Abstract
Purpose
To assess the safety and efficacy of gemcitabine (G) and carboplatin (C) with (arm A) or without (arm B) daily oral cediranib as first-line therapy for advanced non-small cell lung cancer (NSCLC).
Methods
A lead-in phase to determine the tolerability of G 1000 mg/m2 on days 1 and 8, and C on day 1 at AUC 5 administered every 21 days with cediranib 45 mg once daily was followed by a 2 (A):1 (B) randomized phase II study. The primary endpoint was confirmed overall response rate (ORR), with 6-month progression-free survival (PFS6) rate in arm A as secondary endpoint. Polymorphisms in genes encoding cediranib targets and transport were correlated with treatment outcome.
Results
Based on the safety assessment, 30mg daily cediranib was used in the phase II portion. A total of 58 and 29 evaluable patients were accrued to arms A and B. Patients in A experienced more grade 3+ non-hematologic adverse events, 71% vs 45%, p=0.01. The ORR was 19% (A) vs. 20% (B) (p=1.0). PFS6 in A was 48% (95% CI: 35%-62%), thus meeting the protocol specified threshold of at least 40%. The median OS was 12.0 vs. 9.9 months (p=0.10). FGFR1 rs7012413, FGFR2 rs2912791, and VEGFR3 rs11748431 polymorphisms were significantly associated with decreased OS (HR 2.78-5.01, p=0.0002-0.0095).
Conclusions
The trial did not meet its primary endpoint of ORR but met its secondary endpoint of PFS6. Combination with cediranib 30 mg daily resulted in increased toxicity. Pharmacogenetic analysis revealed an association of FGFR and VEGFR variants with survival.
INTRODUCTION
Platinum-based combination chemotherapy offers a modest survival advantage over best supportive care for good PS patients with advanced non-small cell lung cancer (NSCLC).1-3 The Eastern Cooperative Oncology Group (ECOG) E4599 trial,4 showed that addition of the anti-angiogenic agent bevacizumab to carboplatin and paclitaxel prolonged survival compared to chemotherapy alone and validated the decades-long hypothesis that the angiogenesis pathway plays a critical role in tumorigenesis and that its inhibition can result in clinical benefit.5 Because of toxicity, patients with squamous cell histology and history of hemoptysis were excluded from this pivotal trial.6,7
Cediranib (AZD2171) is an oral tyrosine kinase inhibitor of all three VEGFRs [VEGF (vascular endothelial growth factor) receptors], PDGFR [PDGF (platelet-derived growth factor) and FGFR 1, 4 [FGF (fibroblast growth factor) receptor], that has shown promising antitumor activity against a number of malignancies including NSCLC in phase I studies. N0528 was conceptualized shortly after the phase I study combining cediranib with carboplatin/paclitaxel reported in 2006 that full single-agent dose of cediranib at 45 mg once daily maybe administered with standard doses of the combination. 8,9 Another phase Ib study of cediranib with cisplatin/gemcitabine in NSCLC reported in 2007, a few weeks prior to activation of N0528, that the protocol-defined maximum tolerated dose was not reached at the 45 mg level, although 30 mg once daily dosing appeared to be better tolerated with protracted dosing.10,11 Variations in toxicities and response have been observed in all these studies8-11. These phenotypic variations are often traceable to underlying genetic variations as a result of polymorphic genes encoding proteins that metabolize, transport or are targets for the drug. Cediranib targets VEGFR, PDGFR and FGFR family members and inhibits the function of the ABC drug transporters such as ABCB1 and ABCC1, hence affecting drug efflux.12,-14 Metabolism and transport mechanisms are often shared among drugs; therefore genetic variation could affect the bioavailability of more than one drug when they are used in combination, as is the case for ABC drug transporters in this combination therapy. There is no report to date indicating any association of polymorphisms in cediranib-related genes with treatment efficacy or toxicity, therefore this exploratory pharmacogenetics correlative study focused on single nucleotide polymorphisms (SNPs) of 9 of the cediranib-related genes in the VEGF-receptor and FGF-receptor families, VEGFA, and the ABC transporter genes. We did not examine any cediranib metabolism genes in this initial exploratory study. .We thus evaluated the efficacy, tolerability and safety of cediranib with the more commonly used regimen of carboplatin and gemcitabine in patients with advanced NSCLC and examined angiogenesis markers in addition to cediranib molecular target genes (VEGFR 1-3 and FGFR1-3), VEGFA and ABC family transport genes (ABCB1 and ABCC1), and correlated these markers with clinical outcomes.
MATERIALS AND METHODS
Patients and Evaluations
Eligible patients had ECOG performance status 0-1 and histologic or cytologic confirmation of measurable, chemotherapy naïve, stage IIIB (with malignant pleural effusion) or stage IV NSCLC (AJCC staging 6th edition criteria). Squamous histology was allowed. At the time of study concept , the evidence of harm for anti-VEGF therapies in this subset of patients were in the context of fatal hemoptysis, The controversy surrounding this limited data then was deemed inconclusive. Thus this study allowed squamous histology as long as other exclusion criteria typically associated with this histology were not present (hemoptysis, cavitary lesions). Prior neoadjuvant or adjuvant therapy for lung cancer was allowed if > 12 months had elapsed prior to registration. Patients needed to have adequate bone marrow, hepatic and renal function. Patients who were pregnant, with hemoptysis, cavitary lesions, untreated or symptomatic brain metastases, poorly-controlled hypertension or proteinuria ≥ 500 mg/24 hours were excluded. Tumor measurements by RECIST were obtained at least every 6 weeks. The protocol was approved by institutional review boards, and all patients were required to give written informed consent under Federal and institutional guidelines. Common Terminology Criteria for Adverse Events (CTCAE) version 3 was used to grade the severity of toxicities encountered during study period.
