with disabilities, full-risk arrangements are probably best avoided until States and health plans have more experience in setting rates and providing care for this vulnerable and high-cost population. Full-risk contracts would place the burden of inexperience not only on a vulnerable population and the health plans that care for them but also, potentially, on the State budget. Diagnostic adjustment of rates allows plans to specialize and innovate for people with disabilities, but considerable predictable variance in expenditure remains unaccounted for by diagnosis, as can be seen from the higher R2 for regressions using prior expenditure and the predictive ratios significantly different from 1.0 for the biased samples. Even with diagnostic adjustment, a health plan might enroll a favorable selection of members, intentionally or not, and earn large undeserved profits at the expense of the State budget. Alternatively, the reputation, design, or location of a plan could cause it to enroll an adverse selection relative to its adjusted payments.
Arrangements that share risk for profits and losses between State and plan can help focus plans on efficiency and protect both sides from large losses that can result from inadequate adjustment of rates. Further, risk-sharing arrangements will blunt the incentives for efficiency that capitation is intended to produce—perhaps to the benefit of people with disabilities for whom managed care is still an experiment.
As an example, health plans contracting with Ohio Medicaid for people with disabilities are responsible for 90 percent of the first 5-percent margin (profit or loss). For the next 10 percent of margin, the State and plan split the margin 50-50, and for profits and losses greater than 15 percent of the capitation, the State assumes 90 percent of the risk. Profit or loss is defined as the difference between medical expenditures and the medical-services portion of the capitation. The plan should be at full risk for administrative expenses.
Risk-sharing arrangements require Medicaid and plans to agree on the definition of expenditures for which risk will be shared. An advantage of capitation is the flexibility it provides plans in determining payment rates to providers and the services that will be delivered. Under full-risk arrangements, if a plan wants to pay at higher-than-Medicaid rates for a given service to increase its use or wants to provide a traditionally non-covered service such as respite care, home improvements, or alternative forms of personal care, these decisions are entirely up to the plan. Under risk-sharing arrangements, however, the Medicaid program will be appropriately concerned about how the plan is spending dollars that the State may have to partially reimburse.
Risk-sharing arrangements should specify that for most services, including inpatient care, settlement will be at Medicaid rates, that for a few services, such as primary care, higher rates will be subject to State approval, and that for benefits provided by the plan that are not in the Medicaid benefit package, the plan will document their cost-effectiveness. Although Medicaid programs may prefer the simplicity of full-risk contracts, they should ask themselves why some plans are also eager to assume full risk.
Discussion
Comparison With Other Systems
Risk adjustment by diagnosis is one among a number of alternative approaches, including risk adjustment by demographic information, functional status, self-reported health status, and prior expenditures. Most researchers have focused on diagnostic variables because of important shortcomings in the alternatives. Adjustment by demographic variables has little ability to encourage health plans to serve people with serious illness or disability. Functional and self-reported health status have modest predictive power (Hornbrook and Goodman, 1995) but are not generally available for large groups. Prior-expenditure data are readily available and can be used to make the most accurate predictions, but the use of these data would recreate the strong incentives to overservice that capitation is supposed to change.
Several groups have been working to refine diagnostic approaches. Arlene Ash, Randall Ellis, and colleagues have been developing diagnostic cost groups (DCGs) to allow the Health Care Financing Administration to risk adjust capitated payments for Medicare beneficiaries (Ash et al., 1989; Ellis and Ash, 1995; Dunn, 1995). The DPS approach is similar to DCGs, in that both systems use groups of diagnoses identified as being predictive of next-year costs. Variants of the DCG models have evolved to include ambulatory diagnoses and to count multiple conditions from different diagnostic categories. One of the most important differences between DCGs and DPS is the population base: DCGs were created using Medicare data, mostly persons over 65 years of age, including only a small proportion of people with disabilities. By contrast, DPS was developed on data from Medicaid recipients with disabilities under 65 years of age and may be more accurate in predicting costs for this population.