Study Treatment
All patients received gemcitabine 1000 mg/m2 on days 1 and 8 and carboplatin dosed to an area under the serum concentration-time curve (AUC) of 5 on day 1 via intravenous infusion every 3 weeks for a maximum of 6 cycles. Patients in arm A also received once daily oral cediranib in combination with chemotherapy. Patients in arm A with at least stable disease after the initial 6 cycles could continue on maintenance cediranib until disease progression.
Accrual to the study was suspended for a minimum of 3 weeks after the 6th patient was randomized to arm A for safety analysis. These patients were evaluated weekly during the first cycle of treatment for the occurrence of any dose-limiting toxicity(DLT) defined as: CTC grade 3 or higher non-hematologic toxicities (nausea, vomiting or diarrhea were DLTs if severity was grade 3 or higher despite maximal use of anti-emetic support or anti-diarrheal agents, respectively), grade 4 thrombocytopenia, febrile neutropenia or grade 4 neutropenia greater than 5 days, or any toxicity requiring cediranib dose interruption of more than 2 weeks.
Correlative studies
DNA was extracted from blood samples at baseline for analysis of single-nucleotide polymorphisms (SNPs) in the following cediranib target genes; FGFR1, FGFR2, FGFR3, VEGFA, FLT1 (VEGFR1), KDR (VEGFR2), FLT4 (VEGFR3) and the ATP- binding cassette (ABC) transporter genes ABCB1 and ABCC1. These genes were chosen because they play a role in the transport of and serve as targets for Cediranib. Acquisition of tagSNPs and genotyping were similar to that previously described.15 Plasma was processed at baseline and prior to cycle 3 for analysis using the Bio Plex Pro human angiogenesis magnetic bead-based multiplex assay for angiopoietin- 2, folllistatin, GCSF, HGF, IL-8, leptin, PDGF-BB, PECAM, VEGF-A in the laboratory of Dr. Debabrata Mukhopadhyay (Mayo Clinic, Rochester, MN). The assay was carried out according to the manufacturer's instructions and all samples, including standards, were plated in triplicate. Data were acquired using the Luminex software system.
Statistical Methods
A 2:1 randomization scheme (A: B) was utilized to assess the primary endpoint of confirmed objective overall response rate (ORR) using RECIST 1.0.16 A maximum of 28 patients randomized to arm B would provide an estimate of the ORR within 19 percentage points with 90% confidence. Using a one-stage Fleming design, a sample size of 56 patients in arm A provided 86% power to detect a true ORR of at least 40% (if the observed ORR in arm B was 25%), with a type I error rate of 0.09. Arm A would be declared promising if at least 19 successes were observed. For the secondary endpoint of 6-month progression-free survival (PFS6) rate, assuming an exponential model, a sample of 56 evaluable patients in arm A also provided 86% power to test that the true PFS6 rate is at most 25% versus the alternative hypothesis that the PFS6 rate is at least 40% with a type I error rate of 0.09. Secondary endpoints included adverse event profile, overall survival, progression-free survival, and duration of response.
Confirmed response, per RECIST 1.0, was defined as a complete or partial response (CR, PR) noted on two consecutive evaluations at least 4 weeks apart. PFS6 rate considered patients who terminated treatment prior to 6 months post randomization for reasons other than disease progression as failures regardless of their progression status at 6 months. Overall survival (OS) time was defined as the time from registration to death due to any cause. Progression-free survival (PFS) was defined as the time from registration to the first date of disease progression or death as a result of any cause. PFS was censored at the date of the last contact for patients alive and progression free at the time of this analysis. The distributions of PFS and OS were estimated using the Kaplan-Meier (KM) method, and compared between the arms in an exploratory fashion using the stratified log rank test (adjusting for the stratification factors of ECOG performance status (0 vs. 1) and history of adjuvant/neo-adjuvant therapy (yes vs. no). The adverse events (regardless of attribution) were summarized as maximum severity per subject and type, across the duration of intervention for each arm and compared using Fisher's exact test.
Aggregate fluorescence values for the angiogenesis markers (analytes) (measured on a continuous scale) were analyzed. Exploratory Cox proportional hazards model, adjusted for plate number and well location, was used to assess the impact of the levels of the analytes at baseline, prior to cycle 3 (using a landmark approach), and percentage increase from baseline to cycle 3 (using a landmark approach) on OS and PFS. Logistic regression models were used to compare the AE patterns between the SNP subgroups. KM curves and Cox proportional hazards models were used to compare the OS and PFS distributions between the different tagSNP subgroups.
P-values ≤ 0.05 were considered statistically significant for the primary efficacy and AE comparisons, and p-values ≤ 0.02 were considered promising for the correlative analyses. No adjustments for multiple comparisons were performed for the exploratory correlative analyses.
RESULTS
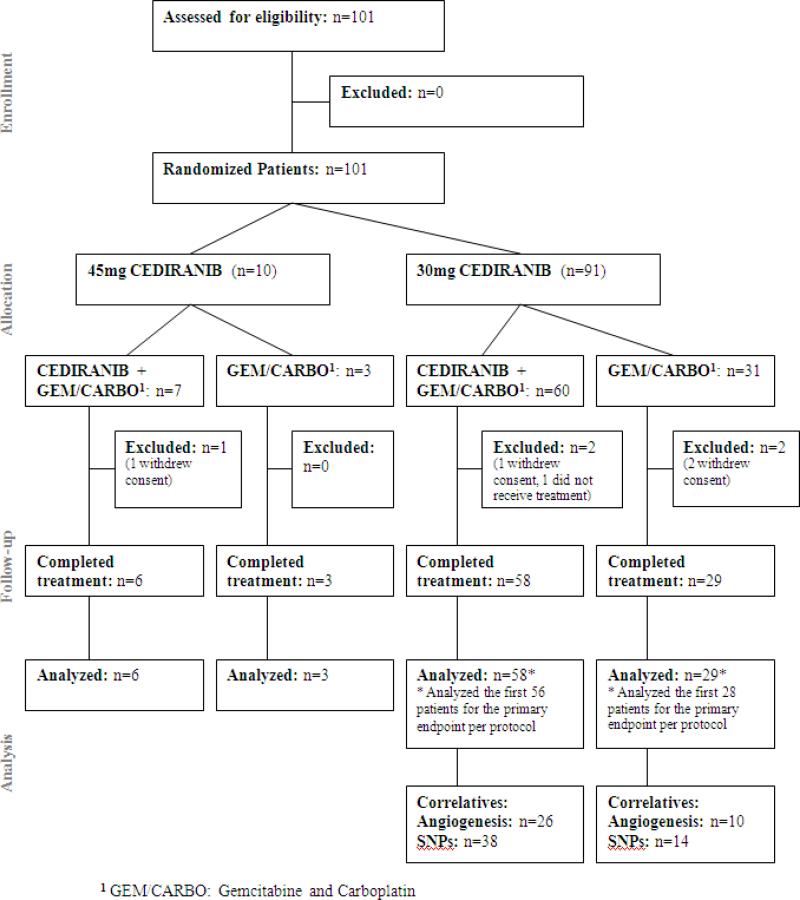
N0528 was activated on June 15, 2007, and completed accrual on December 5, 2008. Data for this report were frozen on September 13, 2011. The CONSORT trial flow diagram is shown in Figure 1.
Figure 1.
CONSORT trial flow diagram
Lead-in phase
10 patients were accrued (7 in arm A, 3 in arm B). Cediranib 45 mg was administered once daily in Arm A. One patient in arm A withdrew consent prior to starting therapy and was excluded. One patient treated on arm A had a protocol defined DLT (grade 3 diarrhea/nausea causing a dose interruption of > 14 days in cycle 1), and only one of the six patients on arm A completed two cycles at full doses of cediranib. Thus the starting cediranib dose of 45mg was not tolerable and was reduced to 30mg once daily on a continuous schedule for the phase II portion of the study. These patients were not included in the analysis for the phase II portion of the study.
Phase II portion
Patient and treatment characteristics
Ninety-one (91) patients were randomized (60 arm A, 31 arm B). Two patients from each arm were removed from the analysis due to either withdrawal of consent or never receiving study treatment (Figure 1). Data from 58 patients in arm A and 29 patients in arm B are included here. Patient characteristics, treatment and follow-up data are summarized in Table 1. There were no significant differences in baseline patient characteristics between the arms except for distribution of histologic subtype.
Table 1.
Baseline Patient Characteristics and Treatment / Follow-up
| A (N=58) | B (N=29) | p-value | |
|---|---|---|---|
| Patient Characteristics | |||
| Age | 0.481 | ||
| Median | 65.0 | 64.0 | |
| Range | (46.0-81.0) | (45.0-82.0) | |
| Gender | 0.752 | ||
| Female | 26 (44.8%) | 12 (41.4%) | |
| Male | 32 (55.2%) | 17 (58.6%) | |
| Performance Score | 0.872 | ||
| 0 | 33 (56.9%) | 17 (58.6%) | |
| 1 | 25 (43.1%) | 12 (41.4%) | |
| Race | 0.433 | ||
| White | 55 (94.8%) | 26 (89.7%) | |
| Black or African American | 3 (5.2%) | 2 (6.9%) | |
| Unknown: Patient unsure | 0 (0.0%) | 1 (3.4%) | |
| Prior Adjuvant Treatment | 1.003 | ||
| Yes | 2 (3.4%) | 1 (3.4%) | |
| No | 56 (96.6%) | 28 (96.6%) | |
| Cell Type | 0.023 | ||
| Squamous | 9 (15.5%) | 8 (27.6%) | |
| Adenocarcinoma | 22 (37.9%) | 16 (55.2%) | |
| All Other | 27 (46.6%) | 5 (17.2%) | |
| Treatment/Follow-up | |||
| Follow-up Time in Months for Alive Patients | 0.421 | ||
| Median | 33.4 | 31.9 | |
| Range | (3.9-38.1) | (30.8-33.1) | |
| Reason End Treatment | 0.00023 | ||
| Completed Study Per Protocol | 5 (8.6%) | 10 (34.5%) | |
| Refused Further Treatment | 5 (8.6%) | 2 (6.9%) | |
| Adverse Event | 32 (55.2%) | 3 (10.3%) | |
| Disease Progression | 13 (22.4%) | 11 (37.9%) | |
| Died on Study† | 1 (1.7%) | 2 (6.9%) | |
| Other | 2 (3.4%) | 1 (3.4%) | |
| Treatment Cycles Received | 0.911 | ||
| Median | 3.5 | 4.0 | |
| Range | (1.0-20.0) | (1.0-6.0) | |
Wilcoxin-rank Sum
Chi-Square
Fisher Exact
Reasons: disease progression (arm A); Death NOS and Death from pulmonary hemorrhage, both deemed not related to study treatment (arm B).
Clinical Outcomes: Primary and Secondary Endpoints
The confirmed ORR in arms A and B among all the evaluable patients was 19% (n=11; 95% CI: 10-31%) and 20% (n=6; 95% CI: 8-40%) (p=1.0). The PFS6 rate among the first 56 evaluable patients in arm A was 46% (26; 95% CI: 33-60%), thus meeting the protocol-specified secondary endpoint. The PFS6 rate among all evaluable patients in arms A and B was 48% (95% CI: 35-62%) and 38% (95% CI: 21-58%) (p=0.49), respectively.