Another leading effort in diagnostic risk adjustment is the system developed by Jonathan Weiner, Gerry Anderson, Barbara Starfield, and colleagues, who have integrated elements of two models, Ambulatory Care Groups (ACGs) and Payment Amounts for Capitated systems (PACs), to predict total costs for Medicare beneficiaries over 65 years of age. A distinctive element of the ACG system is that diagnoses are grouped by the nature of their chronicity, e.g., time-limited, likely to recur, or chronic and unstable, rather than simply by body system or disease type (Weiner et al., 1991). Although this approach certainly does a good job of separating the very healthy from the sick, it is unclear how well it will distinguish among people with different degrees of illness.
In upcoming work, we plan to compare how DPS and the new versions of DCGs and ACGs perform in assessing risks for Medicaid recipients with disability. Perhaps all the systems will achieve fairly similar results, or perhaps DPS will prove more accurate because of its development on a population with disabilities.
Is It Good Enough?
No method of risk adjustment can perform so well that purchasers are free to ignore selection. Smart buyers must always pay close attention to quality, marketing, and disenrollment, lest they waste money on health plans that avoid the sick. Like other methods of diagnostic adjustment, DPS is not a panacea, but it should certainly foster better care for people with disabilities than does the current practice of paying the FFS average. Medicaid programs that implement DPS will reward providers who innovate for people with disabilities and who excel in caring for those with greater needs. A key element in fostering the development of improved systems of care is a method of risk adjustment that provides resources commensurate with need.
Technical Note
Data and Methods Used in the Regression
Diagnostic categories are as assigned in DPS version 2.1. The percentages of recipients are based on an analysis of 536,245 disabled recipient years from the five States that are included in the prospective regression: Colorado, Michigan, Missouri, New York, and Ohio. In the prospective regression, each observation is for a recipient with 12 months of eligibility in a base year and at least 1 month of eligibility in the subsequent year. Base years are 1993 in Colorado; 1991 and 1992 in Michigan; 1991, 1992, and 1993 in Missouri; 1992 in New York; and 1991 and 1992 in Ohio. Recipients are excluded if they are institutionalized, receiving home and community-based waiver services, enrolled in Medicare or enrolled in an HMO in the second year, or if they were in an HMO or enrolled in Medicare in the base year. In the concurrent regression, each observation is for a recipient with 12 months of eligibility. Data are included from 1994 in Colorado; 1991-93 in Missouri; 1992 and 1993 in Michigan; 1991 and 1992 in Ohio; and 1993 in New York.
For the fully counted major categories, the percent of recipients indicated in Table 3 is the percent with a diagnosis in one or more of the subcategories.
Expenditures are normalized to the average fiscal year 1993 expenditure level across the five States. In the prospective regression, the dependent variable is the expenditure in the second year, and the independent variables are the diagnostic categories in the preceding year. Also included in the regressions are seven age-gender dummy variables, a variable for part-year eligibility in the second year, and a variable for each State. For persons eligible for part of the year, we used annualized expenditures for the dependent variable. In the concurrent regression, the dependent variable and the independent variable are measured in the same year.
The values shown in Table 3 for the baseline amount are the percentage of recipients with no DPS diagnosis and the average predicted expenditures for them.
Not shown in the table are the values for the age-gender variables, which were, for the prospective regression: under 1 year of age, −$560; age 1-14, −$140; age 15-24 male, −$165; age 15-24 female, $207; age 25-44 male, $0 (the reference category); age 25-44 female, $350; age 45-64 male, $240; age 45-64 female, $349. The value for the part-year variable was $2,105.
Acknowledgments
We wish to acknowledge the contributions of our clinical advisers: Peter Connolly, M.D., David Dorfman, M.D., Jonathan Epstein, M.D., Anne Epstein, M.D., Mary Glover, R.N.P., Robert Master, M.D., David Miller, M.D., Margaret Myers, M.D., and Robert Smith, M.D.