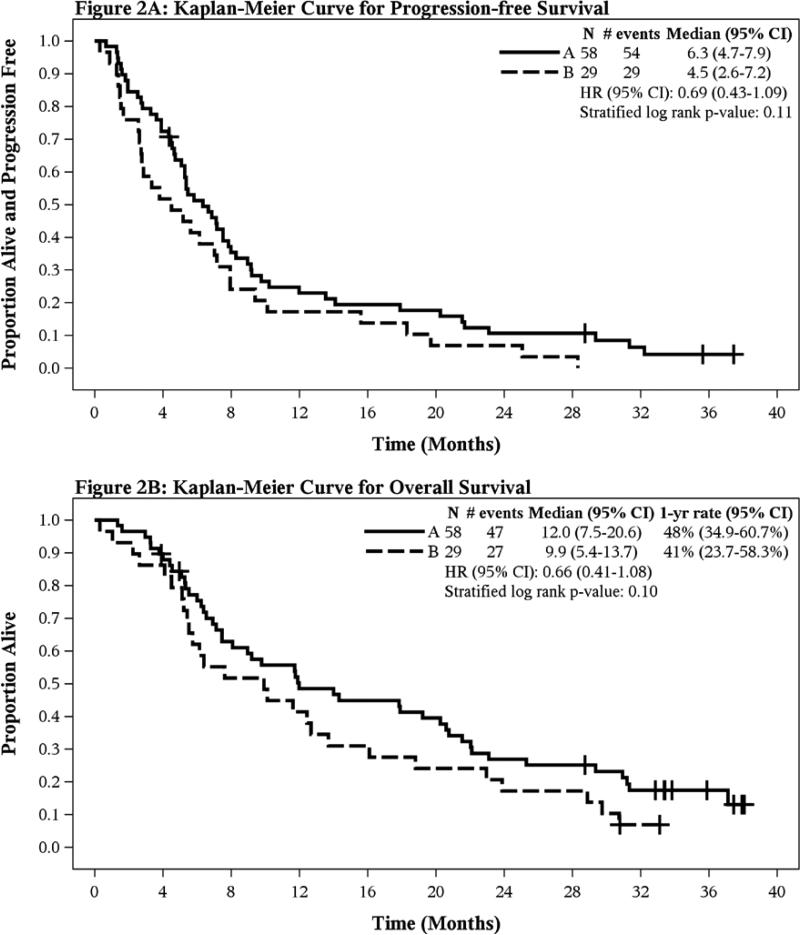
Median PFS was 6.3 months (A, 95% CI 4.7-7.9) versus 4.5 months (B, 95% CI 2.6-7.2), (stratified log rank p=0.11) (Figure 2A). Median OS was 12 months (A, 95% CI 7.5-20.6) versus 9.9 months (B, 95% CI 5.4-13.7), (stratified log rank p=0.10) (Figure 2B).
Figure 2.
Kaplan-Meier Curves in Overall Population for (A)Progression-Free Survival and (B)Overall Survival
Adverse Events
A total of 54 (A, 93%) and 26 (B, 90%) patients had grade 3 or higher AEs (Table 2). Grade 3+ non-hematologic AEs were higher in arm A (71% vs 45%, p=0.01). The most common grade 3/4 non-hematologic AEs in arm A were fatigue (13.8%) and dyspnea (10.3%). The most common grade 3/4 AEs in arm B was fatigue (10.3%). Grade 2 AEs (at least possibly related to treatment) as maximum occurrence were reported in 4 patients in arm A and 2 patients in arm B. In arm A, these were fatigue (5.6%), hypothyroidism (1.8%); oral mucositis (1.8%); neutropenia (3.7%), anorexia (1.8%), and dyspnea (1.8%). Grade 2 anemia was reported in both groups (3.7% in arm A, 7.7% in arm B).
Table 2.
Adverse Events by Arm
| A (N=58) | B (N=29) | p-value | |
|---|---|---|---|
| Overall in all patients | |||
| Grade 3+ | 54 (93.1%) | 26 (89.7%) | 0.681 |
| Grade 4+ | 38 (65.5%) | 16 (55.2%) | 0.342 |
| Grade 3+ Hematologic | 44 (75.9%) | 20 (69.0%) | 0.492 |
| Grade 4+ Hematologic | 32 (55.2%) | 12 (41.4%) | 0.222 |
| Grade 3+ Non-Hematologic | 41 (70.7%) | 13 (44.8%) | 0.012 |
| Grade 4+ Non-Hematologic | 11 (19.0%) | 5 (17.2%) | 0.842 |
| Occurring in at least 10% of patients | |||
| Fatigue Grade 3+ | 8 (13.8%) | 3 (10.3%) | 0.741 |
| Anemia Grade 3+ | 6 (10.3%) | 8 (27.6%) | 0.032 |
| Leukopenia Grade 3+ | 28 (48.3%) | 12 (41.4%) | 0.542 |
| Neutropenia Grade 3+ | 35 (60.3%) | 13 (44.8%) | 0.172 |
| Thrombocytopenia Grade 3+ | 33 (56.9%) | 13 (44.8%) | 0.282 |
Fisher Exact
Chi-Square
Treatment was discontinued due to AE in a greater proportion of arm A patients (55% versus 10%, p=0.0001). Of the 49 patients in arm A who started cycle 2, 18 (37%) continued cediranib 30 mg once daily, 22 (45%) decreased to 20 mg, 7 were at 15mg, and 2 did not receive any further cediranib. Of the 38 patients who started cycle 3, 5(13%) were receiving cediranib 30 mg once daily, 25 (66%) were at 20 mg, 7 were at 15mg, and 1 did not receive any cediranib. The most common reasons for dose adjustments were: non-hematologic AEs (14%) and thrombocytopenia (12%) in cycle 1. Neutropenia and/or thrombocytopenia were the most common reasons for dose adjustments in the subsequent cycles. There was no significant interaction of AE with histology although this analysis is limited by the small number (n=17) of patients with squamous cell histology.
Correlative Outcomes
Thirty-six (36) patients (26 Arm A, 10 Arm B) had complete angiogenesis marker analyses and 52 (38 in Arm A, 14 in Arm B) patients had sufficient DNA for SNP analysis. A comparison between patients with and without adequate specimens for the correlative studies revealed no significant differences in the underlying clinical and demographic variables.
Angiogenesis markers
Because of small sample size, data were combined across arms for this analysis. Baseline levels of the angiogenesis markers had no prognostic value. Patients with higher levels of follistatin prior to start of cycle 3 had improved subsequent OS and PFS (HR=0.81 for a 20-unit increase; p≤0.02). Patients with higher levels of IL-8 and PDGF-BB prior to start of cycle 3 had worse OS (HR=1.37 for a 10-unit increase in IL-8; adjusted HR = 1.21 for a 50-unit increase in PDGF-BB; p≤0.02). Patients with a higher percentage increase in VEGF-A prior to start of cycle 3 compared to baseline had better subsequent PFS (HR=0.88 for 20-unit increase; p=0.02).
Genetic Polymorphisms: TagSNPs and distribution of variants
One hundred and twenty (120) tagSNPs generated from the 9 genes with minor allele frequency (MAF) >5% were successfully genotyped. The distribution of genotypes is shown in the supplementary data (TableS1). Except for one SNP, all other SNPs genotyped were in Hardy-Weinberg equilibrium (HWE). Genotypes observed in <5 patients were regrouped and if the regrouped frequency was ≤ 10%, the SNP was excluded from the analyses with the clinical outcomes. When the genotypes were relevant to arm A alone (FLT1, KDR, FLT4 (VEGFR1-3), VEGFA, FGFR1-3), data were analyzed within arm A, and combined across arms otherwise (ABCB1, ABCC1).
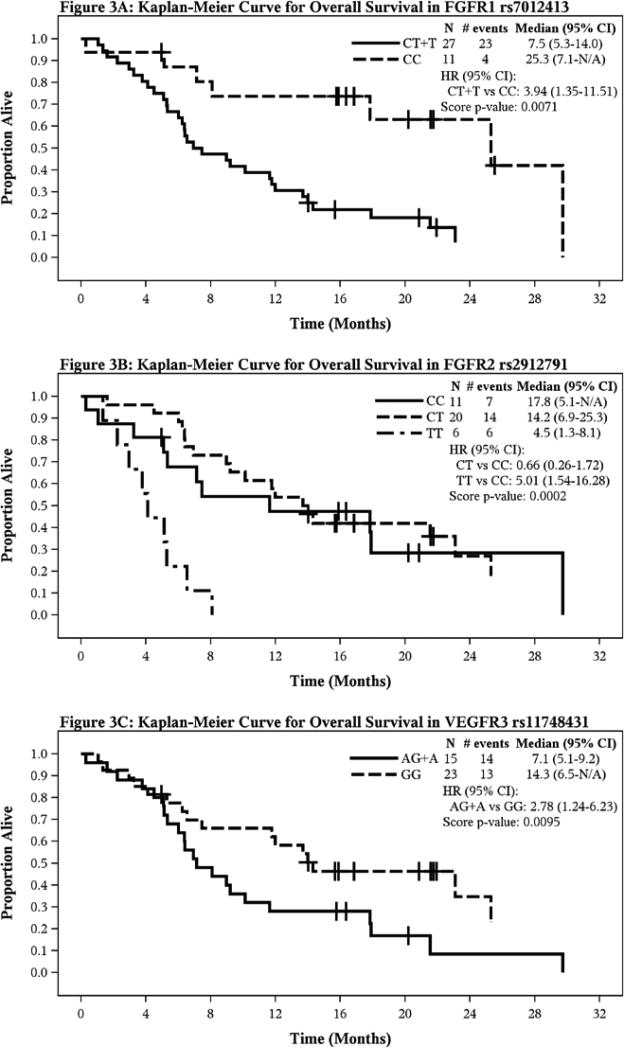
At p≤ 0.02, four polymorphisms, rs2235015 in ABCB1; rs17542768 and rs2071616 in FGFR2 and rs3024987 in VEGFA were significantly associated with reduced toxicities (Table 3), while four other polymorphisms in FGFR1 ( rs7012413), FGFR2 ( rs2912791, rs2981429) and FLT4/VEGFR3 (rs11748431) were significantly associated with survival outcomes (Table 4). Variant alleles in FGFR1 rs7012413, FGFR2 rs2912791 and FLT4 (VEGFR3) rs11748431 SNPs correlated with inferior OS, however, the variant allele in FGFR2 rs2981429 SNP was associated with superior PFS (Table 4), Figure 3 shows the KM plots for OS according to pertinent SNP.
Table 3.
Genotype and Adverse Event Endpoints1
| Adverse Event (Treatment Arm) | Genotype (N) | N Events (% Between Allele Groups) | Odds Ratio (95% CI) | P-value2 |
|---|---|---|---|---|
| Grade 3+ Adverse Arm A | FGFR2: rs17542768 | |||
| GA+GG (10) | 7 (20.6) | 0.086 (0.008-0.963) | 0.0194 | |
| AA (28) | 27 (79.4) | Reference | -- | |
| Grade 3+ Hematologic Arm A | FGFR2: rs2071616 | |||
| GA+AA (21) | 12 (42.9) | 0.083 (0.009-0.750) | 0.0101 | |
| GG (17) | 16 (57.1) | Reference | -- | |
| VEGFA: rs3024987 | ||||
| TC+TT (12) | 5 (17.9) | 0.093 (0.018-0.491) | 0.0023 | |
| CC (26) | 23 (82.1) | Reference | -- | |
| Grade 3+ Non-Hematologic Arm A | FGFR2: rs17542768 | |||
| GA+GG (10) | 4 (14.8) | 0.145 (0.029-0.712) | 0.0117 | |
| AA (28) | 23 (85.2) | Reference | -- | |
| Grade 3+ Non-Hematologic Arm A+B | ABCB1: rs2235015 | |||
| GT+TT (16) | 6 (18.8) | 0.185 (0.051-0.668) | 0.0074 | |
| GG (34) | 26 (81.3) | Reference | -- |
Only p-values < 0.02 shown
Fisher's Exact P-value
Table 4.