The research described in this article was supported by grants from the Robert Wood Johnson Foundation, the Pew Charitable Trusts, the Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services, and the National Institutes on Disability and Rehabilitation Research, U.S. Department of Education. The research also depended on data generously provided by the Medicaid programs of Colorado, Michigan, Minnesota, Missouri, New York, Ohio, and Wisconsin. Richard Kronick and Lora Lee are with the Department of Family and Preventative Medicine, University of California, San Diego (UCSD). Tony Dreyfus is with the Boston University School of Public Health. Zhiyuan Zhou was with UCSD when this research was performed, and is now with Upjohn Pharmaceuticals. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the UCSD, the Boston University School of Public Health, Upjohn Pharmaceuticals, or the Health Care Financing Administration.
Footnotes
The analysis includes recipients who were continuously eligible for all 12 months of the State fiscal year 1994 (July 1993-June 1994). Recipients with disability were excluded if they were institutionalized, receiving home- and community-based waivered services, or had Medicare coverage. If these recipients had been included, the concentration of expenditures among the disabled would have increased.
These regressions include recipients who were continuously eligible for 24 months: in Colorado, fiscal years 1993 and 1994; in Michigan, calendar years 1992 and 1993. Recipients with disability exclude those who were institutionalized, receiving home and community-based waiver services, or enrolled in Medicare in either year. If these recipients had been included, the R2 statistics would have been substantially higher.
These regressions have the same exclusions as the Colorado and Michigan regressions and used data from 1992 and 1993; for Minnesota we used data from 1991 and 1992. In Wisconsin, recipients are from Milwaukee County only, ages 15-64 years.
DPS now includes many three-digit codes that are frequently used despite the ICD-9-CM instructions to add additional digits for more specificity. To sharpen the accuracy of DPS estimates and plan diagnostic reporting, Medicaid programs could exclude these general three-digit codes from future versions of the model.
See the Medicaid Source Book (Congressional Research Service, 1993) for an excellent description of Medicaid eligibility criteria.
We also have data from Minnesota and from Milwaukee County, Wisconsin. Because of time constraints, we were unable to include these data in the analyses.
The benefits covered in State contracts with plans vary across States. For example, some States carve out mental health services; others make the plan responsible for only the first 20 outpatient visits or 30 inpatient days. Some States exclude home and community-based services from contracts, others do not. We have ignored these State-to-State variations.
The exclusion of recipients in HMOs or institutions should not substantially bias our estimates of expected costs of people who might enroll in HMOs. Few recipients with disability are already in HMOs and, controlling for diagnoses, their health care needs probably do not differ much from other recipients.
We analyzed the residuals from an unweighted regression, found that the standard deviation of the residuals was approximately 4 times larger for persons eligible for 1 month than for persons eligible for 12 months and weighted each observation by 1 − 0.067 multiplied by (12 − number of eligible months). The small number of recipients who died were included in the analysis along with other recipients with part-year eligibility.
The concurrent regressions include observations from 1994 in Colorado, 1991-93 in Missouri, 1992-93 in Michigan, 1991-92 in Ohio, and 1993 in New York.
We include indicator variables for seven age-gender categories and an additional variable indicating whether the recipient was eligible for a full 12-month period. The demographic categories are: under 1 year of age (either sex), age 1-14 (either sex), age 15-24 male, age 15-24 female, age 25-44 male, age 25-44 female, age 45-64 male, and age 45-64 female.
Almost all the standard errors reported from an ordinary least-squares regression are at most one-tenth the magnitude of the parameters; most parameters are 20 to 40 times the estimated standard error. As discussed in the “Methods” section, however, the reported standard errors are underestimates.
The intercept from the regression is $2,040. For all those without a diagnosis, we predicted expenditures using only the demographic variables and averaged them to obtain the figure of $1,998.
Prevalence is measured for recipients in the prospective regression, e.g., with eligibility for all of fiscal year 1992 and at least 1 month of 1993. Because of the high mortality of people with AIDS, if recipients eligible for less than the full year had been included, a higher prevalence would have been found.