Genotype and Time to Event Endpoints1
| Time Event (Treatment Arm) | Genotype (N) | N Events (% Between Allele Groups) | Median (95% CI)2 | Hazard Ratio (95% CI)3 | P-value4 |
|---|---|---|---|---|---|
| Overall Survival Arm A | FGFR1: rs7012413 | ||||
| CT+TT (27) | 23 (85.2) | 7.5 (5.3-14.0) | 3.94 (1.35-11.51) | 0.0071 | |
| CC (11) | 4 (14.8) | 25.3 (7.1-N/A) | Reference | -- | |
| FGFR2: rs2912791 | |||||
| CT (20) | 14 (51.9) | 14.2 (6.9-25.3) | 0.66 (0.26-1.72) | 0.0002 | |
| TT (6) | 6 (22.2) | 4.5 (1.3-8.1) | 5.01 (1.54-16.28) | -- | |
| CC (11) | 7 (25.9) | 17.8 (5.1-N/A) | Reference | -- | |
| FLT4(VEGFR3): rs11748431 | |||||
| AG+AA (15) | 14 (51.9) | 7.1 (5.1-9.2) | 2.78 (1.24-6.23) | 0.0095 | |
| GG (23) | 13 (48.1) | 14.3 (6.5-N/A) | Reference | -- | |
| Progression-Free Survival Arm A | FGFR2: rs2912791 | ||||
| CT (20) | 17 (53.1) | 8.5 (4.7-14.1) | 0.55 (0.24-1.27) | 0.0010 | |
| TT (6) | 6 (18.8) | 2.2 (1.3-5.5) | 3.64 (1.17-11.26) | -- | |
| CC (10) | 9 (28.1) | 5.8 (0.7-7.1) | Reference | -- | |
| FGFR2: rs2981429 | |||||
| CT (20) | 17 (51.5) | 8.4 (4.7-14.1) | 0.27 (0.11-0.66) | 0.0106 | |
| TT (8) | 7 (21.2) | 6.1 (0.7-17.9) | 0.41 (0.15-1.16) | -- | |
| CC (9) | 9 (27.3) | 4.7 (1.3-6.3) | Reference |
Only p-values < 0.02 shown
Kaplan-Meier method
Cox model
Score P-value
Figure 3.
Kaplan-Meier Curves for Overall Survival according to polymorphic variants (A) FGFR1 rs7012413, (B) FGFR2 rs2912791 and (C) VEGFR3 rs11748431
DISCUSSION
The results of E4599 have spurred clinical development of angiogenesis inhibitors in lung cancer therapy. However, significant progress has yet to be made, particularly for small molecule VEGFR inhibitors. The phase III ESCAPE study of first-line carboplatin and paclitaxel with or without the multi kinase inhibitor sorafenib,17 was terminated early due to lack of benefit at interim analysis. Subgroup analyses showed an association between squamous histology and excess mortality and inferior survival with sorafenib. The phase III MONET1 study of carboplatin and paclitaxel with or without motesanib, another multikinase inhibitor, suffered a similar fate.18 Interim analysis in 2008 led to its temporary suspension because of more deaths in squamous histology patients on the treatment arm. The study was reopened in February 2009 to patients with nonsquamous histology only, but final analysis of the 1090 non-squamous patients showed no benefit to the addition of motesanib.19
BR.24 was a phase II/III double-blind study investigating the addition of 45 mg daily cediranib to first-line carboplatin and paclitaxel.20 The study was amended early to cediranib 30 mg daily and to exclude poor PS patients due to toxicities of hypertension, thrombocytopenia, hemoptysis, dermatologic and GI AEs. Despite the increase in serious AEs including fatalities in the cediranib arm, the phase II interim analysis revealed a significantly higher response rate with a HR of 0.77 for progression-free survival in the cediranib arm (95% CI, 0.56-1.08) regardless of histology. However, due to the poor tolerance of even the 30 mg dose, the phase III placebo-controlled BR.29 study utilized cediranib at 20mg in combination with carboplatin and paclitaxel. Our experience similarly reflected increased toxicity of cediranib at the 30 mg once daily dosing in combination with carboplatin and gemcitabine, with nearly half of initial cohort requiring dose reduction to 20 mg once daily by the second cycle of treatment and nearly fivefold increased rate of treatment discontinuation due to toxicity in this arm compared to the control group.
While this current study is not powered for survival estimates, results presented here affirm the majority of the findings in BR.24 with PFS and OS trends favoring the cediranib arm (PFS: HR 0.69, 95% CI 0.43 to 1.09; OS: HR 0.66, 95% CI 0.41-1.08) but with more toxicity in the cediranib arm. Of note, the aforementioned BR.29 trial was recently halted due to futility in achieving its overall survival endpoint based on interim analysis of the PFS data despite achieving an increased ORR with the combination of cediranib at the reduced 20 mg daily dose. 21 The need to further dose reduce cediranib to 20 mg in most patients attests to the frequent observation that doses established with short-term use during early clinical development may not prove feasible in clinical practice with chronic dosing. Moreover, such toxicities can undermine overall clinical benefit despite improvement in surrogate endpoints, as seen in BR.29. Our analysis of angiogenesis biomarkers supported observations from other studies. Follistatin is a single-chain glycoprotein that can enhance endothelial cell proliferation.22-23 Whereas baseline follistatin levels had no prognostic value, patients with higher post-treatment levels had better survival which may be explained by mouse models demonstrating increased apoptosis and suppression of angiogenesis and metastasis in follistatin-dependent tumors.22,24 In contrast, elevated levels of plasma IL-8, a proinflammatory chemokine,25-26 had been correlated with poor outcomes in a variety of clinical settings,27-29 a finding confirmed in our study. PDGF-BB is a mitogenic factor that synergizes with FGF2 to promote tumor neovascularization.30-32 Worse outcome among patients with higher post-treatment PDGF-BB may be attributable to the activation of angiogenic switch, such as through FGF signaling, which had been postulated to accelerate the development of a more aggressive phenotype.33,34 On the other hand, serum or plasma VEGF levels are not consistently predictive of outcome and its predictive/prognostic value maybe dependent on tumor type.35-42
Existing literature has reported prognostic and predictive associations of VEGFA and VEGFR polymorphic variants to therapy with anti-VEGF agents.43-45 and these results further contribute to those observations. We observed that an intron 1 polymorphism in VEGFA was associated with reduced hematologic toxicity and an intron 1 polymorphism inVEGFR3 was associated with decreased survival. Furthermore an intron 5 polymorphism in ABCB1 was also associated with reduced risk of non-hematologic toxicities in both treatment groups. A variety of ABCB1 polymorphisms had been correlated with treatment response and toxicities using platinum-based regimens in lung cancer patients.46-48 Finally, reports indicate that FGFR1 amplification is a common genetic event and associated with tumor growth and survival in sqamous cell lung cancer.49-51 Based on our exploratory analysis, we further report that certain genetic variants of FGFR1 and FGFR2 may also be correlated not only with treatment toxicity but also with prognostic outcomes to cediranib therapy in NSCLC. Thus an FGFR1 intron 1 polymorphism was associated with decreased survival while polymorphisms in introns 2, 4 and 6 of FGFR2 were associated with better survival and reduced toxicities.
In summary, cediranib in combination with gemcitabine and carboplatin at the 30mg daily dose tested in this study was significantly more toxic than chemotherapy alone. Furthermore, this combination did not demonstrate improvement in ORR compared to chemotherapy alone in an unselected NSCLC population. The prognostic significance of FGFR1 and VEGFR polymorphisms should be further investigated in future studies involving VEGFR/FGFR kinase inhibitors.
Acknowledgments
Supported by U10 CA25224 from NCI and by a grant from Astra-Zeneca Pharmaceuticals
Footnotes
Presented in abstract form at the 46th Annual Meeting of the American Society of Clinical Oncology in Chicago, Illinois, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfister DG, Johnson DH, Azzoli CG, et al. American society of clinical oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Azzoli CG, Baker S, Jr., Temin S, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: Analysis of eastern cooperative oncology group trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 8.Laurie SA, Arnold A, Gauthier I, et al. Final results of a phase I study of daily oral azd2171, an inhibitor of vascular endothelial growth factor receptors (vegfr), in combination with carboplatin I + paclitaxel (t) in patients with advanced non-small cell lung cancer (nsclc): A study of the national cancer institute of Canada clinical trials group (ncic ctg). J Clin Oncol. 2006;24:18s. doi: 10.1200/JCO.2007.14.4741. (abstr 3054) [DOI] [PubMed] [Google Scholar]
- 9.Laurie SA, Gauthier I, Arnold A, et al. Phase I and pharmacokinetic study of daily oral azd2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: The national cancer institute of Canada clinical trials group. J Clin Oncol. 2008;26:1871–1878. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 10.Goss GD, Laurie S, Shepherd F, et al. Phase I study of daily oral azd2171, a vascular endothelial growth factor receptor inhibitor (VEGRFI), in combination with gemcitabine and cisplatin (g/c) in patients with advanced non-small cell lung cancer (ANSCLC): A study of the NCIC clinical trials group. J Clin Oncol. 2007;25:18s. doi: 10.1200/JCO.2007.14.4741. (abstr 7649) [DOI] [PubMed] [Google Scholar]
- 11.Goss G, Shepherd FA, Laurie S, et al. A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: A study of the national cancer institute of Canada clinical trials group. Eur J Cancer. 2009;45:782–788. doi: 10.1016/j.ejca.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Tian C, Ambrosone CB, Darcy KM, et al. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124(3):575–81. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedge SR, Kendrew J, Hennequin LF. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 14.Tao LY, Liang YJ, Wang F, et al. Cediranib (recentin, AZD2171) reverses ABCB1- and ABCC1-mediated multidrug resistance by inhibition of their transport function. Cancer Chemother Pharmacol. 2009;64:961–969. doi: 10.1007/s00280-009-0949-1. [DOI] [PubMed] [Google Scholar]
- 15.Adjei AA, Mandrekar SJ, Dy GK, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small cell lung cancer: NCCTG and SWOG study N0426. J Clin Oncol. 2010;28:614–619. doi: 10.1200/JCO.2009.23.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 18.Amgen [May 19, 2012];Takeda Say Lung Cancer Drug Study Will Resume (Update2) Available at: http://www.bloomberg.com/apps/news?pid=newsarchive&sid=aUYBejA4gl3M&refer=japan.