For unknown reasons, among persons eligible for the full year in Colorado, a high proportion (16 percent) have no claims at all for the year, compared with 6-7 percent in the other States. This larger percentage with no claims largely accounts for the smaller percentages with identified diagnoses in Colorado.
We used parameter estimates from the combined regression and each of the five State regressions to calculate six predicted expenditure amounts for each recipient. The correlations of these six variables average approximately 0.90.
The independent variables in the regression with prior expenditures include a continuous variable for prior expenditures as well as four dummy variables if prior expenditures were below $300 per year, between $300 and $1,000, between $1,000 and $4,000, or more than $9,000, to account for non-linearities in the relationship between prior and current expenditures.
Other alternatives include paying the FFS average as a final rate and applying the case-mix assessment only for second-year payments or making a preliminary assessment soon after enrollment begins (see Medicaid Working Group [1995] for more details on implementation under these circumstances).
In both the prospective and concurrent models, we used the same classification system, which was developed using prospective analysis.
Reprint Requests: Dr. Richard Kronick, Department of Family and Preventive Medicine, University of California, San Diego, La Jolla, California 92093-0622, email: rkronick@ucsd.edu.
References
- Ash A, Porell F, Gruenberg L, et al. Adjusting Medicare Capitation Payments Using Prior Hospitalization. Health Care Financing Review. 1989;10(4):17–29. [PMC free article] [PubMed] [Google Scholar]
- Congressional Research Service. Medicaid Source Book: Background, Data, and Analysis (a 1993 Update) Washington, DC.: U.S. Government Printing Office; Jan, 1993. [Google Scholar]
- Dunn DL, Rosenblatt A, Taira DA, et al. A Comparative Analysis of Methods of Health Risk Assessment. Chicago: Society of Actuaries; Dec, 1995. Final Report. [Google Scholar]
- Ellis R, Ash A. Refinements to the Diagnostic Cost Group (DCG) Model. Inquiry. 1995/96 Winter;32:418–429. [PubMed] [Google Scholar]
- Enthoven AC. Theory and Practice of Managed Competition in Health Care Finance. New York: North Holland; 1988. [Google Scholar]
- Epstein AM, Cumella E. Capitation Payment: Using Predictors of Medical Utilization to Adjust Rates. Health Care Financing Review. 1988 Fall;10(1):51–70. [PMC free article] [PubMed] [Google Scholar]
- Hornbrook MC, Goodman MJ. Assessing Relative Health Plan Risk With the RAND-36 Health Survey. Inquiry. 1995 Spring;32:56–74. [PubMed] [Google Scholar]
- Kronick R, Zhou Z, Dreyfus T. Making Risk Adjustment Work for Everyone. Inquiry. 1995 Spring;32:41–55. [PubMed] [Google Scholar]
- Master R, et al. The Community Medical Alliance: An Integrated System of Care in Greater Boston for People With Severe Disability and AIDS. Managed Care Quarterly. 1996 Spring;4(2):26–37. [PubMed] [Google Scholar]
- Medicaid Working Group. Nov, 1995. Risk-Adjusted Reimbursement for People With Disabilities: A Diagnostic Approach Proposed for Missouri. [Google Scholar]
- Newhouse JP, et al. Adjusting Capitation Rates Using Objective Health Measures and Prior Utilization. Health Care Financing Review. 1989;10(3):41–54. [PMC free article] [PubMed] [Google Scholar]
- van Vliet RCJA, van de Ven WPMM. Capitation Payments Based on Prior Hospitalizations. Health Economics. 1993;2:177–188. doi: 10.1002/hec.4730020210. [DOI] [PubMed] [Google Scholar]
- van Vliet RCJA. Predictability of Individual Health Care Expenditures. Journal of Risk and Insurance. 1992;59(3):443–461. [Google Scholar]
- Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and Application of a Population-Oriented Measure of Ambulatory Care Case-Mix. Medical Care. 1991;29(5):452–471. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]