- 19.Scagliotti G, Vynnychenko I, Ichinose Y, et al. An international, randomized, placebo-controlled, double-blind phase III study (MONET1) of motes nib plus carbolated/palliate(C/P) in patients with advanced nonsquamous non-small cell lung cancer (NSCLC). J Clin Oncol. 2011;29:18s. doi: 10.1200/JCO.2011.41.4987. (abstrLBA7512) [DOI] [PubMed] [Google Scholar]
- 20.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]
- 21.Laurie SA, Solomon BJ, Seymour L, et al. A randomized double-blind trial of carboplatin plus paclitaxel (CP) with daily oral cediranib (CED), an inhibitor of vascular endothelial growth factor receptors, or placebo (PLA) in patients (pts) with previously untreated advanced non-small cell lung cancer (NSCLC): NCIC Clinical Trials Group study BR.29. J Clin Oncol. 2012:30S. abstr 7511. [Google Scholar]
- 22.Mccarthy SA, Bicknell R. Inhibition of vascular endothelial cell growth by activin-A. J Biol Chem. 1993;268:23066–23071. [PubMed] [Google Scholar]
- 23.Krneta J, Kroll J, Alvex F, et al. Dissociation of angiogenesis and tumor genesis in follistatin- and activin-expressing tumors. Cancer Res. 2006;66:5686–5695. doi: 10.1158/0008-5472.CAN-05-3821. [DOI] [PubMed] [Google Scholar]
- 24.Ogino H, Yano S, Kakiuchi S, et al. Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in NK cell-depleted SCID mice. Clin Cancer Res. 2008;14:660–667. doi: 10.1158/1078-0432.CCR-07-1221. [DOI] [PubMed] [Google Scholar]
- 25.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 26.Huang D, Ding Y, Zhou M, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopetz S, Hoff Pm, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellmunt J, Gonzalez-Larriba JL, Prior C, et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22:2646–2653. doi: 10.1093/annonc/mdr023. [DOI] [PubMed] [Google Scholar]
- 29.Orditura M, De Vita F, Catalano G, et al. Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: relationship with prognosis. J Interferon Cytokine Res. 2002;22:1129–1135. doi: 10.1089/10799900260442557. [DOI] [PubMed] [Google Scholar]
- 30.Westermark B, Heldin CH. Platelet-derived growth factor. Structure, function and implications in normal and malignant cell growth. Acta Oncol. 1993;32:101–105. doi: 10.3109/02841869309083897. [DOI] [PubMed] [Google Scholar]
- 31.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 32.Nissen LJ, Cao R, Hedlund EM, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DePrimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rini Bi, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 37.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 38.Pena C, Lathia C, Shan M, et al. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: results from sorafenib phase III treatment approaches in renal cancer global evaluation trial. Clin Cancer Res. 2010;16:4853–4863. doi: 10.1158/1078-0432.CCR-09-3343. [DOI] [PubMed] [Google Scholar]
- 39.Jayson GC, de Haas S, Delmar P, et al. Evaluation of plasma VEGFA as a potential predictive pan-tumour biomarker for bevacizumab.. European Multidisciplinary Cancer Congress; September 23-27, 2011; 2011. Abstract 804. [Google Scholar]
- 40.Van Cutsem E, Jayson G, Dive C, et al. Analysis of blood plasma factors in the AVITA phase III randomized study of bevacizumab with gemcitabine-erlotinib in patients with metastatic pancreatic cancer.. European Multidisciplinary Cancer Congress. Abstract 803. 2011 European Multidisciplinary Cancer Congress; September 23-27, 2011.2011. [Google Scholar]
- 41.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab-an Eastern Cooperative Oncology Group study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 42.Mok T, Gorbunova V, Juhasz E, et al. Biomarker analysis in BO21015, a phase II randomized study of first-line bevacizumab combined with carboplatin-gemcitabine or carboplatin-paclitaxel in paitents with advanced or recurrent non-squamous non-small cell lung cancer.. European Multidisciplinary Cancer Congress. Abstract 9003. 2011 European Multidisciplinary Cancer Congress; September 23-27, 2011.2011. [Google Scholar]
- 43.Kim DH, Xu W, Kamel-Reid S, et al. Clinical relevance of vascular endothelial growth factor (VEGFA) and VEGF receptor (VEGFR2) gene polymorphism on the treatment outcome following imatinib therapy. Ann Oncol. 2010;21:1179–1188. doi: 10.1093/annonc/mdp452. [DOI] [PubMed] [Google Scholar]
- 44.Heist RS, Zhai R, Liu G, et al. VEGF Polymorphisms and Survival in Early-Stage Non–Small-Cell Lung Cancer. J Clin Oncol. 2008;26:856–862. doi: 10.1200/JCO.2007.13.5947. [DOI] [PubMed] [Google Scholar]
- 45.Schneider BP, Wang M, Radovich M, et al. Association of Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor-2 Genetic Polymorphisms With Outcome in a Trial of Paclitaxel Compared With Paclitaxel Plus Bevacizumab in Advanced Breast Cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohn JW, Lee Sy, Lee SJ, et al. MDR1 polymorphisms predict the response to etoposide-cisplatin combination chemotherapy in small cell lung cancer. Jpn J Clin Oncol. 2006;36:137–141. doi: 10.1093/jjco/hyi231. [DOI] [PubMed] [Google Scholar]
- 47.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Huo X, Lin Y, et al. Association of MDR1 and ERCC1 polymorphisms with response and toxicity to cisplatin-based chemotherapy in non-small cell lung cancer patients. Int J Hyg Environ Health. 2010;213:140–145. doi: 10.1016/j.ijheh.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Goeke F, Franzen A, Menon R, et al. Rationale for treatment of metastatic squamous cell carcinoma of the lung using FGFR Inhibitors. Chest. 2012 Apr 12; doi: 10.1378/chest.11-2943. [DOI] [PubMed] [Google Scholar]
- 50.Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One. 2011;6(6):e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss J, Sos ML, Seidel D. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010 Dec 15;2(62):62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]